Abstract
Introduction
In children, acute pain occurs predominantly during infectious illnesses or after surgery. Chronic pain, especially headache and abdominal pain, is becoming increasingly common among children and adolescents.
Methods
Selective literature review, also including evidence-based guidelines and recommendations.
Results
Simple self-reporting and behavioral pain scales are easy to use to assess the intensity of acute pain. To evaluate chronic pain, on the other hand, more complicated, multi-dimensional instruments are necessary (e.g., semi-structured interviews). The most commonly used analgesics are ibuprofen and paracetamol (acetaminophen). When paracetamol is used, its narrow therapeutic window should be kept in mind. Perioperative pain should be treated with balanced analgesia involving a combination of non-pharmacological treatment strategies, non-opioid drugs, opioids, and regional anesthesia. Chronic pain in children can only be treated successfully over the long term with multidisciplinary team intervention based on this biopsychosocial model.
Discussion
Pain not only causes children momentary suffering but also threatens to impair their normal development. Therefore, every effort should be made to prevent pain and to treat it effectively once it arises.
Keywords: pain, children, adolescents, pain scale, medication, treatment
In children, acute pain occurs predominantly during infectious illnesses, painful interventions or after surgery (e1). Chronic pain generally takes the form of headache and abdominal pain, usually with several reported pain locations (1, e2). In this article the authors confine themselves to the most common painful conditions. The learning goals are:
Differentiating the various presentations and causes of pain
Becoming familiarized with assessment tools and measures for assessing pain in children of all age groups (infants to adolescents) with acute and chronic pain
Internalizing the principles of pain therapy adapted to children’s age and type of pain.
A separate article devoted to painful interventions will be published at a later date.
The methodological basis of this article was a selective literature review mainly in the Medline database (via PubMed), confined to original articles in German and English, systematic reviews, and evidence-based meta-analyses. The Cochrane Database was also consulted. Unless otherwise indicated, the evidence level is stated as specified by the Oxford Centre of Evidence-based Medicine (Version: May 2001) (www.cebm.net) (table 6).
Table 6. Regional anesthesia and analgesia techniques according to surgical intervention.
| Operation | Regional anesthetic technique | Evidence level*1 |
|---|---|---|
| Urogenital | ||
| Circumcision | Caudal anesthesia or subpubic penile block | A (very high) |
| Hypospadias correction | Caudal anesthesia | A (very high) |
| Orchidopexy | Caudal anesthesia | A (very high) |
| Herniotomy (open) | Wound infiltration, ilioinguinal block; caudal anesthesia | A (very high) |
| Abdominal | ||
| Extensive abdominal surgery | PDA | B (high) |
| Appendectomy (open) | Wound infiltration additionally to other analgesic measures | D (low) |
| Fundoplication (open) | PDA | D (low) |
| Orthopedic | ||
| Lower extremity | PCEA | C (medium) |
| Upper extremity (hand, forearm) | Brachial plexus block | D (low) |
| Spine | PDA, poss. with two catheters | C (medium) |
| Various | ||
| Thoracotomy | PDA | B (high) |
*1 Detailed definition of Evidence level on the internet, evidence levels: A (very high; high quality meta-analyses, randomized controlled trials [RCTs]);
B (high; systematic review articles); C (medium; good case control or cohort studies); D (low, case series).
PDA, peridural anesthesia and postoperative analgesia; PCEA, patient controlled epidural analgesia. Modified from (7)
Acute pain
Pain in children.
In children, acute pain occurs predominantly during infectious illnesses, painful interventions, or after surgery.
Chronic pain most commonly takes the form of headache and abdominal pain.
A large number of acute diseases of childhood (otitis media, pharyngitis, burns, aphthous stomatitis, etc.) are associated with pain. Pain and fever are the commonest causes of an unscheduled pediatric consultation (e3). Almost half the children suffering from otitis media experience severe pain; the mean pain score is 7.5 on a visual analog scale of 0 to 10 (VAS 0–10); 0 = no pain, 10 = maximal pain) (e4). The mean duration of pain can be significantly, but only inconsiderably reduced by antibiotic treatment: from 3.3 to 2.8 days (e5). Antibiotic therapy has little influence on pain intensity in the first 24 hours. Regardless of whether it is decided to treat the child with antibiotics, additional analgesic therapy, for example with ibuprofen, should be initiated for a variable period of one to seven days (recommendation level A) (2). Severe acute pain is also experienced postoperatively. Compared to adults, pediatric patients receive fewer and/or incorrectly dosed analgesics in daily routine (e1). Severe pain experiences in childhood can – even if they cannot subsequently be consciously recalled – lead via the development of a pain memory to unusual sensory processing of pain and altered behavioral patterns persisting well beyond convalescence. If adequate pain prevention is neglected in a single painful intervention, this results in higher analgesic requirements, increased stress and pain as well as a greater proportion of failed analgesia and sedation in subsequent interventions (2).
