Abstract
Introduction
Screening colonoscopy is an effective means for early detection of colorectal carcinoma. Any exhaustive evaluation of the method must take further factors into account: epidemiology of colorectal adenomas and carcinomas in the target population, acceptance by the patients, structure, process, and outcome quality, and health economics.
Methods
The internet-based colonoscopy database of the Bavarian Association of Statutory Health Insurance Physicians (ASHIP) for the year 2006 includes data on 86.05% of all outpatient colonoscopies performed in Bavarian ASHIP patients, or a total of 245 263 documented examinations.
Results
The rate of participation in preventive colonoscopies was low (1.5%) and showed considerable geographical variation. The rate of detection of histologically confirmed colorectal neoplasia in symptom-free screened individuals was almost 26.0%. Some 1.3% of those screened had colorectal carcinoma. In 76.31% of the participants a completely clean gut was achieved. The incidence of bleeding, perforation, and cardiorespiratory complications was 0.22%, 0.03%, and 0.06%, respectively.
Discussion
The complication rate of outpatient colonoscopy is on the order of tenths of a percent, while the process quality is high. The rate of detection of colorectal adenoma and carcinoma is high and the projected benefits for public health are considerable, but the rate of participation is too low.
Keywords: epidemiology, colonoscopy, screening, prevention, quality control
Screening colonoscopy is an effective means for early detection of colorectal carcinoma and its precursor lesions. Its superiority to other screening methods lies in its high sensitivity and specificity, and the ability it provides to perform polypectomy or biopsy (1). Any public health evaluation of the method must consider the following factors:
Acceptance: Since 2002, statutory health insurance (SHI) members in Germany aged 55 and older are eligible for a total of two screening colonoscopies spaced 10 years apart. Ideally, 10% of persons in each age decade should undergo screening each year.
Epidemiology of colorectal neoplasia: a higher a priori probability means higher detection rates and a larger proportion of true positives among suspicious cases. Limiting screening to older persons or high risk groups can be helpful. Screening strategies used in other populations cannot be adopted uncritically.
Structure quality: The inconvenience, shortcomings, and risks that are acceptable in the medical treatment of disease are unacceptable in the context of preventive care. Because the persons undergoing screening are free of symptoms and generally healthy, ensuring systematic quality control is an ethical obligation. In Germany, structural factors, such as training and equipment, are regulated by the Quality Control Agreement in Colonoscopy (Qualitätssicherungsvereinbarung zur Koloskopie) of 24 July 2006 (www.kbv.de/rechtsquellen/2500.html).
Process quality: Even if the screening infrastructure is working properly, certain processes can perform suboptimally. For this reason, reimbursement for screening colonoscopy in Germany has been tied since 2002 to standardized documentation and epidemiological evaluation of all screening procedures (3). Patient safety is crucial. A measure that has demonstrated low risk in individual cases can, when implemented on a population-wide basis, lead to many complications that outweigh its benefits.
Outcome quality: Surrogate criteria for the success of the screening program include detection rates and the benignity or malignancy of the newly detected lesions. Over the long term, evidence should be required of a decline in the overall incidence of colorectal cancer and colorectal cancer mortality.
Health Economics: Are the costs and risks of screening outweighed by the benefits?
In this article, the authors present their findings on patient acceptance, process quality, and the safety of screening colonoscopy, in addition to data on the epidemiology of colorectal adenomas. The findings are based on colonoscopy data from the Bavarian Association of Statutory Health Insurance Physicians (ASHIP) from the year 2006. The data include 86.05% of the outpatient colonoscopies performed among SHI members in Bavaria during that year.
