Abstract
Introduction
Non-invasive mechanical ventilation (NIV) has been used to treat acute respiratory failure (ARF) for approximately 20 years. This guideline addresses the indications for, and limitations of, NIV as treatment for ARF according to evidence-based criteria.
Methods
A panel of experts from 12 scientific medical societies reviewed circa 2900 publications. The panel judged the clinical relevance of these studies and assessed the evidence presented in each, then held two interdisciplinary consensus conferences to formulate guideline recommendations and algorithms.
Results
Whenever possible, NIV should be preferred to invasive mechanical ventilation, in order to avoid the risk of ventilator and tube-associated complications such as nosocomial pneumonia (grade of recommendation A). Particularly in patients with hypercapnic ARF, NIV reduces the rate of hospital-acquired pneumonia, the length of hospital stay and mortality in the intensive care unit and in the hospital (grade of recommendation A). NIV (or continuous positive airway pressure) is also recommended in cardiogenic pulmonary edema (grade of recommendation A), as treatment for ARF in immunocompromised patients (grade of recommendation A), to prevent postextubation failure, to facilitate weaning in patients with hypercapnic ARF (grade of recommendation A), and to improve dyspnea in palliative care (grade of recommendation C). NIV is not generally recommended in patients with hypoxic ARF because of its high failure rate of 30% to over 50% in such patients.
Discussion
Although evidence indicates that NIV can be used as the treatment of first choice for several indications, it is still underutilized in the acute setting. These guidelines provide evidence-based information about the indications for, and limitations of, NIV in the treatment of ARF.
Keywords: non-invasive positive pressure ventilation, evidence-based medicine, therapy, acute respiratory distress syndrome, respiratory insufficiency
Introduction
When treating acute respiratory failure, physicians must ask what form of ventilation is best for the individual patient, i.e., what form of ventilation provides the greatest benefit with the lowest risk. In past decades, mechanical ventilation by way of an invasive access route – an endotracheal tube or tracheostomy – became established as life-saving treatment for acute respiratory failure (ARF). Over the last 20 years, however, a great deal of clinical research has shown that non-invasive ventilation (NIV), too, is a valuable form of treatment for ARF (table 1). NIV is not yet firmly established in acute care in all institutions. According to an international study, 4.9% of 5183 ventilated patients in intensive care units were treated with NIV (1). The NIV rate was only 16.9% among patients who were ventilated because of exacerbations of chronic obstructive pulmonary disease (COPD), despite the fact that good evidence supporting the use of NIV for this indication has been available for several years (2). The utility of NIV is still variably assessed depending on the indication (2, 3).
Table 1. Strength of recommendations for the use of non-invasive ventilation (NIV) for the treatment of conditions involving acute respiratory failure (ARF), according to indication.
| Strength of indication for the use of NIV | Indication for NIV in ARF |
|---|---|
| High (multiple controlled studies) | COPD exacerbations |
| Acute cardiogenic pulmonary edema | |
| ARF in immunosuppressed patients | |
| Weaning from the ventilator in COPD patients | |
| Intermediate (few controlled studies/many case series) | Postoperative respiratory failure |
| Avoidance of extubation failure | |
| Do-not-intubate order | |
| Weak or not to be recommended | Acute respiratory distress syndrome (ARDS) |
| Trauma | |
| Cystic fibrosis |
On this background, 12 scientific medical societies in Germany have now formulated a guideline for the use of NIV in the treatment of ARF. The leading role was played by the German Society for Pulmonology and Respiratory Medicine (Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin, DGP) under the aegis of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V., AWMF). This class S3 guideline is based on a systematic analysis of the literature and an interdisciplinary consensus process (e1).
This guideline contains verified indications, contraindications, and criteria for the monitoring and termination of NIV, as well as the advantages and disadvantages of NIV compared with invasive ventilation. The main goal of this guideline is to encourage broader use of this form of treatment in acute care medicine on the basis of the scientific evidence. In this article, the method of guideline development is briefly described and the core recommendations are stated. The full-length version of the guideline can be viewed online at the AWMF website (http://awmf.org) and will be published in August 2008 in the German-language medical journal Pneumologie (the official journal of the DGP).
Methods
This guideline was developed over a period of three years, from early 2005 to late 2007. A total of 28 experts representing 12 medical specialty societies (box 1) formed six working groups, each of which was devoted to a different indication for NIV.
Box 2. List of key words searched.
Each search was performed on sets of key words including terms from the common search list and terms from the specific working list of one of the working groups, connected with the "AND" operator.
Common search list for all working groups:
NIV, NPPV, NIPPV, non invasive ventilation, non-invasive ventilation, noninvasive ventilation, noninvasive positive pressure, noninvasive positive-pressure, noninvasive mechanical ventilation, mask ventilation, nasal ventilation
Specific search lists for each working group:
-
Working Group 1 – hypercapnic ARF:
hypercapnia, hypercapnic respiratory failure, hypercapnic exacerbation, arterial hypercarbia, acute respiratory failure, acute respiratory insufficiency, acute exacerbation, exacerbation of chronic obstructive pulmonary disease, COPD
-
Working Group 2 – hypoxemic ARF:
non hypercapnic respiratory failure, acute hypoxemic respiratory failure, respiratory failure, acute cardiogenic pulmonary oedema, acute cardiac failure, pulmonary edema, decompensated heart failure, pneumonia, ARDS, ALI, non-COPD
-
Working Group 3 – change of ventilation interface:
acute respiratory failure, weaning from respirator, weaning from mechanical ventilation, extubation failure, NIV failure, invasive mechanical ventilation, reintubation, ventilator-associated pneumonia, VAP
-
Working Group 4 – NIV in the perioperative phase:
acute respiratory failure, extubation failure, post-operative hypoxemia, postoperative, surgery, surgical patients, early extubation
-
Working Group 5 – technique, logistics, and site of ventilation:
ventilators, mode, setting, interfaces, mask, helmet, pressure support, location, intensive care unit, ICU, intermediate care unit, normal ward, respiratory ward, hospital ward, education
-
Working Group 6 – palliative use of NIV:
palliation, cancer, malignancy, end stage disease, do not intubate, DNI, do not resuscitate, DNR
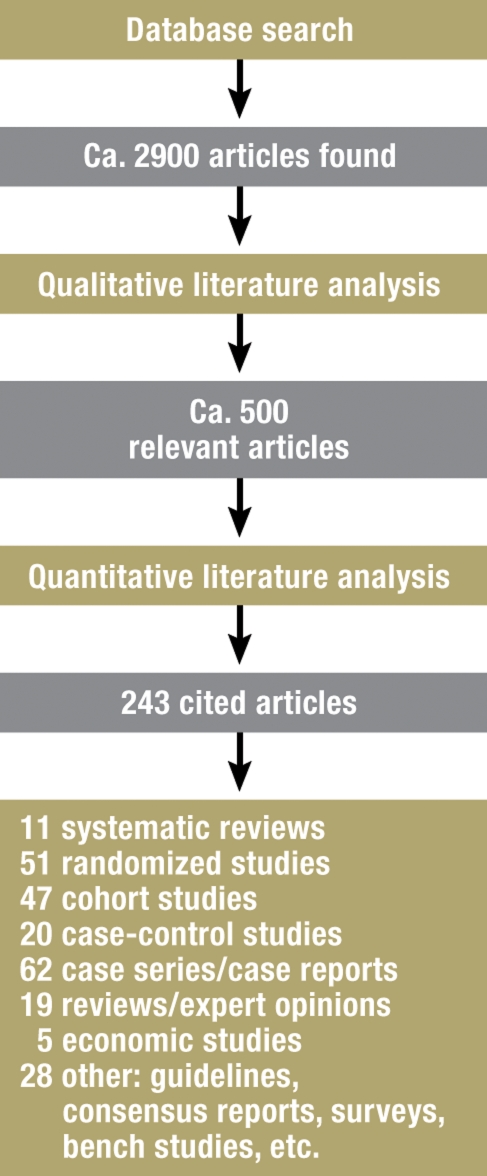
A central coordinating group carried out the literature searches and provided logistical aid with the analysis of the literature. A search on the terms listed in box 2 in the Medline and Cochrane databases, performed in September 2005, yielded 2425 hits. This catalogue of the relevant literature was updated multiple times up to June 2007, resulting in the addition of 476 further references for a total of about 2900 (figure 1). Further, unquantified references were derived from the reference lists of review articles, from the personal archives of the participating experts, and from informal knowledge about forthcoming publications. The number of references evaluated by each individual working group ranged from 350 to 1050.