Chronic pain
Pain experience.
The experience of extreme pain in childhood and adolescence can result in the development of a pain memory.
Chronic and recurrent pain is a frequent phenomenon during childhood (1, e1, e2) and may be associated with increased anxiety and depression, a restricted level of physical/psychosocial functioning, and frequent school absenteeism (e6). The parents of the affected children are frequently under severe emotional stress and have a tendency to exhibit pain exacerbating reactions (e7, e8). Persisting pain during childhood predisposes towards the development of chronic pain in adulthood (3, 4, e9–e13).
Pain assessment
Pain is a subjective phenomenon. Verbal self-reporting is therefore the gold standard for qualitative and quantitative pain measurement both for children of school age and adults (recommendation level B) (5, e14). For pain perception see ebox 1.
e-Box 1. Pain perception in children.
From about three years of age onwards, children are capable of expressing their pain in concrete terms (e54).
The notion of pain (causes and assessment) follows Piaget’s stages of cognitive development (e55).
From the "concrete operational stage" (the stage from which childhood thought is predominantly bound to specific events) onwards, children are capable of forming concepts about causes of pain.
As regards recall processes, Chen (e56) has demonstrated that children at the age of three years are already able to remember painful procedures.
Von Baeyer et al. have described four different ways in which children handle memories of pain (e57):
Habituation
No change through the memory
No memorization pattern
Sensitization which can take place at the physiological and psychological level, for example by the formation of anxiety-triggered avoidance behavior
Reinforcing factors include operant mechanisms such as increased parental attention when pain occurs.
Newborns, infants and young children: The Childhood Discomfort and Pain Scale (KUSS, Kindliche Unbehagen- und Schmerz-Skala) is suitable for assessing postoperative pain in non-mechanically ventilated newborns up to the end of the fourth year (table 1) (6).
Table 1. Childhood Discomfort and Pain (KUSS) Scale*1.
| Observation | Rating | Points |
|---|---|---|
| Crying | Not at all | 0 |
| Groaning, whining, whimpering | 1 | |
| Shouting | 2 | |
| Facial expression | Relaxed, smiling | 0 |
| Mouth distorted | 1 | |
| Mouth and eyes grimacing | 2 | |
| Trunk posture | Neutral | 0 |
| Unsettled | 1 | |
| Rearing up, arching | 2 | |
| Leg posture | Neutral | 0 |
| Struggling, kicking | 1 | |
| Drawn up to body | 2 | |
| Motor restlessness | Not present | 0 |
| Moderate | 1 | |
| Restless | 2 | |
| Points total: | ||
*1KUSS, Kindliche Unbehagen- und Schmerz-Skala;
The scale is suitable for newborns and young children up to the end of the
fourth year of age. Modified from (5).
Only one statement is allowed for each variable. The observation period is 15 seconds. Only data from this time window are to be recorded, even if the child’s behavior subsequently changes. Repeated observations at defined time intervals have greater information value than a single observation. A check on the degree of alertness is part of each observation. A sleeping child has no acute analgesic therapy requirement. Analgesic therapy requirement begins with four points. Its urgency increases with rising points score
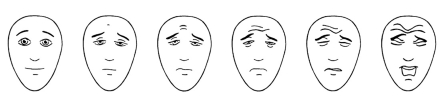
Preschool and school children: The KUSS can be used in children without knowledge of German, whose general or linguistic development is delayed, or who are situationally impaired by anxiety. Depending on the developmental status and previous experience, a face pain scale for self rating should be used in children from five years of age (figure). Of all the face pain scales, preference should be given to those which are free of emotional content. A face with tears running down can have different meanings; younger children may possibly use this variant to express high pain intensity, while older children do not wish to cry despite having pain – whether because of cultural or family values – and therefore report falsely low values. A laughing face at the beginning of the scale may possibly result in a false high pain score because sick children even without pain rarely see themselves as "happily" laughing. The "Faces Pain Scale – Revised" (7) shows good psychometric characteristics and is most suitable for this age group (8, e15).