Patients and methods
The Bavarian ASHIP colonoscopy database
Since 2002 the Central Research Institute of Ambulatory Health Care in Germany (ZI, Zentralinstitut für die kassenärztliche Versorgung) has implemented epidemiological quality control measures on a nationwide basis. For the most part, these have involved paper documentation based on the findings sheet in the Cancer Screening Guidelines (2). Scanning the documents has proved to be a source of error, and the submission of incomplete pathological findings has also become problematic. Until recently, physicians were required to send the documentation sheets at the end of each calendar quarter; as a result, findings that were not yet available by this date could no longer be taken into account. Moreover, the Bavarian ASHIP uses the term "curative colonoscopy" to describe the following symptomatic, or non-screening, procedures: diagnostic evaluations of clinical signs and symptoms; interventions to treat previously diagnosed lesions; and examinations conducted as part of follow-up for colorectal carcinoma or adenoma. Until recently, data on these procedures were not recorded by the ZI (3).
In light of these shortcomings, the Bavarian ASHIP implemented revised procedures at the beginning of 2006 to obtain a more comprehensive overview of public health. As part of a structural agreement, the documentation requirement was expanded to include curative colonoscopy. In general, procedures are documented via internet.
As of early 2007, a total of 432 users were able to access, via a secure web portal, a central database containing partially anonymized patient data. The choice of which information to record follows federal guidelines and includes demographic data such as the first three digits of the zip code of residence; indicators of process quality (e.g., success of colon cleaning and thoroughness of the examination); macroscopic and histological findings; diagnoses; acute complications; and recommendations for further diagnosis and treatment. In cases where curative colonoscopy is performed, the indication for the procedure is recorded. Pathology findings can be added later at any time (e.g., following a longer hospital stay due to carcinoma surgery).
According to Federal Ministry of Health figures, 83.2% of the Bavarian population was covered by SHI in 2006 (www.bmg.bund.de). In turn, the database contained records for 86.05% of the outpatient colonoscopies reimbursed by SHI in 2006. Data on patients covered by private insurance were not recorded. We thus estimate that the database includes approximately 72% of all outpatient colonoscopies performed in Bavaria in 2006.
Statistics
To calculate participation rates at the local level, Bayern’s municipalities were assigned to 79 regions based on the first three digits of the zip codes recorded in the database. Age- and gender-specific participation rates were calculated using demographic data available from the Bavaria State Statistical Office. Regional differences were analyzed using a spatial Poisson model (4) and presented in the form of a map. Calculations were performed with WinBUGS (www.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.shtml). The quantitative analyses of process quality and the epidemiology of colorectal neoplasia used 95% confidence intervals (CI) for prevalence and incidence rates and logistic regression (5). Analyses were performed using SAS 9.1.3 for Linux (SAS Institute, Cary, NC, USA).
Results
Demographics
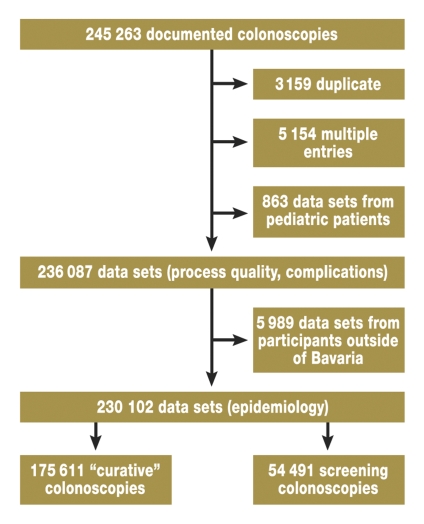
Figure 1 shows the steps in the data selection process by which 245 263 examinations in 2006 were reduced to 175 611 curative colonoscopies and 54 491 screening colonoscopies. Table 1 gives information about age and gender distribution, as well as the prevalence of colorectal lesions, in the patient cohort under study.
Figure 1.
Data selection flow-chart. "Duplicates" are old versions of updated data sets. In cases where multiple examinations were performed in 2006 ("multiple entries"), only the first examination (index colonoscopy) was considered. Data from pediatric patients were excluded. The remaining data sets were used to analyze acute complications and process quality ("data sets process quality, complications"). To analyze adenoma and carcinoma epidemiology, the data were restricted further to include only patients whose place of residence was in Bavaria ("data sets epidemiology").
