Abstract
Introduction
Malignant tumors of the musculoskeletal system are rare, and their symptoms are non-specific. The diagnosis of primary malignant tumors of bone or soft tissue by tissue biopsy is necessary before multimodal treatment with chemo- and/or radiotherapy and resection can be provided. These biopsies are straightforward surgical procedures; they must, however, be performed according to the guidelines if high rates of error and complications are to be avoided.
Methods
Selective literature review.
Results
Biopsies are either incisional or excisional. There are guidelines for the performance of both kinds. The biopsy channel is inevitably contaminated with tumor cells and thus must be completely removed together with the tumor. Excisional biopsies are indicated only for the histopathological diagnosis of small (< 5 cm), epifascial soft-tissue tumors and small, slowly growing bony tumors that are considered most likely to be benign. If in doubt, an incision biopsy should be performed.
Discussion
The complication rate of tumor biopsies is known to be higher when they are performed in an institution without extensive experience in the treatment of sarcoma. Thus, patients with musculoskeletal tumors that are suspected of being malignant should be referred to a suitable tumor center for biopsy.
Keywords: tumor, bone, soft tissue, sarcoma, biopsy
Malignant tumors of the musculoskeletal system, i.e., bone and soft tissue sarcomas, are rare: bone sarcomas account for less than 1% of all tumors (1). The incidence of soft tissue sarcomas is similar (2, 3). An orthopedic surgeon who is not specialized in musculoskeletal tumors sees – as a statistical average – less than one patient with a tumor of the musculoskeletal system (MSS) every three years (3, 4).
These clinicians therefore cannot generally be assumed to possess knowledge of the basic diagnostic and therapeutic principles.
The management of patients with malignant tumors of the musculoskeletal system is based on an interdisciplinary model including standard schedules with neo and adjuvant chemotherapy and optional radiotherapy in combination with resection. Treatment success rates have been greatly improved by this approach (5).
For the commonest adult bone sarcoma – chondrosarcoma – there is, with only a few exceptions, no adjuvant therapeutic option. Treatment consists of resection alone.
For soft tissue sarcomas (STS) it is necessary to distinguish childhood from adult sarcomas. In childhood and adolescence, soft tissue sarcomas account for 10% of malignant tumors. In histopathological terms, these are often rhabdomyosarcomas located centrally on the trunk. In adults, soft tissue sarcomas mainly develop peripherally on the leg (6) and, depending on the entity, show variable age distribution. Histopathologically, these are mainly liposarcomas or pleomorphic soft tissue sarcomas (formerly malignant fibrous histiocytomas), followed by synoviosarcomas and leiomyosarcomas.
The standard therapy of soft tissue sarcomas in adults is resection in healthy tissue (= R0 resection; International Union Against Cancer [UICC]) or compartment resection depending on the tumor site, combined with post- or preoperative radiotherapy (7). Neo and/or adjuvant chemotherapies have not so far been convincingly successful in adult soft tissue sarcomas (8, 9). In childhood soft tissue sarcomas, chemotherapy is one of the main components of therapy alongside resection (10). Nevertheless, resection of the tumor in healthy tissue (R0 resection in the UICC tumor classification [TNM] system [5]) is a "conditio sine qua non." Sarcomas on the extremities or trunk can usually be resected by compartment resection or, if located extracompartmentally, by wide resection. R0 resection is possible for cervical, intra-abdominal or intrathoracic STS, but no comparable compartments exist (6, 11).
For all sarcomas it is generally the case that inadequate resection, i.e. non R0 resection, significantly worsens the treatment outcome. Adjuvant therapies cannot compensate for this situation (5).
If a sarcoma is suspected, diagnostic evaluation must be performed to a high standard, otherwise adequate resection may be difficult or impossible.
A targeted diagnostic procedure is the basis of sarcoma therapy. Because of the unspecific symptoms and the limited information value of imaging techniques, a soft tissue or bone tumor must be diagnostically confirmed by biopsy.
Besides imaging based diagnostics (radiography in two planes, magnetic resonance imaging [MRI]), biopsy is the most important procedure for obtaining representative tissue for histopathological analysis.
The purpose of this publication is to present the basic criteria and types of biopsy techniques based on a selective literature review, because this simple surgical procedure is subject to many possible types of error. These errors are described below in the context of case reports.
Types of biopsy
A distinction is drawn between incisional and excisional biopsies (12):
Incisional biopsy involves obtaining a small, most representative possible part of the tumor. Needle or punch biopsies are distinguished from open biopsies.