Figure: Faces Pain Scale for children aged about 5 years onwards (7).
Instructions for taking a medical history of the child’s pain situation: "These faces show how much something can hurt. This face (point to left-most face) shows no pain. The faces show more and more pain (point to each from left to right) up to this one (point to right-most face) – it shows very much pain. Point to the face that shows how much you hurt (right now)."
To convert to a points system and document in the patient curve, score the chosen face 0, 2, 4, 6, 8 or 10, counting left to right. From a score of four onwards there is an analgesic requirement for acute pain. (www.painsourcebook.ca). Figure with kind permission of ISAP
Consequences of pain experience.
Chronic pain in children causes frequent school absenteeism and if left untreated predisposes towards chronic pain in adulthood.
Chronic pain: Questionnaires record further pain dimensions such as pain quality, concomitant symptoms, pain-inducing/exacerbating conditions, pain related impairment, emotional distress, quality of life, pain related coping, and self rating of therapy related improvement. Pain relevant factors in the social environment (family, kindergarten, school) are components of the interview guide. For younger children or when there is a wish for additional information, the parent’s reported information is also included. For chronic pain, it is indispensable under diagnostic and therapeutic aspects to keep a pain diary designed suitably for children (e14).
Postoperative pain therapy in children and adolescents
Younger children perceive the same injuries as more painful than older children (e16). Multimodal preventive and therapeutic concepts for perioperative pain integrate psychological, pharmacological, and physical techniques (immobilization etc.). The best basis for ensuring effectiveness of pain therapy is good psychological preparation by provision of age-appropriate information.
Principles of non-pharmacological treatment
The purpose of psychological interventions is to prevent children developing anxieties and to beneficially influence pain experience through cognitive and emotional processes. Several methods are indispensable for this purpose:
Since situational factors influence children’s pain experience to a particular degree, optimal background conditions such as age-appropriate briefing, procedures without long waiting times, and child-appropriate room design are important.
To reduce or prevent anxieties and negative emotional stress factors, cognitive and behavioral therapeutic methods such as age-appropriate education, breathing exercises, role playing, external attention directing or imaginative techniques – adjusted to the individual situation – are to be included (5, e17) (recommendation level A for individual psychological interventions for painful procedures).
Principles of pharmacological treatment
KUSS scale.
Acute pain in newborns, infants, and young children is assessed with the KUSS scale. Preschool and school children can report their pain themselves.
Therapeutic administration of placebos in response to children’s acute expressions of pain are not generally productive and ethically highly questionable (see ebox 2). In non-sedated children, intramuscular and subcutaneous injections are to be strictly avoided. Depending on age and culture, rectal administration is often unwelcome. Plasma level concentrations and onset of action – of paracetamol for example – are unpredictable after rectal administration. The absorption of orally administered medications may be delayed in the immediate postoperative period. The intravenous administration of analgesics allows rapid titration according to pain in the recovery room. In the immediate postoperative period on the ward, analgesics should be given according to a fixed time schedule ("by the clock") and additionally on demand – for a variable period depending on the scale of the operation. Analgesics should then be administered according to demand based on the pain score.
e-Box 2. Additional information on administration of placebos in pediatric pain therapy.
It is common practice at German pediatric centers to administer short infusions with NaCL 0.9% or oral placebos (especially vitamin C) to children thought to be "feigning pain." If the children show marked pain reduction, this may result in the treating team feeling justified in their belief that the pain reported by the child does not have a "biological" but rather a "psychological" origin. The fact is overlooked that up to 50% of persons with extremely severe pain (children and adults with migraine attacks, postoperatively, acute abdomen, etc.) show a 50% placebo induced pain reduction (e58–e60). Placebo administration often leads to pain reduction which can, however, be explained in terms of factors such as classical conditioning processes or expectation effects (e61). Despite these effects, placebo use in children and adolescents can lead to loss of trust not only among children and adolescents but also parents (e62).