Table 1. Colorectal lesion prevalence and basic demographic data for patients in Bavaria who underwent outpatient colonoscopy in 2006.
| Patients in Bavaria who underwent screening colonoscopy in 2006 | Patients in Bavaria who underwent "curative" colonoscopy in 2006 | ZI figures for 2006 as comparison | |
|---|---|---|---|
| Number of cases | 54 491 | 175 611 | 529 699 |
| Percentage of women | 55.80 | 57.05 | 54.70 |
| Age (mean ± SD): men | 64.9 ± 6.8 | 57.3 ± 14.6 | |
| Age (mean ± SD): women | 64.3 ± 6.8 | 56.3 ± 15.3 | |
| Prevalence of low-grade adenomas (%) [95% CI] | 17.32 [17.00–17.64] | 13.57 [13.41–13.73] | 13.70 [13.61–13.79] |
| Prevalence of advanced adenomas (%) [95% CI] (diameter ≥ 10 mm, high-grade intraepithelial neoplasia, villous or tubulovillous histology, or combination thereof) | 7.36 [7.14–7.58] | 5.57 [5.46–5.68] | 4.00 [3.95–4.05] |
| Prevalence of carcinoma (%) [95% CI] | 1.27 [1.18–1.37] | 1.59 [1.53–1.65] | 0.90 [0.88–0.92] |
| Overall prevalence of histologically confirmed adenoma and carcinoma (%) [95% CI] | 25.95 [25.58–26.32] | 20.73 [20.54–20.92] | 22.00 [21.89–22.12] |
SD, standard deviation; 95% CI, 95% confidence interval
As comparison: nationwide figures for screening colonoscopy for the year 2006 published by the Central Research Institute of Ambulatory Health Care in Germany (ZI, Zentralinstitut für die kassenärztliche Versorgung) (27).
Use of screening colonoscopy
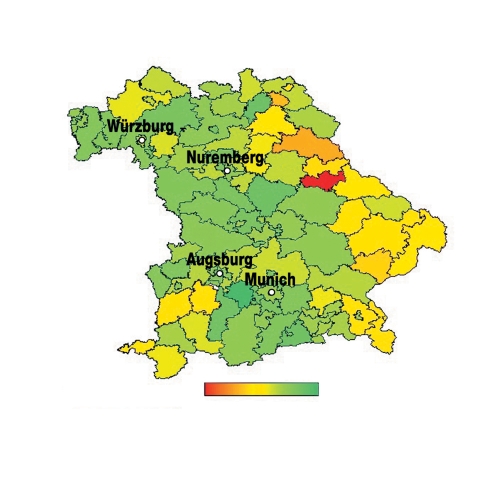
Table 2 shows the age- and gender-specific participation rates for screening colonoscopies. Of the approximately 3.713 million Bavarians in the eligible age group, only 1.50% took part in the screening program in 2006. The participation rates differed only marginally according to gender (men: 1.49%; women: 1.51%). However, the age-related patterns were complex. Women aged 55 to 64 years were more likely to participate than their male counterparts (2.35% vs. 1.74%). In men and women aged 65 to 74 years, participation rates were very similar (1.68% vs. 1.65%). In elderly patients, participation rates were markedly lower among women (0.43% vs. 0.70%). These findings remained unchanged after adjusting for regional effects. Figure 2 shows an estimate of regional effects based on a spatial Poisson model. The highest rates of participation were observed in greater metropolitan regions, and the lowest rates in the border region of eastern Bavaria. The differences were considerable, with participation rates varying by a factor of 2.6 between the eight regions with the highest outpatient colonoscopy use and the eight regions with the lowest outpatient colonoscopy use.