In needle or punch biopsies, a distinction is made between a fine needle and a trocar or core needle biopsy depending on the instrument. Different amounts of tissue can be collected by these techniques. Depending on the amount and consistency of the material, cytological and in most cases also histological analysis is possible (13). Multiple biopsies should be avoided because this is presumed to promote tumor cell dissemination.
In open incisional biopsies, the tumor is opened and a piece of tissue about 1 x 1 x 1 cm in size is obtained. If the available institutional facilities allow, the tissue is forwarded immediately without fixation to the pathologist for rapid section diagnosis. The pathologist can then immediately state whether tumor tissue is present. The intervention is concluded and most of the tumor is left in place.
Cellular contamination of the biopsy site is unavoidable. Biopsy procedures are therefore subject to guidelines (2) designed to ensure that the subsequent surgical resection is optimally executed (2, 12):
The sampling site should be defined on the basis of the imaging results in a conference of radiologists, pathologists, and surgeons with the aim of obtaining the most reliably representative, whenever possible non-ossified or necrotic material.
Arrest of blood supply to extremities may be performed if considered necessary. Bandaging the extremity to produce ischemia could cause tumor compression leading to dissemination of tumor cells. It appears preferable not to arrest the blood supply because hemostasis occurs immediately and not until after opening the tourniquet. Hematomas are to be avoided because they lead to extensive contamination of the surrounding area.
Only longitudinal incisions should be made on extremities. The most important criterion is to select the incision site such that it can be removed as an en bloc resectate together with the tumor in the later tumor resection procedure, because the biopsy scar is regarded as being contaminated with tumor cells. For bone sarcomas without soft tissue infiltration, the intervention is made intentionally through one of the muscle compartments. Dissection in the fascial recess between the muscle compartments is disadvantageous because the recesses promote contamination with tumor cells. Surgical dissection is performed in direct approach to the tumor, dissection towards the side is to be avoided. In biopsies, proximity to vessels and nerves is to be avoided due to the risk of contamination. If the bony lesion has a soft tissue component, the biopsy should be taken from this tissue provided that other criteria are not violated. A similar procedure is followed for STS.
After collecting sufficient tissue, subtle hemostasis is performed. The use of a drain is essential, but the drainage channel should not additionally cross or contaminate healthy tissue or compartments. This means that the drain should exit immediately from the wound angle or about 1 cm in an extension of the incision.
Skin closure should be atraumatic with intracutaneous suture or a narrow transcutaneous suture.
Special situation of suspected truncal, thoracic, and abdominal/retroperitoneal sarcoma
These soft tissue tumors are often located such that although biopsies are possible with access through pleura and the peritoneal space, they would result in uncontrollable dissemination of tumor cells. At such sites, tumors can only be removed with difficulty and with functional losses. In such cases, preoperative transcutaneous biopsy for confirmation of diagnosis often has to be forgone and surgical exploration before tumor resection initiated primarily with specimen collection and rapid sectioning, for example in order to rule out the presence of a lymphoma.
The procedure is different for excisional biopsies: the tumor is removed totally by enucleation and subjected to histopathological analysis. This approach is only indicated for small tumors (<5 cm), superficially localized and thus of low suspected malignancy.
If an excisional biopsy is performed on the assumption of a benign tumor, it is important to consider the possibility that despite the absence of suspicious signs, it could nevertheless be malignant. Consequently, the criteria for incisional biopsies also have to be followed for excisional biopsies.
Avoiding contamination of the healthy peritumor area is the paramount consideration, always remembering that a complete resection within wide margins is always mandatory for malignant tumors.
To improve orientation in subsequent control MRIs prior to repeat resection, if necessary, the extent of the wound cavity can be marked using metallic vascular clips (if possible titanium clips which do not interfere with MRI scans). This facilitates planning of repeat resection when the tumor has already been removed and the previously operated, tumor cell-contaminated area (previously the wound cavity) has to be removed by "wide repeat resection" (3).
If, despite the absence of suspicious signs – small tumor, superficial localization, slow growth – doubts still remain regarding the tumor status, incisional biopsy is to be preferred to excisional biopsy.
Who performs the biopsy?