If a placebo is administered to an affected child as a means of distinguishing between "biological" and "psychological" pain, this contradicts the scientifically established fact that pain is always to be understood as being of bio-psycho-social etiology (e63). Giving placebo in response to a child’s expressions of acute pain provides no information about the underlying causes of the pain, can prejudice the relationship between the treating physician, patient and parents and also prevents the use of an adequate multimodal pain therapy in which psychological elements are an important component (1). Giving a placebo may for a brief period of time free the treating team from its inability to deal professionally with the child’s expressions of pain, but usually does not lead to a positive solution of the underlying conflict – the child is expressing pain and wants pain therapy; I as the treating person do not believe he is in pain and do not wish to give him analgesic therapy – but is more likely to further exacerbate this conflict. Particularly when the treating person perceives a child’s expressions of pain appear inadequate in relation to the triggering cause (severe pain, small blood sample), inconsistent with the child’s behavior (reporting maximum pain rating but "smiling child," complaints of severe headache and wish to play at the computer) or occurring only at certain times (headache on Mondays; in the evening severe postoperative pain but active play behavior during the day), the team should understand the expression of pain as a "message" that is important to understand and which warrants a professional response (e46) – and not the administration of a placebo.
Monitoring: Standardized monitoring and documentation protocols are recommended for this purpose. After titration to the required dose, a pain measurement should be performed every two to four hours (at rest and during exercise) with continuous infusion of analgesics.
Analgesics
Modern evidence-based perioperative analgesic regimens are stratified according to the scale, localization, and extent of the operation. They often comprise a combination of regional anesthesiological interventions and systemically acting analgesics (balanced procedure, recommendation level A to C depending on the operation studied). The evidence situation is essentially unsatisfactory in this indication.
Non-opioids
The choice of non-opioids is based on the pathophysiology of the pain and on contraindications. For inflammatory pain, non-steroidal anti-inflammatory drugs (NSAIDs) are used, for spasmodic abdominal pain metamizole and if there is an increased hemorrhagic risk, paracetamol or metamizole. A comparison of the two most commonly used non-opioids paracetamol and ibuprofen is provided in table 2.
Table 2. Comparison of advantages and disadvantages of ibuprofen and paracetamol in pain therapy*1.
| Ibuprofen | Paracetamol | |
|---|---|---|
| Advantages | ||
| Potent analgesic | Rectal administration form also available for young children | |
| Wide therapeutic range | Approved for infants <6 months | |
| Low toxicity on overdose | No antiplatelet effect | |
| Palatable syrups | Intravenous formulation available | |
| Better tolerability in children <2 years with history of obstructive bronchitis | ||
| Dose certainty (3 × 10 mg/kg/BW/day) | ||
| Long duration of action (8 h) | ||
| Disadvantages | ||
| Only approved from age 6 months | Dose uncertainty | |
| Risk of acute renal failure if pre-existing relevant dehydration |
|
|
| Relatively unpalatable oral formulation |
*1 5, 15, 16, 25, e19, e48–e53; BW, body weight
Pain prevention strategies.
Non-pharmacological pain prevention strategies are important because anxiety can intensify pain.
Paracetamol: Paracetamol (PCM) has no clinically relevant inhibitory effect on platelet aggregation. It has no anti-inflammatory component. The adverse effects typical of non-steroidal anti-inflammatory drugs, such as gastrointestinal mucosal injury, are absent. Paracetamol is hepatically glucuronidated and/or sulfated. Overdose results in formation of the highly toxic N-acetyl-p-benzoquinone imine (NAPQI). Genetic changes (polymorphisms of CYP2E1; autosomal recessive inherited deficit of glutathione synthesis) may make affected children more susceptible to the paracetamol associated hepatotoxicity.
Paracetamol.
The low analgesic potency, variable absorption after rectal administration, and the narrow therapeutic range should be considered when using paracetamol.
PCM is approved for use from birth onwards. The analgesic potency of paracetamol is rated by many authors as lower than that of ibuprofen or other NSAIDs (5, e18). Postoperatively, paracetamol monotherapy is only weakly effective (e19). Dose recommendations for paracetamol are given in table 3.
Table 3. Recommended dosages for paracetamol*1.