Table 2. Age- and gender-specific rates for use of preventive colonoscopy in eligible population.
| Age (years) | Men | Women | Total | |||
|---|---|---|---|---|---|---|
| Participation rate (%) [95% CI] | Population (millions) | Participation rate (%) [95% CI] | Population (millions) | Participation rate (%) [95% CI] | Population (millions) | |
| 55–64 | 1.74 [1.71–1.77] | 0.704 | 2.35 [2.32–2.39] | 0.713 | 2.05 [2.03–2.07] | 1.416 |
| 65–74 | 1.65 [1.62–1.68] | 0.610 | 1.68 [1.65–1.71] | 0.693 | 1.66 [1.63–1.68] | 1.303 |
| ≥ 75 | 0.70 [0.67–0.73] | 0.343 | 0.43 [0.41–0.45] | 0.650 | 0.52 [0.51–0.53] | 0.994 |
| Total | 1.49 [1.47–1.51] | 1.657 | 1.51 [1.48–1.52] | 2.056 | 1.50 [1.49–1.51] | 3.713 |
95% CI, 95% confidence interval
Figure 2.
Geographical variation in age- and gender-adjusted participation rates for preventive colonoscopy (green: regions with higher rates; red: regions with lower rates)
Process quality and complications
The colon was completely emptied leaving no residue in 76.31% of patients; liquid was present, but could be removed by suction in 22.22% of patients; and semisolid or solid amounts of stool were seen in 1.47% of patients. In total, 97.43% of all colonoscopies were complete, and 98.87% of all colonoscopies were video recorded. Finally, 92.85% of the procedures took place with sedation/analgesia. In a multivariate analysis of predictors of colonoscopy completion (i.e., gender, age, indication, degree of colon cleanliness, and premedication), the following were associated with a higher completion rate: male gender, middle age, screening indication, adequate colon cleanliness, and use of sedation/analgesia. Among the 236 087 procedures performed, bleeding was documented in 520 cases (0.22%), of which 10 required non-conservative treatment. A total of 69 cases of intestinal perforation were recorded (0.03%), 50 of which necessitated surgery. Cardiorespiratory complications occurred in 152 patients (0.06%) and led to three fatalities. The authors analyzed age, gender, indication, analgesia/sedation, and biopsy or polypectomy as potential risk factors. All complications were more frequent in older age groups. The most important risk factors for bleeding and perforation were biopsy and polypectomy.
Epidemiology of colorectal lesions
Table 1 provides basic demographic data on the 54 491 screening patients and 175 611 curative colonoscopy patients. Due to age limits for screening, curative colonoscopy patients were, on the average, younger and showed greater age variability. The most important finding was an unexpectedly high prevalence of histologically confirmed lesions among screening patients, which at 25.95% clearly exceeds the comparative prevalence figures for the rest of Germany in 2005 or 2006 (6). The prevalence rates in both screening populations were higher than the rate seen in Bavarian patients undergoing curative colonoscopy (20.73%). Among Bavarian patients, one-third of the lesions detected were identified as advanced adenomas, with a diameter of more than 10 mm, high-grade intraepithelial neoplasia (IEN), and/or (tubulo)villous histology. Carcinoma was diagnosed in 1.27% of the patients undergoing screening and 1.59% of the patients undergoing curative colonoscopy.
Regardless of their indication (i.e. screening vs. curative), adenoma bearers in Bavaria had similar characteristics. In both groups, men were overrepresented. In total, 43.8% of adenoma bearers in the screening population and 45.1% of adenoma bearers in the curative colonoscopy population were women. Multiple adenomas were identified in 50.2% of all screening patients and 49.1% of all curative colonoscopy patients. The majority of adenomas were tubular (81.9% vs. 80.9%) and smaller than 10 mm (88.2% vs. 89.2%).