To obtain optimal therapeutic results, it is advisable to refer patients with suspected sarcoma before the biopsy to a center experienced in sarcoma management (14, 15). If a biopsy is to be performed at a different institution than that in which the surgical procedure will later be carried out, this institution must also comply with the principles of biopsy. It is valuable to discuss the biopsy access route with the surgeon and the department responsible for the patient’s future management. Needle biopsies should therefore not be performed except at centers. It is especially important to document the access route. For open incisional biopsies, the resulting cutaneous scar marks the access route. If malignancy is suspected, incisional biopsy is the procedure of choice.
Case reports
Problems with biopsies not performed in conformity with the aforementioned criteria are usually attributable to the fact that although the diagnosis could be made, e.g., by sonographically guided needle biopsy, the biopsy site was documented inadequately or not at all and is not identifiable by a scar. The surgeon cannot later remove the biopsy channel during the operation, which increases the risk of an implantation metastasis.
Localizing the biopsy channel frequently also creates difficulties. If it is not included in the later incision pattern for tumor resection, according to applicable standards, this is either difficult or even impossible.
The problems that can occur if the biopsy principles are not followed are illustrated by means of case reports (boxes 1 and 2).
Box 1. Problems with biopsies I.
-
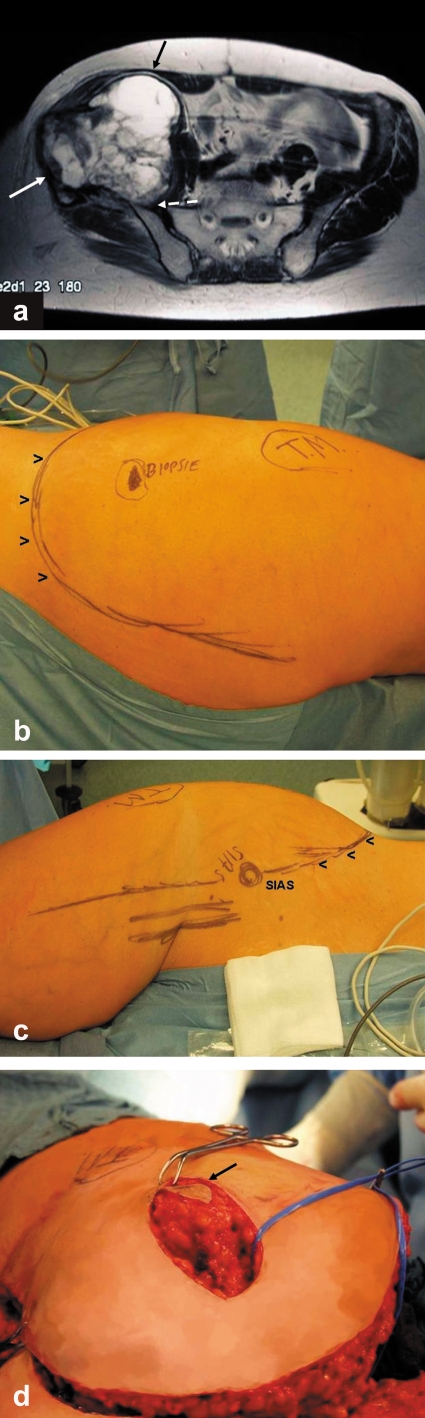
An incisional biopsy was performed (figure 1b) in a 50-year-old woman with an extensive pelvic tumor (figure 1a). Histological analysis revealed an "undifferentiated sarcoma without further classification." The indication for resection was then established. "Wide" or R0 resection and hip transposition plasty were performed.
Problem:
Since the biopsy site is not in the area of the incision for tumor resection (figures 1b and c), additionally the former biopsy channel has to be dissected intraoperatively in such a way that it can be removed together with the tumor (figure 1d). This prolongs the operation time and necessitates a second incision. Both increase the risk of infection and of relapse.
Comments:
The correct procedure would have been to position the incisional biopsy within the incision pattern for the tumor resection.
Box 2. Problems with biopsies II.