Figure 1.
Literature search flowchart
With the aid of electronic evaluation tables, more than 90% of the abstracts were accessible over Medline through hyperlinks. This manner of working reduced the need for individual searching and paperwork. Further support from the coordinating group consisted of the administration and distribution of meta-information about the literature search and general instructions about the evaluation of scientific evidence. All participants were informed about the current state of the project in a newsletter that was sent a total of 16 times.
Assessment of relevance and qualitative evaluation of scientific evidence
The goal of the initial selection procedure was the identification of publications that would be relevant to the development of the guideline from among the 2900 publications that were revealed by the search. In this phase, all publications were assessed according to criteria of clinical relevance. Publications were excluded if
the patients treated did not have ARF (e.g., in studies of outpatient ventilation treatment),
treatment with NIV was not the subject of the study, or
the publication was clearly not sufficiently evidence-based.
The latter aspect will be discussed more fully in what follows. In general, publications in English or in German were considered. Every article was evaluated by two experts. Differing judgments over inclusion versus exclusion were resolved internally in each working group. The percentage of publications selected as sources for evaluation of the scientific evidence varied from 15% to 25% among the working groups.
Even in this initial phase, account had to be taken of the fact that the use of NIV for different indications is supported by different levels of evidence. For some indications, the use of NIV has already been shown to be beneficial by randomized studies with large numbers of patients. Thus, for these indications, observational studies and other publications with similar results could be excluded from the further analysis.
Systematic review articles, i.e., review articles with a documented search and meta-analysis of original publications, were accepted for further analysis as long as the data from the publications cited in them were homogeneous. Non-systematic review articles, on the other hand, were classified as expert opinions, and only the original publications that they cited were accepted for further analysis.
Evaluation of scientific evidence
The quantitative evaluation of the scientific evidence provided by each publication was performed by two experts according to the recommendations of the Oxford Centre of Evidence-Based Medicine (CEBM) (4). The classification system used, based on study design and the quality of execution of each study, is shown in table 2. Uniformity of criteria was ensured by the use of standardized checklists (one for original articles and one for systematic reviews). The guideline cited a total of 243 publications (figure 1).
Table 2. Strength-of-evidence rating scheme of the Centre for Evidence-Based Medicine (abridged).
| Recommendation grade | Evidence level | Study design |
|---|---|---|
| A | Ia | Meta-analysis of randomized controlled trials (RCTs) |
| Ib | Single RCT (with narrow confidence interval) | |
| Ic | All-or-none principle | |
| B | IIa | Meta-analysis of well-designed cohort studies |
| IIb | Single well-designed cohort study or RCT of lesser quality | |
| IIc | Outcome research | |
| IIIa | Meta-analysis of case-control studies | |
| IIIb | Single case-control study | |
| C | IV | Case series or cohort/case-control studies of lesser quality |
| D | V | Expert opinion without explicit evaluation of scientific evidence or based on physiological models or laboratory research |
Two interdisciplinary consensus conferences were held in July 2006 and April 2007 under the auspices of the AWMF. Representatives of the nursing professions and of manufacturers of ventilation apparatus also participated (the latter without vote). The first conference established the central recommendations and algorithms of the guideline; in the second conference, the recommendations were cast in their final wording and the strength of the recommendations was established on the basis of the state of the evidence for each.
Results
Invasive and non-invasive ventilation
There is no doubt that invasive mechanical ventilation is often an indispensable life-saving measure, but it is also associated with the risk of endotracheal tube or ventilator associated pneumonia, leading to high mortality as well as to markedly elevated costs (e2). Invasive ventilation should be avoided in favor of NIV or early extubation whenever possible so that this serious complication can be avoided (grade A recommendation) (e3). NIV has major advantages over invasive ventilation with respect to both respiratory mechanics and infectious disease (table 3).
Table 3. Characteristics of invasive and non-invasive ventilation.
| Complications and clinical aspects | Invasive ventilation | Non-invasive ventilation |
|---|---|---|
| Ventilator (endotracheal tube) associated pneumonia | Increased risk after 3 or 4 days of ventilation | Rare |
| Additional work of breathing due to the endotracheal tube | Yes (during spontaneous breathing and in case of inadequate compensation for the endotracheal tube) | No |
| Early and late tracheal damage | Yes | No |
| Sedation | Often necessary | Rarely necessary |
| Intermittent application | Rarely possible | Often possible |
| Effective coughing possible | No | Yes |
| Eating and drinking possible | Difficult with tracheostomy, not possible with intubation | Yes |
| Communication possible | Difficult | Yes |
| Upright body posture | Limited feasibility | Often possible |
| Difficult weaning from ventilator | 10% to 20% | Rare |
| Airway access | Direct | Difficult |
| Pressure sites on the face | No | Sometimes |
| Back-breathing of CO2 | No | Rare |
| Leakage | Very little | Usually present to a greater or lesser extent |
| Aerophagy | Very little | Sometimes |
One advantage of invasive mechanical ventilation in respiratory failure, and particularly in acute respiratory distress syndrome (ARDS), is the greater constancy of pressure, because the lung can collapse if the positive airway pressure is interrupted even for a very short time (e4, 5). NIV, therefore, should not be used if there are important clinical reasons to ventilate invasively (grade B recommendation). NIV is absolutely contraindicated (grade D recommendation) in the following situations:
Lack of spontaneous breathing; gasping
Anatomical or functional airway obstruction
Gastrointestinal bleeding or ileus (6).