Prevalence of colorectal adenoma and carcinoma in defined risk groups
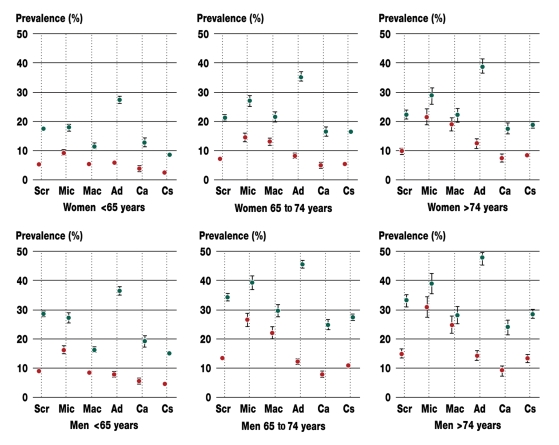
The prevalence of colorectal lesions was analyzed in six subgroups: persons undergoing screening colonoscopy, patients with fecal occult blood, patients after macroscopic bleeding, patients undergoing adenoma surveillance, patients undergoing carcinoma follow-up, and patients with other clinical symptoms (diarrhea, constipation, incomplete bowel evacuation, altered stool frequency, anemia of uncertain etiology, weight loss). The last five of the listed subgroups are thus made up of patients who underwent curative colonoscopy. Table 3 describes the different subpopulations. Figure 3, in turn, illustrates the inhomogeneity of the age- and gender-specific prevalence profiles. Important to note here are the higher risk for men and the rise in prevalence associated with advancing age. Among younger patients, the prevalence of adenoma did not differ between those with fecal occult blood and those who underwent screening colonoscopy. The former, however, were more likely to have advanced neoplasia. In persons aged 65 years and older, the presence of fecal occult blood was associated with an increased prevalence of adenoma and a disproportionate rise in the prevalence of advanced lesions. Patients with macroscopic bleeding were less likely than patients with fecal occult blood to have adenoma or advanced neoplasia. In persons under the age of 65, the risk of having an advanced lesion after macroscopic bleeding was no higher than it was in symptom-free screening patients. Adenomas were most prevalent among patients undergoing adenoma surveillance. However, advanced lesions were no more likely in this group than they were in screening patients or in patients with fecal occult blood or macroscopic bleeding. In patients undergoing carcinoma follow-up, the likelihood of having lesions was approximately the same as that seen in screening patients. The presence of clinical symptoms without fecal occult blood or macroscopic bleeding was not associated with an increased risk of lesions, but rather seemed to have a protective effect.
Table 3. Evaluating the prevalence of colorectal adenoma and advanced lesions – characteristics of the individual risk groups.
| Number of cases | Percentage women | Age ± SD (years) | Percentage of persons with adenomas [95% CI] | Percentage of persons with carcinomas [95% CI] | |
|---|---|---|---|---|---|
| Preventive colonoscopy in symptom-free persons | 54 491 | 55.8 | 64.6 ± 6.8 | 24.7 [24.3–25.1] | 1.3 [1.2–1.4] |
| "Curative" colonoscopies | |||||
| Fecal occult blood positive | 13 531 | 56.2 | 59.9 ± 12.4 | 26.4 [25.7–27.0] | 4.1 [3.8–4.4] |
| Macroscopic bleeding | 24 477 | 53.7 | 52.6 ± 15.3 | 16.6 [16.1–17.1] | 3.6 [3.4–3.9] |
| Adenoma surveillance | 25 347 | 47.5 | 64.2 ± 9.9 | 37.1 [35.6–36.8] | 0.5 [0.4–0.6] |
| Carcinoma follow-up | 11 071 | 46.1 | 67.8 ± 10.0 | 19.6 [18.9–20.4] | 1.5 [1.3–1.7] |
| Other clinical symptoms | 101 185 | 61.6 | 54.3 ± 15.5 | 14.2 [14.0–14..4] | 1.1 [1.0–1.1] |
SD, standard deviation; 95% CI, 95% Confidence interval
Figure 3.