-
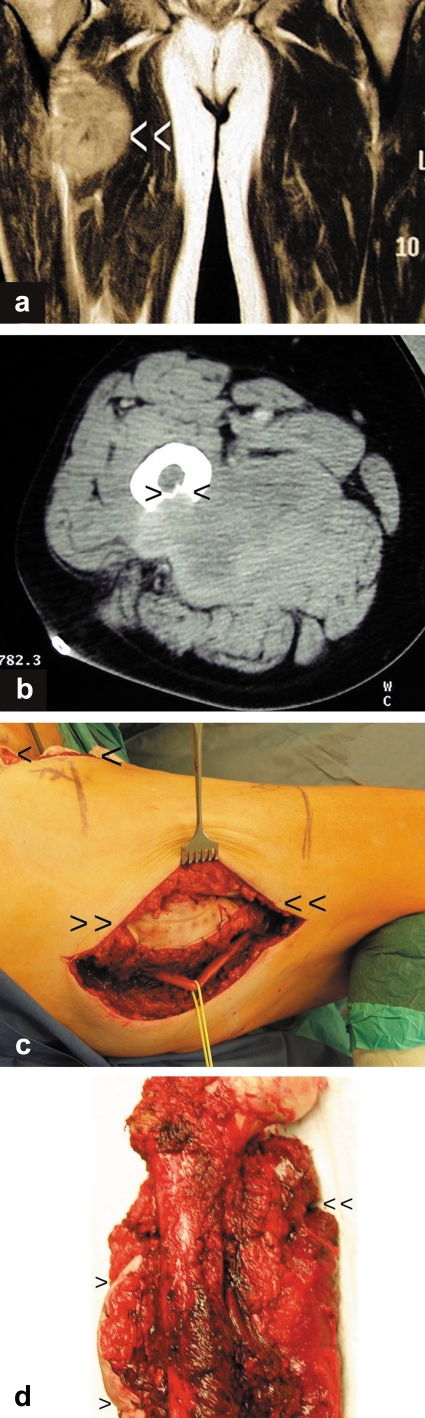
In a 59-year-old man, a soft tissue tumor in the adductor compartment of the right thigh with osseous involvement of the femur was diagnosed by magnetic resonance imaging and computed tomography (figure 2a and b). Incisional biopsy was performed from the lateral side with additional injury to the flexor compartment. Due to the anatomical conditions, dissection was also necessary deep between the femur and the sciatic nerve. The histological diagnosis revealed a "low-grade malignant fibrosarcoma" (figure 2c and d).
For oncological reasons, the en bloc resection required not only resection of the adductor compartment and the proximal femur but also of the lateral incision access.
Comments:
Imaging clearly shows that the tumor is located in the soft tissues of the adductor compartment. The site at which the femur is arroded is at the boundary to the flexor compartment.
It is not clear why the incisional biopsy was performed from the lateral side when the medial soft tissues were affected, the flexor compartment was opened, and the intervention was performed between the femur and the sciatic nerve. This resulted in a further compartment being contaminated and the risk of injuring or contaminating the nerve was increased. The ideal approach would have been an incisional biopsy along the later anteromedial access route over the adductors.
Discussion
Sarcomas are rare. Various studies (Schnurr C, Pippan M, Hartmut S, Delank KS, Eysel P: Erstellung eines soziodemografischen Risikoprofils zur Diagnoseverzögerung bei Knochentumoren [Establishment of a demographic risk profile for delayed diagnosis of bone tumors]), Dt. Kongress f. Orthopädie u Unfallchirurgie, 2.–6.10.2006, Berlin) show that there is a considerable delay in the diagnosis both of bone and soft tissue sarcomas. About four months pass until the first visit to a physician, and the consultation is also followed by an average delay of six months (two to 79 months) until diagnosis. The commonest reason for this delay is an incorrect clinical or sonographic assessment (16).
Secondary symptoms such as a sudden decrease in performance, fatigue, loss of appetite, and other paraneoplastic symptoms commonly associated with malignant conditions are largely absent with sarcomas. This is why the most important diagnostic activity is to consider the possibility of a sarcoma at all. Epidemiological data are of assistance in this regard: pain and swelling in the region of the knee joint should prompt the physician to consider the possibility of an osteosarcoma, especially in patients in the second decade. Rapidly growing, hardly mobile, space occupying lesions, which in adults occur mainly in the thigh, should be highly suspected of being STS.
MRI is the imaging technique of choice, while sonography is only marginally informative. A radiographic examination should always be performed. In this way, bony lesions can often be defined by a differential diagnostic approach. For soft tissue tumors, bony arrosions and soft tissue calcifications can be detected. If tumors show rapid growth, a deep subfascial location and a maximum extent >5 cm, this suggests an STS.
Patients with a tumor of uncertain status should be referred before the biopsy to a center with experience in sarcoma management. The development of diagnostic strategies at interdisciplinary conferences at a center experienced in the management of sarcomas is known to produce better results with lower complication rates (14, 17).
An optimal biopsy is indispensable for an adequate resection. Its defective execution and inadequate documentation can lead not only to a delay in diagnosis but may also complicate the surgical treatment and may reduce the survival rate.