If a relative contraindication is present (box 3), a trial of NIV can be considered in individual cases, as long as the patient is meticulously observed at close intervals and can be intubated without delay if necessary.
Box 3. Contraindications.
Absolute contraindications
Lack of spontaneous breathing; gasping
Anatomical or functional airway obstruction
Gastrointestinal bleeding or ileus
Relative contraindications
Coma
Massive agitation
Massive retention of secretions despite bronchoscopy
Severe hypoxemia or acidosis (pH < 7.1)
Hemodynamic instability (cardiogenic shock, myocardial infarction)
Anatomical and/or subjective difficulty gaining access to the airway
Status post upper gastrointestinal surgery
Techniques of non-invasive ventilation
Negative and positive pressure ventilation – The favored mode of ventilation for the treatment of ARF is positive pressure ventilation with inspiratory pressure support (assisted mode). This is often combined with positive end-expiratory pressure (PEEP). A minimum respiratory frequency must be set to protect the patient against apnea, and oxygen should be given in whatever amount necessary to ensure a saturation of 85% to 90% (grade D recommendation) (2). Inspiratory pressure support with a mask or helmet (e5) reduces the patient’s work of breathing; furthermore, the application of positive pressure raises transpulmonary pressure and thereby enlarges the end-expiratory lung volume. This effect prevents alveolar closure and promotes the recruitment of alveolar volume (atelectases). Negative pressure respiration, e.g., with a "tank," is only rarely used at present (e6).
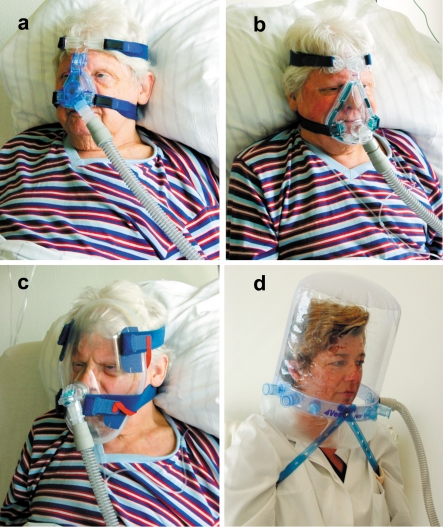
Interface – There is a broad spectrum of ventilation interfaces in the area of the face, including nose masks, full face masks, and total face masks (illustration a–c). Mouth-nose masks are preferred mainly in the initial phase of treatment (e7). A ventilation helmet enclosing the entire head (illustration d) is mainly used for patients with hypoxemic ARF (e8). The advantages and disadvantages of the different types of ventilation interface are summarized in table 4.
Illustration: Different types of ventilation interface: (a) nose mask, (b) full face mask, (c) total face mask, and (d) ventilation helmet
Table 4. The advantages and disadvantages of common types of ventilation interface.
| Aspect | Nose mask | Nose-mouth mask | Helmet |
|---|---|---|---|
| Oral leakage | – | + | + |
| Volume monitoring | – | + | – |
| Initial response of blood gases | 0 | + | 0 |
| Speaking | + | – | 0 |
| Expectoration | + | – | – |
| Risk of aspiration | + | 0 | + |
| Aerophagy | + | 0 | 0 |
| Claustrophobia | + | 0 | 0 |
| Dead space (compressible volume) | + | 0 | – |
| Noise and limitation of hearing | + | + | – |
+, advantage; o, neutral; –, disadvantage
Ventilation apparatus and modes of ventilation – The spectrum of ventilation apparatus ranges from easy-to-use portable equipment to technically demanding intensive-care ventilators (e9). Unlike patients treated with at-home ventilation because of chronic ventilatory insufficiency, patients with ARF are often markedly agitated and have a pronounced respiratory drive; thus, they are only rarely treated with controlled (i.e., time-based) ventilation and much more often with ventilation that is triggered by the patient’s own respiratory efforts (e9).
Important practical considerations for NIV
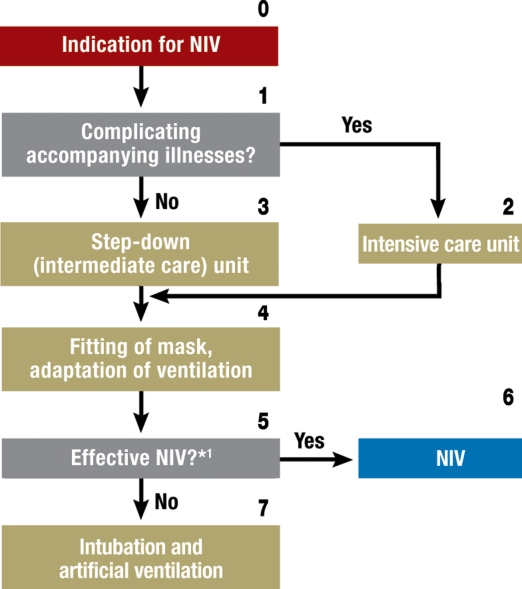
The manner in which NIV is used to treat ARF is largely independent of the specific indication and is presented in figure 2. In particular, the adequacy of ventilation should be verified during the first one to two hours of treatment, and its beneficial effect should be observed. The criteria for successful ventilation are listed in table 5. Stable, low pH values and a stably elevated PaCO2 (arterial partial pressure of carbon dioxide) can be tolerated for longer than 2 hours during the NIV adaptation phase, as long as the patient’s clinical condition and the other success criteria listed in table 5 are improving (grade C recommendation) (e10).
Table 5. Criteria for the success of non-invasive ventilation.
| Criterion | Success |
|---|---|
| Dyspnea | Decrease |
| Alertness | Gradual improvement |
| Respiratory rate | Decrease |
| Ventilation | Decrease in PaCO2 |
| pH | Increase |
| Oxygenation | Rise of SaO2 to 85% or above |
| Heart rate | Decrease |
The most important parameters for monitoring the course of the adaptation phase are the arterial blood gases, the respiratory rate, the patient’s subjective experience of dyspnea, and the patient’s level of alertness (grade C recommendation) (2, e11–e13). In case NIV fails, it should be terminated immediately and the patient should be intubated without delay (grade C recommendation).