Gender- and age-adjusted prevalence rates for adenoma (green) and advanced adenoma/carcinoma (red) among screening participants (Scr), patients with fecal occult blood (Mic), patients with macroscopic bleeding (Mac), patients undergoing adenoma surveillance (Ad), patients undergoing carcinoma follow-up (Ca), and patients with other clinical signs and symptoms (Cs)
Discussion
The prevalence of colorectal lesions in symptom-free screening patients was almost 26%, which was unexpectedly high and – due to the more advanced age of these patients – even greater than that observed in persons undergoing curative colonoscopy. Although most of the detected lesions showed favorable, tubular histology, more than one percent of the screening and curative colonoscopy patients had cancer. This rate of detection was higher than in the rest of Germany (6, 25). The high rate of detection corresponds to a low number needed to screen, and thus also to a favorable cost-benefit ratio (7): to detect one advanced lesion, only 12 persons (95% CI: 11 to 13) need to be screened. The risk of advanced adenomas developing into cancer should not be underestimated. In the literature, the annual adenoma-to-carcinoma conversion rate was 3% for large adenomas, 17% for adenomas with villous histology, and 37% for adenomas with high-grade IEN (8).
The epidemiology of the adenomas diagnosed in Bavarian patients differed from that described in international studies. Multiple adenomas were more frequent in the Bavarian population than in the US National Polyp Study (NPS) (1, 9, 10), but were less frequent than in the Danish Funen study (11–13). In terms of histology, the adenomas detected in Bavaria were similar to those detected in Denmark, whereas the adenomas in the NPS were more advanced. The adenomas detected in Bavaria were smaller than those in the NPS, the Funen study, and a recent Polish study (14).
The prevalence of adenomas in the six subpopulations confirms their relationship with gender and age. Patients undergoing cancer surveillance did not differ substantially from the screening population with regard to the prevalence of adenomas or advanced lesions. However, the prevalence of advanced adenomas in persons who were older than 65 years and had a positive fecal occult blood test was almost twice as high compared to the screening population. Although this shows that fecal occult blood tests can identify persons with adenomas, it is important to point out that these tests cannot replace preventive colonoscopy. One possible approach, however, would be to encourage the use of fecal occult blood tests in higher age groups as a way to motivate patients who receive a positive test result to take part in a screening colonoscopy. Finally, in patients undergoing adenoma surveillance, there was an increased risk of being diagnosed with an adenoma, but the prevalence of advanced neoplasia was similar to that seen in the screening population. This underlines the value of follow-up care: apparently, the risk in persons with prior adenoma is not eliminated, but reduced to the level observed in the screening population.
The data presented here demonstrate that the quality of the examination procedures was high. Indeed, the rate of complications in outpatient colonoscopies was on the order of tenths of a percent (6, 15). It should be noted, however, that this figure does not take account of complications prior to the procedure – e.g., related to fasting or the bothersome need to clear the colon of solid matter (16) – or late complications after the procedure. A detailed analysis of these complications is the subject of an ongoing project. Currently, there are no follow-up data on patients with bleeding or intestinal perforation requiring surgery.
In light of the potential benefits of screening colonoscopy, and of the quality of the service being offered, the 1.5% participation rate observed in this study is disappointingly low. Nevertheless, this is the same as the average rate seen for all of Germany, and is better than that reported in a recent Polish study (14). Here, too, it must be pointed out that the data presented in this study include only 86% of the colonoscopies reimbursed by SHI, and provide no information about the 17% of patients in Bavaria who have private insurance or are uninsured. If we assume identical preventive care behavior in this group, the overall rate of participation for outpatient screening colonoscopies in Bavaria can be placed at 2.1%. Moreover, if we take into account that there are two curative colonoscopy patients in the eligible age group for each screening patient, then the colonoscopy rate in Bavaria can be estimated at 6.0% per year. Extrapolating this figure to the 10-year interval between the procedures reimbursed by SHI indicates that 40% of the population does not receive colonoscopies. Finally, participation rates decreased with advancing age, particularly among women. Our study does not allow us to speculate on the causes, but in light of the discussion surrounding the use of screening colonoscopies in very elderly persons (17–23), we should consider that women may derive more benefit thanks to their greater life expectancy.