Which type of incisional biopsy is chosen depends on the experience of the individual examiner at the centers. Various studies at specialized centers have shown that needle biopsy is a simple, cost-effective procedure suitable for outpatient use which delivers a correct diagnosis in 95% of cases, at least as regards tumor status (18). Its reliability, however, depends on the consistency of the tumor (19). In many cases, fine needle biopsies allow only a cytological analysis, and tumor grading is often underestimated. Other tumor centers report a reliability equivalent to that of open or trocar biopsy (20).
Open biopsy is regarded as the gold standard (13) and allows experienced examiners to reach a diagnosis in 98% of cases (21), but can lead to an increased rate of complications with hematomas, seromas, and pathological fractures (22).
What improvements in biopsy are to be expected from the use of interventional procedures in combination with new imaging techniques remains to be seen. Initial experience with these techniques, also evaluated at centers, has shown good results (23, 24).
Nevertheless it will continue to be necessary to discuss the procedure in an interdisciplinary conference because new techniques – if used incorrectly in relation to the subsequent resection – can lead to errors.
If adequate diagnosis and therapy have been performed, depending on the histopathological differentiation (grading) and extent (staging), interdisciplinary therapeutic strategies can provide a five-year survival for 60% to 80% of the patients both for bone and soft tissue sarcomas (25). The precondition for optimal therapeutic success for all sarcomas is wide resection or R0 resection (17). This is possible in extremities, on the pelvis, and the trunk in most cases. Limb salvage is frequently possible without compromising the surgical oncological success (3). Abdominal and thoracic sarcomas often cannot be diagnosed and resected according to these criteria. In these cases a biopsy often has to be forgone and the tumor is removed like a soft tissue sarcoma with the widest possible resection margins.
Figure 1.
a) View of the large pelvic tumor at the right iliac bone with soft tissue displacement.
b) The former biopsy site does not lie along the course of the incision for tumor resection (TM: trochanter major). View from dorsal with skin incision pattern marked.
c) View from ventral with incision pattern marked (SIAS: spina iliaca anterior superior).
d) View of incision for tumor resection from cranial: the former biopsy site has to be excised from the soft tissue flap created by the resection incision so that it can be removed while still attached to the tumor.
Figure 2.
a) MRI scan (coronary level) of the soft tissue tumor located in the adductor compartment (white arrow heads).
b) CT scan of the arrosion of the dorsal femur by the sarcoma (black arrow heads).
c) Image of the excision of the incorrectly positioned biopsy site (double arrow heads). The actual incision for tumor resection is located medially over the adductor compartment (single arrow heads). The excised biopsy site must be removed while still attached to the tumor.
d) Resection specimen with lateral marking of the former biopsy site (single arrow heads) and incision site for tumor resection (double arrow heads).
Acknowledgments
Translated from the original German by mt-g.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Delling G. Diagnostik von Knochentumoren. Verh Dtsch Ges Path. 1998;82:121–132. [PubMed] [Google Scholar]
- 2.Deutsche Gesellschaft f. Orthopädie u. orthopädische Chirurgie u. d. Berufsverbandes f. Orthopädie: Leitlinie: Diagnostik muskuloskelettaler Malignome (033/034) www.uni-duesseldorf.de/WWW/AWMF/ll/ll_kjpp.htm. [Google Scholar]
- 3.Steinau HU, Homann HH, Drücke D, Torres A, Soimaru D, Vogt P. Resektionsmethodik und funktionelle Wiederherstellung bei Weichgewebssarkomen der Extremitäten. Chirurg. 2001;72:501–513. doi: 10.1007/s001040051339. [DOI] [PubMed] [Google Scholar]
- 4.Springfield DS, Rosenberg A. Editorial: biopsy: complicated and risky. J Bone Joint Surg. 1996;78:639–643. [PubMed] [Google Scholar]