In the initial phase of the treatment of ARF with NIV, the personnel-to-patient ratio is relatively high at 1:1. Over the further course of treatment, NIV results in a saving of time and effort on the part of the treatment personnel (e14–e17). NIV for the treatment of ARF should preferably be performed in an intensive care unit (grade D recommendation). In cases of isolated respiratory failure (single-organ failure), NIV can also be provided in an intermediate care (step-down) unit. Depending on the local circumstances and resources, NIV might also be provided, in individual cases, on a specialized normal patient ward (e18–e22).
The spectrum of indications
The guideline provides recommendations for the following groups of indications:
Hypercapnic ARF
Hypoxemic ARF
Cardiogenic pulmonary edema
The perioperative phase
Early weaning and the postextubation phase
NIV in pediatrics
Palliation.
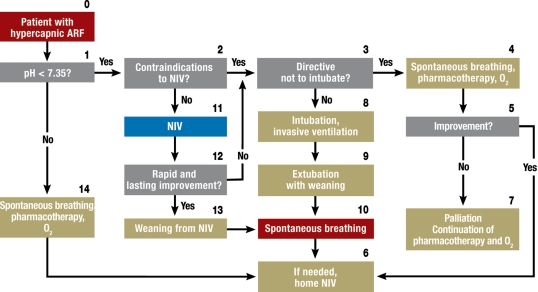
Hypercapnic ARF – The most common cause of hypercapnic ARF, defined as pH <7.35 and PaCO2 >45 mmHg, is an acute exacerbation of COPD (AE-COPD). The elevated airway resistance, dynamic overexpansion of the lungs, and consequent flattening of the diaphragm put excessive demands on the respiratory muscles, leading to imminent exhaustion (e23, e24).
In "mild to moderately severe AE-COPD," with a pH of 7.30 to 7.35, NIV should be applied early (grade A recommendation) (8). When used in combination with standard therapy, NIV already lowers the PaCO2, improves the pH, and lowers the respiratory rate within one hour of the initiation of treatment. NIV reduces the frequency of intubation and lessens both the duration of hospital stay and mortality (9, 10, e25). The effectiveness of NIV has also been demonstrated for AE-COPD with pH in the broader range of 7.20 to 7.35 (8, e26). The clinical algorithm for the use of NIV in hypercapnic ARF is shown in figure 3.
Figure 2.
Algorithm for the application of non-invasive ventilation (NIV).
*1 Possible reasons for failure of non-invasive ventilation: patient’s inability to cooperate, functional glottal closure, mechanical obstruction or swelling in the glottal area.
Hypoxemic ARF – The data are less clear regarding the value of NIV in hypoxemic, rather than hypercapnic, ARF.
An experienced team of researchers has shown that NIV in patients with purely hypoxemic ARF significantly lowers the frequency of intubation, the rate of septic shock, and the 90-day mortality compared to standard therapy (11). NIV has also been used with success to treat mixed hypercapnic and hypoxemic ARF, e.g., in COPD patients with pneumonia (e16, e27–e29).
The use of continuous positive airway pressure (CPAP) and NIV is recommended for immunosuppressed patients with (hemato-)oncological disorders, as well as for patients with AIDS and Pneumocystis jirovecii pneumonia, also known as Pneumocystis carinii pneumonia (grade A recommendation) (12, 13, e30).
NIV should only be used to treat ARDS if the patient is closely monitored in a specialized treatment center (grade D recommendation). The NIV failure rate in a mixed group of patients with hypoxemic ARF was quite high: 30% in patients with community-acquired pneumonia and 50% in patients with ARDS (9, 14). The main causes of NIV failure are to be sought in the complex pathophysiology of hypoxemic forms of ARF and the inadequate constancy of pressure provided by NIV, particularly in the expiratory phase.
Cardiogenic pulmonary edema – There is now clear evidence demonstrating the utility of NIV and CPAP, alongside standard medical therapy, in the treatment of cardiogenic pulmonary edema (15, e31). Patients with hypoxemic ARF due to cardiogenic pulmonary edema should first be given oxygen by the nasal route and should then be treated primarily with CPAP (grade A recommendation). The indicated pharmacotherapy and necessary cardiological interventions, such as catheterization, must also be performed without delay (grade D recommendation) (e32–e34). In this situation, CPAP reduced cardiac preload and afterload, reduces the work of breathing, improves coronary perfusion, and normalizes the ventilation-perfusion ratio. If cardiogenic pulmonary edema is associated not only with hypoxemia, but also with hypercapnia, then CPAP should be provided in combination with inspiratory pressure support, i.e., it should be provided as NIV (e35-e39).
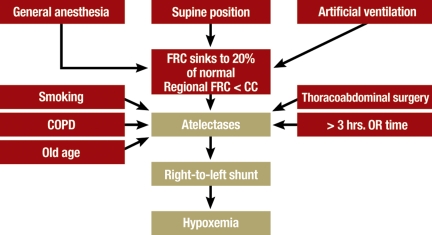
ARF in the perioperative phase – The reduced functional residual capacity in mechanical ventilation (e40) leads to the end-expiratory closure of smaller airways and the formation of atelectases (figure 4), which may persist for a few days after surgery. The severity of the postoperative impairment of pulmonary function is determined, among other factors, by preoperative risk factors such as smoking, COPD, high ASA status (ASA = American Society of Anesthesiologists), and age as well as by the nature and duration of the operative procedure (e41). The frequency of reintubation after major surgical procedures can be as high as 20% (e42, e43). In patients at elevated risk for postoperative hypoxemic ARF, the rate of reintubation and other complications can be significantly lowered by the early use of CPAP and NIV immediately after extubation (grade B recommendation). In some studies regarding the use of NIV to treat postoperative ARF in cardiothoracic surgery, improvements have been reported not only with respect to gas exchange and hemodynamics, but also a lower intubation rate, fewer complications, and reduced mortality (16, e44–e46).
Figure 3.
Algorithm for non-invasive ventilation (NIV) for the treatment of hypercapnic acute respiratory failure (ARF)
NIV can be used to improve ventilation and oxygenation during bronchoscopy (e47–e50) (grade C recommendation). Because of the paucity of clinical data, no general recommendation can be given regarding the use of NIV in the preparatory phase before surgery.
Weaning from the ventilator and the post-extubation phase – Invasively ventilated patients with COPD should be extubated as early as possible and switched to NIV (grade A recommendation). In such patients, extubation followed by NIV was shown to improve the success rate of weaning to a statistically significant extent as compared with continued invasive ventilation in a control group. It also lowered mortality and the rates of reintubation, tracheotomy, and other complications. The utility of NIV in difficult weaning due to hypoxemic (as opposed to hypercapnic) respiratory failure is still being debated.