The causes of the regional variations observed in participation rates are also unclear. The data presented here do not allow us to determine whether these are due, for example, to differences in patient acceptance or to deficits in the care provided by private-practice gastroenterologists. As a result, it is difficult to make any recommendations on cost-effective ways to increase participation rates.
Because the present study is based on routine data from everyday care, a number of limitations need to be considered. The first of these is the possibility of self-selection among high-risk patients, such as those with a family history of cancer, or with initially unspecific symptoms. An accumulation of adenoma patients in the screening group would result in an overestimate of the detection rate, and thus in an overly optimistic assessment of the effect of screening. In addition, the present study does not take account of the selection bias introduced by excluding inpatient colonoscopies and colonoscopies in patients with private insurance. We were unable to evaluate how our conclusion would need to be modified if these factors had been taken into consideration. Finally, aside from conducting validity tests, the authors were unable to verify whether data had been collected correctly. Even though the low complication rates observed in this cohort are in agreement with those reported in the literature (15), underreporting – which would result in an overly optimistic estimate of the cost-benefit ratio for screening colonoscopy – cannot be ruled out.
Due to the inevitably limited scope of the documentation, a number of questions related to process quality remained unanswered, including data on length of examination, choice of instruments (forceps, snare) and how these related to the size of the polyp(s) removed or the location of lesion(s) whose removal led to bleeding or perforation. In this regard, it would be highly desirable to pay greater heed to international standards (24). Moreover, our data source did not allow us to investigate factors related to lifestyle. Doing so, however, would have made it possible to conduct a more detailed analysis of participation rates, compliance with follow-up care, and prevalence profiles.
Some of the questions raised above require further validation in investigations with better study designs. These could employ existing structures for data collection, which would allow, for example, cluster-randomized trials evaluating the effect of specific procedures on the quality of care to be implemented cost-effectively and in a scientifically validated manner.
In addition to completeness and safety, the cost-effectiveness of outpatient colonoscopy depends on participation rates and compliance with post-adenomectomy surveillance programs. The cross-sectional observations presented here provide no data on compliance with these programs. However, over the course of time, the partial anonymization of the patient records will make it possible to derive longitudinal observations from the cross-sectional data.
From a technical viewpoint, the existing infrastructure for data collection could serve as a basis for establishing an adenoma registry for all of Bavaria at a reasonable cost. The registry would serve as a model for the rest of Germany and provide the ideal prerequisites for a comprehensive evaluation of colonoscopy as a tool for preventing colorectal carcinoma.
Acknowledgments
We would like to thank the Munich Center of Health Sciences (LMUinnovativ) for their support.
Translated from the original German by Matthew D. Gaskins.
Footnotes
Conflict of interest statement
Mr. Augustin is an employee of the Bavarian ASHIP (KV Bayern). The other authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Limburg PJ. With screening colonoscopy, what you see is what you get. Gastroenterology. 2007;132:2065–2066. doi: 10.1053/j.gastro.2007.03.099. [DOI] [PubMed] [Google Scholar]
- 3.Sieg A, Theilmeier A. Results of coloscopy screening in 2005 - an internet-based documentation. Dtsch Med Wochenschr. 2006;131:379–383. doi: 10.1055/s-2006-932528. [DOI] [PubMed] [Google Scholar]
- 4.Diggle PJ, Tawn JA, Moyeed RA. Model-based geostatistics. Applied Statistics. 1998;47:299–350. [Google Scholar]
- 5.Agresti A. Categorical data analysis. 2nd ed. New York: Wiley; 2002. [Google Scholar]
- 6.Zentralinstitut für die kassenärztliche Versorgung in Deutschland. Wissenschaftliche Begleitung von Früherkennungs-Koloskopien in Deutschland, Berichtszeitraum 2005 - 3. Jahresbericht, http://www.zi-berlin.de/koloskopie/downloads.php.