- 5.Flege S, Kuhlen M, Paulussen M, Bielack S, Jürgens H. Operative Therapie primär maligner Knochentumoren. Orthopäde. 2003;11:940–948. doi: 10.1007/s00132-003-0555-6. [DOI] [PubMed] [Google Scholar]
- 6.Kettelhack C, Tunn U, Schlag PM. Strategie multimodaler Therapie bei Weichgewebssarkomen des Stammes und der Extremitäten. Chirurg. 1998;69:393–401. doi: 10.1007/s001040050429. [DOI] [PubMed] [Google Scholar]
- 7.Budach V. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. Strahlenther Onkol. 2000;176:482–483. [PubMed] [Google Scholar]
- 8.Bauer S, Schütte J. Stellenwert neoadjuvanter Therapieverfahren und adjuvanter Chemotherapie in der Behandlung von Weichgewebssarkomen. Onkologe. 2002;8:334–341. [Google Scholar]
- 9.Bramwell VHC. Adjuvant chemotherapy for adult soft tissue sarcoma: Is there a standard of care? J Clin Oncol. 2001;19:1235–1237. doi: 10.1200/JCO.2001.19.5.1235. [DOI] [PubMed] [Google Scholar]
- 10.Interdisziplinäre Leitlinie der Deutschen Krebsgesellschaft und der Gesellschaft für Pädiatrische Onkologie und Hämatologie Leitlinie Weichteilsarkome im Kindesalter. (025/007) www.uni-duesseldorf.de/WWW/AWMF/ll/ll_kjpp.htm. [Google Scholar]
- 11.Junginger T, Budach V, Harms D, Hossfeld DK. Weichteilsarkome der Extremitäten, der Brust und Bauchwand und des Retroperitoneums. Dtsch Arztebl. 2001;98(50):A 2850–A 2845. [Google Scholar]
- 12.Bruns J, Yazigee O, Werner M, Delling G, Hossfeld DK. Bioptische Sicherung muskuloskelettaler Tumoren. Der Onkologe. 2006;12:119–127. [Google Scholar]
- 13.Roy-Coquard I, Ranchère-Vince D, Thiesse P, et al. Evaluation of core needle biopsy as a substitute to open biopsy in the diagnosis of soft-tissue masses. Europ J Cancer. 2003;39:2021–2025. doi: 10.1016/s0959-8049(03)00430-1. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson P, Dreinhöfer KE, Rydholm A. Soft tissue sarcoma should be treated at a tumor center. Acta Orthop Scand. 1994;65:47–50. doi: 10.3109/17453679408993717. [DOI] [PubMed] [Google Scholar]
- 15.Nijhuis PHA, Schaapveld M, Otter R, Hoekstra HJ. Soft tissue sarcoma - compliance with guidelines. Cancer. 2001;91:2186–2195. doi: 10.1002/1097-0142(20010601)91:11<2186::aid-cncr1248>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Brouns F, Stas M, De Wever I. Delay in diagnosis of soft tissue sarcoma. Europ J Surg Oncol. 2003;29:440–445. doi: 10.1016/s0748-7983(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 17.Brennan MF. Management of soft tissue sarcoma. Brit J Surg. 1996;83:577–579. doi: 10.1002/bjs.1800830502. [DOI] [PubMed] [Google Scholar]
- 18.Stoker DJ, Cobb JP, Pringel JAS. Needle biopsy of musculoskeletal lesions. J Bone Joint Surg. 1991;73:498–500. doi: 10.1302/0301-620X.73B3.1670457. [DOI] [PubMed] [Google Scholar]
- 19.Jelinek JS, Murphey MD, Welker JA, et al. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002;223:731–737. doi: 10.1148/radiol.2233011050. [DOI] [PubMed] [Google Scholar]
- 20.Bijl AEvd, Taminiau AHM, Hermanns J, Beerman H, Hogendoorn PCW. Accuracy of the Jamshidi Trocar Biopsy in the diagnosis of bone tumors. Clin Orthop. 1997;334:233–243. [PubMed] [Google Scholar]
- 21.Heeten GJ, Oldhoff J, Oosterhuis JW, Schraffordt KH. Biopsy of bone tumors. J Surg Oncol. 1985;28:247–251. doi: 10.1002/jso.2930280402. [DOI] [PubMed] [Google Scholar]
- 22.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. J Bone Joint Surg. 1996;78:656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Puri A, Shingade VU, Agarwal MG, et al. CT-guided percutaneous dore needle biopsy in deep seated musculoskeletal lesions: a prospective study in 128 cases. Skeletal Radiol. 2006;35:138–143. doi: 10.1007/s00256-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 24.Carrino JA, Khurana B, Ready JE, Silvermann SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg. 2007;89:2170–2187. doi: 10.2106/JBJS.F.01230. [DOI] [PubMed] [Google Scholar]
- 25.McKee MD, Liu DF, Brooks JJ, Gibbs JF, Driscoll DL, Kraybill WG. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004;85:68–76. doi: 10.1002/jso.20009. [DOI] [PubMed] [Google Scholar]