Above all in elderly COPD patients with chronic heart failure and hypersecretion, who are at high risk of developing hypercapnic ARF after extubation, the early application of NIV lowers the rates of reintubation and death (e51, e52, 17, 18). Data from randomized controlled studies do not support the use of NIV in patients with hypoxemic ARF after extubation (3, e53). The only positive results to date from the use of NIV in cases of difficult weaning due to hypoxic respiratory failure are derived from small, single-center studies (e54).
NIV in pediatrics – No randomized studies have been published that demonstrate any advantage of NIV over invasive ventilation in children and adolescents, except in the neonatal period. Consequently, the guideline recommendations in this area are based on observational studies.
ARF in children and adolescents can be effectively treated with NIV (grade C recommendation). In ARF due to cystic fibrosis, a trial of NIV should be performed in preference to invasive ventilation, as long as NIV is not contraindicated (grade C recommendation). The same holds for children with neuromuscular disorders (grade C recommendation) and for immunosuppressed children (grade C recommendation). NIV to treat ARF in children should always be performed in an intensive care unit (grade C recommendation).
NIV in palliative care – NIV can be used palliatively to relieve dyspnea and improve the quality of life (grade C recommendation) (e55, e56, 19). Even if a patient has issued an advance directive that he or she should not be intubated, NIV can still be initiated after the patient has been comprehensively informed about it, as long as there is no objection to mechanical ventilation as such (grade B recommendation) (e55, 19, e57, e58, e59, 20, e60). A recent study revealed that about 30% of patients who died in an intermediate-care unit were treated with NIV (19). While being treated with NIV, patients continue to possess a certain degree of autonomy. In such situations, physicians must monitor the treatment with all due care to ensure that the effect of NIV is not merely to prolong the patient’s suffering and/or the dying process (e61).
Discussion
The currently available scientific evidence shows that NIV is markedly superior to invasive ventilation for the treatment of hypercapnic ARF (box 4) and cardiogenic pulmonary edema, as well as hypoxemic ARF in immunosuppressed patients. Further indications are the postoperative prophylaxis against reintubation and difficult weaning after prolonged invasive ventilation for hypercapnic respiratory failure. The high failure rate of NIV (30% to over 50%) in patients with hypoxemic ARF argues against the routine use of NIV for this indication.
Box 4. Clinical take home messages.
Non-invasive ventilation (NIV) is preferable to invasive ventilation whenever possible.
The advantages of NIV are greatest in the treatment of hypercapnic respiratory failure.
The most important parameters of clinical course are PaCO2 (the arterial partial pressure of carbon dioxide), pH, respiratory rate, dyspnea, and alertness; these must show a trend toward improvement in the first 2 hours of NIV.
NIV failure may occur early or after a few days. NIV failure must be recognized in timely fashion so that the patient can be intubated without delay.
Hypoxemic ARF should generally not be treated with NIV except in selected patients and under meticulously controlled conditions.
Despite the good evidence that is available for the above indications, NIV is still underused in the everyday practice of intensive care medicine. This guideline is intended to help NIV become more firmly established in practice.
Limitations and weaknesses of this guideline
The recommendations of evidence-based medicine are usually derived from studies that were performed under controlled conditions on selected groups of patients, often in a single institution, by teams with extensive clinical experience. Thus, the generalization of study results to everyday clinical practice is not justified without further qualification. Furthermore, a lack of experience, staff, and equipment often stand in the way of the successful use of NIV (e62), particularly when it is used for indications in which its efficacy is less well documented. Finally, a guideline is in any case no more than a snapshot of the current state of scientific knowledge. This guideline is only valid until three years after publication; an update will be needed then, at the latest.
Box 1. Participating medical societies and experts.
(1) German Society for Pulmonology and Respiratory Medicine (Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin e.V.) (initiating society)
(2) Working Group for Home Ventilation and Ventilator Weaning (Arbeitsgemeinschaft Heimbeatmung und Respiratorentwöhnung e.V.)
(3) German Society for Anesthesiology and Intensive Care Medicine (Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin e.V.)
(4) German Surgical Society (Deutsche Gesellschaft für Chirurgie e.V.)
(5) German Society for Nursing and Allied Health Care Professions (Deutsche Gesellschaft für Fachkrankenpflege und Funktionsdienste e.V.)
(6) German Geriatric Society (Deutsche Gesellschaft für Geriatrie e.V.)
(7) German Society for Intensive Care in Internal Medicine (Deutsche Gesellschaft für Internistische Intensivmedizin e.V.)
(8) German Cardiological Society (Deutsche Gesellschaft für Kardiologie e.V.)
(9) German Society for Neonatology and Pediatric Intensive Care Medicine (Deutsche Gesellschaft für Neonatologie und pädiatrische Intensivmedizin e.V.)
(10) German Neurological Society (Deutsche Gesellschaft für Neurologie e.V.) and German Society for Neurological Intensive Care and Emergency Medicine (Deutsche Gesellschaft für Neurologische Intensiv- und Notfallmedizin e.V.)
(11) German Society for Palliative Medicine (Deutsche Gesellschaft für Palliativmedizin e.V.)
(12) Spectaris Industrial Association (Industrieverband Spectaris)
The numbers in parentheses indicate the memberships of the listed experts to the societies named above:
Dr. med. Thomas Barchfeld, Fachkrankenhaus Kloster Grafschaft (1,2)
Prof. Dr. med. Heinrich F. Becker, Asklepios Klinik Bambek,
Department of Pulmonology (1)
Dr. med. Martina Bögel*1, Weinmann GmbH, Hamburg (12)
Andreas Bosch*1, Heinen & Löwenstein GmbH, Bad Ems,
1st consensus conference (12)
(Dr. Ulrich Brandenburg, Heinen & Löwenstein GmbH, Bad Ems,
2nd consensus conference [12])
Prof. Dr. med. Carl Criée, Ev. Krankenhaus Göttingen-Weende,
Dept. of Pulmonology (1,2)
Rolf Dubb, Katharinenhospital, Klinikum Stuttgart (5)
Dr. med. Hans Fuchs, University Pediatric Clinic, Ulm (9)
Dr. med. Holger Hein*2, Reinbek, formerly of the Krankenhaus
Grosshansdorf (1,2)
Dr. med. H. J. Heppner, Klinikum Nürnberg Nord, Geriatrics Department (6)
Prof. Dr. med. Uwe Janssens, St. Antonius Hospital, Eschweiler,
Cardiology Dept. (8)
Dr. med. Thomas Jehser, Gemeinschaftskrankenhaus Havelhöhe,
Dept. of Palliative Medicine (11)
Dr. med. Ortrud Karg, Asklepios Fachkliniken, Gauting,
Pulmonology Dept. (1,2)
PD Dr. med. Erich Kilger, Cardiology Dept., Ludwig-Maximilian University,
Munich (3)
Prof. Dr. med. Hermann H. Klein*2, Klinikum Idar-Oberstein GmbH,
Cardiology Dept. (8)
Prof. Dr. med. Dieter Köhler, Fachkrankenhaus Kloster Grafschaft (1,2)
Dr. med. Thomas Köhnlein, Medizinische Hochschule Hannover,
Pulmonology Dept. (1,2)
Prof. Dr. med. Ralf Kuhlen, Helios Klinikum, Berlin Buch,
Department of Intensive Care Medicine (3)
Prof. Dr. med. Martin Max, Centre Hospitalier de Luxembourg (3)
Dr. med. Michael Metze, Universitätsklinik Leipzig, Surgical Center (4)
PD Dr. med. F. Joachim Meyer, Ruprecht-Karl University, Heidelberg.