- 7.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317:307–312. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371–379. [PubMed] [Google Scholar]
- 10.Winawer SJ, Zauber AG, O’Brien MJ, et al. The National Polyp Study - design, methods, and characteristics of patients with newly diagnosed polyps. Cancer. 1992;70(5 Suppl):1236–1245. doi: 10.1002/1097-0142(19920901)70:3+<1236::aid-cncr2820701508>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen OD, Kronborg O, Fenger C. The Funen Adenoma Follow-up Study. Incidence and death from colorectal carcinoma in an adenoma surveillance program. Scand J Gastroenterol. 1993;28:869–874. doi: 10.3109/00365529309103127. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen OD, Kronborg O, Fenger C. The Funen Adenoma Follow-Up Study. Characteristics of patients and initial adenomas in relation to severe dysplasia. Scand J Gastroenterol. 1993;28:239–243. doi: 10.3109/00365529309096079. [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen OD, Kronborg O, Fenger C. A randomized surveillance study of patients with pedunculated and small sessile tubular and tubulovillous adenomas. The Funen Adenoma Follow-up Study. Scand J Gastroenterol. 1995;30:686–692. doi: 10.3109/00365529509096314. [DOI] [PubMed] [Google Scholar]
- 14.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 15.Becker F, N.G., Welke J, Hahn EG, Mansmann U. Follow-up after colorectal polypectomy: a benefit-risk analysis of German surveillance recommendations. Int J Colorectal Dis. 2007;22:929–939. doi: 10.1007/s00384-006-0252-0. [DOI] [PubMed] [Google Scholar]
- 16.Mühlhauser I. Früherkennung und Prävention: Ist Vorbeugen besser als Heilen? Dtsch Arztebl. 2007;104(25):A 1804–A 1807. doi: 10.1016/j.zgesun.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Cooper GS. Con: screening colonoscopy in the extreme elderly is not a wise choice. Am J Gastroenterol. 2006;101:1715–1717. doi: 10.1111/j.1572-0241.2006.00756_2.x. discussion 1717-8. [DOI] [PubMed] [Google Scholar]
- 18.Kahi CJ, Azzouz F, Juliar BE, Imperiale TF. Survival of elderly persons undergoing colonoscopy: implications for colorectal cancer screening and surveillance. Gastrointest Endosc. 2007;66:544–550. doi: 10.1016/j.gie.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch M. Screening colonoscopy in the elderly: more reasons for refusal. Am J Gastroenterol. 2007;102:457–457. doi: 10.1111/j.1572-0241.2006.00904_5.x. author reply 457. [DOI] [PubMed] [Google Scholar]
- 20.Lin OS. Screening colonoscopy in very elderly patients: judgment versus dogma. Am J Gastroenterol. 2007;102:457–458. doi: 10.1111/j.1572-0241.2006.00904_7.x. author reply 458. [DOI] [PubMed] [Google Scholar]
- 21.Lin OS, Kozarek RA, Schembre DB, et al. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. JAMA. 2006;295:2357–2365. doi: 10.1001/jama.295.20.2357. [DOI] [PubMed] [Google Scholar]
- 22.Stevens T, Burke CA. Colonoscopy screening in the elderly: when to stop? Am J Gastroenterol. 2003;98:1881–1885. doi: 10.1111/j.1572-0241.2003.07576.x. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian S, Amonkar MM, Hunt TL. Use of colonoscopy for colorectal cancer screening: evidence from the 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2005;14:409–416. doi: 10.1158/1055-9965.EPI-03-0493. [DOI] [PubMed] [Google Scholar]
- 24.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–2877. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 25.Zentralinstitut für die kassenärztliche Versorgung in Deutschland. Projekt wissenschaftliche Begleitung von Früherkennungs-Koloskopien in Deutschland: Berichtszeitraum 2006 - 4. Jahresbericht, http://www.zi-berlin.de/koloskopie/downloads.php. 2008.