Dept. of Medicine III (1,2)
PD Dr. med. Wolfgang Müllges, University Dept. of Neurology, Würzburg (9)
Prof. Dr. med. Peter Neumann, Ev. Krankenhaus Göttingen-Weende,
Department of Anesthesiology (3)
Prof. Dr. med. Christian Putensen, Rhineland’s Friedrich-Wilhelm University,
Bonn (3)
Prof. Dr. med. Bernd Schönhofer, Klinikum Region Hannover,
Pulmonology Dept. (1,2)
Dr. med. Dierk Schreiter, Universitätsklinik Leipzig, Surgical Center (4)
Dr. med. Jan Storre, Albert-Ludwig University, Freiburg,
Pulmonology Dept. (1,2)
Prof. Dr. med. Tobias Welte, Medizinische Hochschule Hannover,
Pulmonology Dept. (1,7)
Dr. med. Michael Westhoff, Lungenklinik Hemer (1,2)
PD Dr. med. Wolfram Windisch, Albert-Ludwig University, Freiburg,
Pulmonology Dept. (1,2)
Dr. med. Holger Woehrle*2, Martinsried, formerly of the University of Ulm (1,2)
1 Non-voting participant in the consensus conferences
2 Ceased participating before the conclusion of the project
Figure 4.
Causes of postoperative atelectasis. FRC, functional residual capacity; CC, closure capacity
Acknowledgments
The authors thank the German Society of Pulmonology and Respiratory Medicine (Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin, DGP) for financing a position for a scientific assistant as well as the consensus conferences. We also thank the AG Pneumologischer Kliniken, the AG Heimbeatmung und Respiratorentwöhnung, and the German Society of Anesthesiology and Intensive Care Medicine (Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin, DGAI) for their co-financing of the position for a scientific assistant.
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Schönhofer has received lecture honoraria from the ResMed and Heinen & Löwenstein companies. Prof. Kuhlen has received lecture honoraria from the ResMed, Dräger, Tyco, Viasys, and Maquet companies. Prof. Neumann has received lecture fees from the manufacturers Dräger and B+P Beatmungsprodukte GmbH. Dr. Westhoff, Dipl.-Ing. Berndt, and PD Dr. Sitter declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 2.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 3.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 4.Phillips B, Ball C, Sackett D, et al. Levels of Evidence. Oxford Centre for Evidence-Based Medicin. 2001 [Google Scholar]
- 5.Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35:18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 6.British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax. 2001;56:708–712. doi: 10.1136/thorax.56.9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935. doi: 10.1016/s0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- 9.Peter JV, Moran JL, Phillips-Hughes J, et al. Noninvasive ventilation in acute respiratory failure - a meta-analysis update. Crit Care Med. 2002;30:555–562. doi: 10.1097/00003246-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ram FS, Wellington S, Rowe BH, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD004360.pub2. CD004360. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer M, Esquinas A, Leon M, et al. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168:1438–1444. doi: 10.1164/rccm.200301-072OC. [DOI] [PubMed] [Google Scholar]
- 12.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 13.Confalonieri M, Calderini E, Terraciano S, et al. Noninvasive ventilation for treating acute respiratory failure in AIDS patients with Pneumocystis carinii pneumonia. Intensive Care Med. 2002;28:1233–1238. doi: 10.1007/s00134-002-1395-2. [DOI] [PubMed] [Google Scholar]
- 14.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27:1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 15.Winck JC, Azevedo LF, Costa-Pereira A, et al. Efficacy and safety of non-invasive ventilation in the treatment of acute cardiogenic pulmonary edema - a systematic review and meta-analysis. Crit Care. 2006;10:R69–R69. doi: 10.1186/cc4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auriant I, Jallot P, Hervé P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164:1231–1235. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- 17.Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465–2470. doi: 10.1097/01.ccm.0000186416.44752.72. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 19.Nava S, Sturani C, Hartl S, et al. End-of-life decision-making in respiratory intermediate care units: a European survey. Eur Respir J. 2007;30:156–164. doi: 10.1183/09031936.00128306. [DOI] [PubMed] [Google Scholar]
- 20.Chu CM, Chan VL, Wong IW, et al. Noninvasive ventilation in patients with acute hypercapnic exacerbation of chronic obstructive pulmonary disease who refused endotracheal intubation. Crit Care Med. 2004;32:372–377. doi: 10.1097/01.CCM.0000108879.86838.4F. [DOI] [PubMed] [Google Scholar]
- e1.Kopp IB, Lorenz W, Müller W, et al. Methodische Empfehlungen zur Leitlinienerstellung. 2004. http://www.uni-duesseldorf.de/AWMF/ll/index.html. [Google Scholar]
- e2.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- e3.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32:1396–1405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- e4.Neumann P, Berglund JE, Mondejar EF, et al. Effect of different pressure levels on the dynamics of lung collapse and recruitment in oleic-acid-induced lung injury. Am J Respir Crit Care Med. 1998;158:1636–1643. doi: 10.1164/ajrccm.158.5.9711095. [DOI] [PubMed] [Google Scholar]
- e5.Moerer O, Fischer S, Hartelt M, et al. Influence of two different interfaces for noninvasive ventilation compared to invasive ventilation on the mechanical properties and performance of a respiratory system: a lung model study. Chest. 2006;129:1424–1431. doi: 10.1378/chest.129.6.1424. [DOI] [PubMed] [Google Scholar]
- e6.Gorini M, Ginanni R, Villella G, et al. Non-invasive negative and positive pressure ventilation in the treatment of acute chronic respiratory failure. Intensive Care Med. 2004;30:875–881. doi: 10.1007/s00134-003-2145-9. [DOI] [PubMed] [Google Scholar]
- e7.Kwok H, McCormack J, Cece R, et al. Controlled trial of oronasal versus nasal mask ventilation in the treatment of acute respiratory failure. Crit Care Med. 2003;31:468–473. doi: 10.1097/01.CCM.0000045563.64187.20. [DOI] [PubMed] [Google Scholar]
- e8.Antonelli M, Conti G, Pelosi P, et al. New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet - a pilot controlled trial. Crit Care Med. 2002;30:602–608. doi: 10.1097/00003246-200203000-00019. [DOI] [PubMed] [Google Scholar]
- e9.Schonhofer B, Sortor-Leger S. Equipment needs for noninvasive mechanical ventilation. Eur Respir J. 2002;20:1029–1036. doi: 10.1183/09031936.02.00404202. [DOI] [PubMed] [Google Scholar]
- e10.Meduri GU, Conoscenti CC, Menashe P, et al. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest. 1989;95:865–870. doi: 10.1378/chest.95.4.865. [DOI] [PubMed] [Google Scholar]
- e11.Bott J, Carroll MP, Conway JH, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557. doi: 10.1016/0140-6736(93)90696-e. [DOI] [PubMed] [Google Scholar]
- e12.Ambrosino N, Foglio K, Rubini F, et al. Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: correlates for success. Thorax. 1995;50:755–757. doi: 10.1136/thx.50.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Meduri GU, Abou-Shala N, Fox RC, et al. Noninvasive face mask mechanical ventilation in patients with acute hypercapnic respiratory failure. Chest. 1991;100:445–454. doi: 10.1378/chest.100.2.445. [DOI] [PubMed] [Google Scholar]
- e14.Nava S, Evangelisti I, Rampulla C, et al. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest. 1997;111:1631–1638. doi: 10.1378/chest.111.6.1631. [DOI] [PubMed] [Google Scholar]
- e15.Bott J, Baudouin SV, Moxham J. Nasal intermittent positive pressure ventilation in the treatment of respiratory failure in obstructive sleep apnoea. Thorax. 1991;46:457–458. doi: 10.1136/thx.46.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Confalonieri M, Potena A, Carbone G, et al. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–1591. doi: 10.1164/ajrccm.160.5.9903015. [DOI] [PubMed] [Google Scholar]
- e17.Kramer N, Meyer TJ, Meharg J, et al. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151:1799–1806. doi: 10.1164/ajrccm.151.6.7767523. [DOI] [PubMed] [Google Scholar]
- e18.Corrado A, Roussos C, Ambrosino N, et al. Respiratory intermediate care units: a European survey. Eur Respir J. 2002;20:1343–1350. doi: 10.1183/09031936.02.00058202. [DOI] [PubMed] [Google Scholar]
- e19.Leger P, Laier-Groeneveld G. Infrastructure, funding and follow-up in a programme of noninvasive ventilation. Eur Respir J. 2002;20:1573–1578. doi: 10.1183/09031936.02.00405802. [DOI] [PubMed] [Google Scholar]
- e20.Schonhofer B. Respiratory high-dependency units in Germany. Monaldi Arch Chest Dis. 1999;54:448–451. [PubMed] [Google Scholar]
- e21.Schönhofer B, Wagner TO. Ort der maschinellen Beatmung im Beatmungszentrum - Intensivstation, Intermediate care oder spezialisierte Normalstation. Pneumologie. 2006;60:376–382. doi: 10.1055/s-2006-932134. [DOI] [PubMed] [Google Scholar]
- e22.Becker HF, Schönhofer B, Vogelmeier C. Intermediate-Care-Units und nichtinvasive Beatmung. Med Klin (München) 2006;101:334–339. doi: 10.1007/s00063-006-1043-7. [DOI] [PubMed] [Google Scholar]
- e23.Crieé CP, Laier-Groeneveld G. Die Atempumpe. Atemw- Lungenkrkh. 1995:94–101. [Google Scholar]
- e24.Vassilakopoulos T, Zakynthinos S, Roussos C. Respiratory muscles and weaning failure. Eur Respir J. 1996;9:2383–2400. doi: 10.1183/09031936.96.09112383. [DOI] [PubMed] [Google Scholar]
- e25.Keenan SP, Sinuff T, Cook DJ, et al. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med. 2003;138:861–870. doi: 10.7326/0003-4819-138-11-200306030-00007. [DOI] [PubMed] [Google Scholar]
- e26.Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707. doi: 10.1007/s00134-002-1478-0. [DOI] [PubMed] [Google Scholar]
- e27.Wysocki M, Tric L, Wolff MA, et al. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest. 1995;107:761–768. doi: 10.1378/chest.107.3.761. [DOI] [PubMed] [Google Scholar]
- e28.Martin TJ, Hovis JD, Costantino JP, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–813. doi: 10.1164/ajrccm.161.3.9808143. [DOI] [PubMed] [Google Scholar]
- e29.Domenighetti G, Gayer R, Gentilini R. Noninvasive pressure support ventilation in non-COPD patients with acute cardiogenic pulmonary edema and severe community-acquired pneumonia: acute effects and outcome. Intensive Care Med. 2002;28:1226–1232. doi: 10.1007/s00134-002-1373-8. [DOI] [PubMed] [Google Scholar]
- e30.Hilbert G, Gruson D, Vargas F, et al. Noninvasive continuous positive airway pressure in neutropenic patients with acute respiratory failure requiring intensive care unit admission. Crit Care Med. 2000;28:3185–3190. doi: 10.1097/00003246-200009000-00012. [DOI] [PubMed] [Google Scholar]
- e31.Moritz F, Brousse B, Gellée B, et al. Continuous positive airway pressure versus bilevel noninvasive ventilation in acute cardiogenic pulmonary edema: a randomized multicenter trial. Ann Emerg Med. 2007;50:666–675. doi: 10.1016/j.annemergmed.2007.06.488. [DOI] [PubMed] [Google Scholar]
- e32.Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- e33.Hamm CW. Akutes Koronarsyndrom (ACS). Teil 1: ACS ohne persistierende ST-Hebung. Z Kardiol. 2004;93:72–90. doi: 10.1007/s00392-004-1064-2. [DOI] [PubMed] [Google Scholar]
- e34.Hamm CW. Akutes Koronarsyndrom (ACS). Teil 2: ACS mit ST-Hebung. Z Kardiol. 2004;93:324–341. doi: 10.1007/s00392-004-0109-x. [DOI] [PubMed] [Google Scholar]
- e35.Masip J, Betbese AJ, Paez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet. 2000;356:2126–2132. doi: 10.1016/s0140-6736(00)03492-9. [DOI] [PubMed] [Google Scholar]
- e36.Rusterholtz T, Kempf J, Berton C, et al. Noninvasive pressure support ventilation (NIPSV) with face mask in patients with acute cardiogenic pulmonary edema (ACPE) Intensive Care Med. 1999;25:21–28. doi: 10.1007/s001340050782. [DOI] [PubMed] [Google Scholar]
- e37.Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med. 2003;168:1432–1437. doi: 10.1164/rccm.200211-1270OC. [DOI] [PubMed] [Google Scholar]
- e38.Köhler D, Pfeifer M, Criée C. Pathophysiologische Grundlagen der mechanischen Beatmung. Pneumologie. 2006;60:100–110. doi: 10.1055/s-2005-919155. [DOI] [PubMed] [Google Scholar]
- e39.Chadda K, Annane D, Hart N, et al. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30:2457–2461. doi: 10.1097/00003246-200211000-00009. [DOI] [PubMed] [Google Scholar]
- e40.Wahba RW. Perioperative functional residual capacity. Can J Anaesth. 1991;38:384–400. doi: 10.1007/BF03007630. [DOI] [PubMed] [Google Scholar]
- e41.Smetana G. Current concepts: Preoperative pulmonary evaluation. N Engl J Med. 1999;340:937–944. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- e42.Stock MC, Downs JB, Gauer PK, et al. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest. 1985;87:151–157. doi: 10.1378/chest.87.2.151. [DOI] [PubMed] [Google Scholar]
- e43.Kindgen-Milles D, Buhl R, Gabriel A, et al. Nasal continuous positive airway pressure: A method to avoid endotracheal reintubation in postoperative high-risk patients with severe nonhypercapnic oxygenation failure. Chest. 2000;117:1106–1111. doi: 10.1378/chest.117.4.1106. [DOI] [PubMed] [Google Scholar]
- e44.Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235–241. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- e45.Azoulay E, Alberti C, Bornstain C, et al. Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29:519–525. doi: 10.1097/00003246-200103000-00009. [DOI] [PubMed] [Google Scholar]
- e46.Hoffmann B, Jepsen M, Hachenberg T, et al. Cardiopulmonary effects of non-invasive positive pressure ventilation (NPPV) - a controlled, prospective study. Thorac Cardiovasc Surg. 2003;51:142–146. doi: 10.1055/s-2003-40320. [DOI] [PubMed] [Google Scholar]
- e47.Antonelli M, Conti G, Riccioni L, et al. Noninvasive positive-pressure ventilation via face mask during bronchoscopy with BAL in high-risk hypoxemic patients. Chest. 1996;110:724–728. doi: 10.1378/chest.110.3.724. [DOI] [PubMed] [Google Scholar]
- e48.Antonelli M, Pennisi MA, Conti G, et al. Fiberoptic bronchoscopy during noninvasive positive pressure ventilation delivered by helmet. Intensive. Care Med. 2003;29:126–129. doi: 10.1007/s00134-002-1554-5. [DOI] [PubMed] [Google Scholar]
- e49.Da Conceicao M, Genco G, Favier JC, et al. [Fiberoptic bronchoscopy during noninvasive positive-pressure ventilation in patients with chronic obstructive lung disease with hypoxemia and hypercapnia] Ann Fr Anesth Reanim. 2000;19:231–236. doi: 10.1016/s0750-7658(00)00213-6. [DOI] [PubMed] [Google Scholar]
- e50.Trachsel D, Erb TO, Frei FJ, et al. Use of continuous positive airway pressure during flexible bronchoscopy in young children. Eur Respir J. 2005;26:773–777. doi: 10.1183/09031936.05.00029405. [DOI] [PubMed] [Google Scholar]
- e51.Carlucci A, Gregoretti C, Squadrone V, et al. Preventive use of non-invasive mechanical ventilation to avoid post-Extubation respiratory failure: a randomised controlled study. Eur Respir J. 2001;33(18 suppl):306–306. [Google Scholar]
- e52.Hilbert G, Gruson D, Portel L, et al. Noninvasive pressure support ventilation in COPD patients with postextubation hypercapnic respiratory insufficiency. Eur Respir J. 1998;11:1349–1353. doi: 10.1183/09031936.98.11061349. [DOI] [PubMed] [Google Scholar]
- e53.Keenan SP, Powers C, McCormack DG, et al. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA. 2002;287:3238–3244. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- e54.Kilger E, Briegel J, Haller M, et al. Effects of noninvasive positive pressure ventilatory support in non-COPD patients with acute respiratory insufficiency after early extubation. Intensive Care Med. 1999;25:1374–1380. doi: 10.1007/s001340051084. [DOI] [PubMed] [Google Scholar]
- e55.Cuomo A, Delmastro M, Ceriana P, et al. Noninvasive mechanical ventilation as a palliative treatment of acute respiratory failure in patients with end-stage solid cancer. Palliat Med. 2004;18:602–610. doi: 10.1191/0269216304pm933oa. [DOI] [PubMed] [Google Scholar]
- e56.Shee CD, Green M. Non-invasive ventilation and palliation: experience in a district general hospital and a review. Palliat Med. 2003;17:21–26. doi: 10.1191/0269216303pm659oa. [DOI] [PubMed] [Google Scholar]
- e57.Meduri GU, Fox RC, Abou-Shala N, et al. Noninvasive mechanical ventilation via face mask in patients with acute respiratory failure who refused endotracheal intubation. Crit Care Med. 1994;22:1584–1590. [PubMed] [Google Scholar]
- e58.Meert AP, Berghmans T, Hardy M, et al. Non-invasive ventilation for cancer patients with life-support techniques limitation. Support Care Cancer. 2006;14:167–171. doi: 10.1007/s00520-005-0845-0. [DOI] [PubMed] [Google Scholar]
- e59.Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med. 2004;32:2002–2007. doi: 10.1097/01.ccm.0000142729.07050.c9. [DOI] [PubMed] [Google Scholar]
- e60.Nava S, Cuomo AM. Acute respiratory failure in the cancer patient: the role of non-invasive mechanical ventilation. Crit Rev Oncol Hematol. 2004;51:91–103. doi: 10.1016/j.critrevonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- e61.Schönhofer B, Köhler D, Kutzer K. Ethische Betrachtungen zur Beatmungsmedizin unter besonderer Berücksichtigung des Lebensendes. Pneumologie. 2006;60:408–416. doi: 10.1055/s-2006-932137. [DOI] [PubMed] [Google Scholar]
- e62.Nouira S, Marghli S, Belghith M, et al. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet. 2001;358:2020–2025. doi: 10.1016/S0140-6736(01)07097-0. [DOI] [PubMed] [Google Scholar]