| Single initial dose at start of therapy (mg/kg) | Follow-on dose (mg/kg) | Dose interval (h) | Max. daily dose (mg/kg/day) | |
|---|---|---|---|---|
| Rectal | ||||
| Premature infants 28th–30th GW | 20 | 15 | 12 | 35 |
| Premature infants 31st–38th GW | 20 | 15 | 12 | 45 |
| Newborns and infants up to age 6 months | 30 | 15 | 8 | 60 |
| Infants after 6th month | 35–45 | 15–20 | 6–8 | 60 |
| Young children >1 year | 35–45 | 15–20 | (4–)6 | 75 |
| Children >6 years | 35–45 | 15–20 | (4–)6 | 90 Absolute maximum 4000 mg/day |
| Oral | ||||
| Newborns and infants up to age 6 month | 20 | 20 | 8 | 60 |
| Infants after 6th month | 30 | 10–20 | (4–)6 | 60 |
| Young children >1 year | 30 | 15 | (4–)6 | 75 |
| Children >6 years | 30 | 15 | (4–)6 | 90 Absolute maximum 4000 mg/day |
| Intravenous | ||||
| All age groups | 15 | 15 | 6 | 60 Absolute maximum 4000 mg/day |
After rectal administration the peak plasma level is only reached after 2 to 3 hours because of slow and variable absorption (e18).
For oral or rectal administration a saturation dose should be given on starting treatment. For intravenous therapy the saturation dose is not required.
Maximal analgesia is achieved 1 to 2 hours after rapid (within 10 min) intravenous administration (e18). Because of the narrow therapeutic range the age adapted maximum daily dose should not be exceeded and not administered for longer than 48 hours;
Non-steroidal anti-inflammatory drugs (NSAIDs): The NSAID most widely used in pediatrics is ibuprofen (table 4). Like all non-selective NSAIDs, it inhibits cyclooxygenase (COX) type I and II and secondarily also platelet aggregation. For interventions with an increased hemorrhagic risk (tonsillectomy, large wound areas etc.) and in patients with hemorrhagic tendency a careful risk assessment is required. The influence of COX I and II inhibitors on the incidence of relevant bleeding after tonsillectomy has not yet been conclusively determined: a Cochrane analysis concludes that the risk of relevant post-tonsillectomy bleeding is not increased by NSAIDs (9, e20–e22). Children rarely develop gastrointestinal mucosal injury and nephrotoxicity after short-term ibuprofen therapy (less than seven days). These adverse effects do not occur more often than with paracetamol. Individual cases of acute renal failure have been reported when dehydrated children received NSAIDs. In children with mild bronchial asthma, the risk of an allergic reaction to NSAIDs is classified as low. Ibuprofen is only approved for use in children older than six months since in younger infants the risk of reduced cerebral or renal blood flow may be increased.
Table 4. Recommended dosages for several selected NSAIDs and metamizole*1.
| Substance | Dosage route | Single dose (mg/kg) | Dose interval (h) | Max. daily dose (mg/kg/day) |
|---|---|---|---|---|
| Ibuprofen | Oral/rectal | 10–15 | 8 | 30–40 Absolute: 2400 mg/day |
| Diclofenac | Oral/rectal | 1–2 | 8–12 | 3 Absolute: 150 mg/day |
| Metamizole | Oral/rectal/ short i.v. infusion/ slow drip infusion | 10–15 | 4–6 | 60–75 Absolute: 5000 mg/day |
*1 modified from (11)
Metamizole: Because of its spasmolytic properties metamizole is particularly suitable for visceral pain or colicky pain. Caution is advised in patients with a history of asthma or allergies and when the cardiovascular situation is unstable. During intravenous therapy, a considerable fall in blood pressure and even shock can occur due to hypersensitivity reactions or allergies.
Metamizole should only be administered as a short infusion and always with close monitoring of blood pressure values. More useful than repeated short infusions is a long-term infusion with a dosage of 2.5 to 3.0 mg/kg/h. Metamizole is – depending on the route of administration and the product – approved for use from the age of three months onwards. A risk assessment for agranulocytosis in children receiving metamizole therapy is not possible at present – only one such case has been reported to date (10)
Opioids
Non-steroidal anti-inflammatory drugs.
Gastrointestinal mucosal injury and nephrotoxicity rarely occur in children during short-term treatment with ibuprofen.
Choice of non-opioid.
The choice of the non-opioid is based on the pathophysiology of the pain and the possible contraindications.
Indications of non-opioids.
Inflammatory pain => NSAIDs
Spasmodic (abdominal) pain => metamizole
Increased hemorrhagic risk => PCM or metamizole
Newborns, infants, and children with pre-existing cerebral impairment react with particular sensitivity to opioids, which therefore have to be initiated at low doses and titrated upwards slowly until maximal control of the symptoms amenable to pharmacotherapy is achieved. Besides respiratory depression and sedation, the same adverse effects are essentially seen in children as in adults (nausea, vomiting, constipation, pruritus, urinary retention, lowered seizure threshold). The risk of dependence is to be classified as low. An extensive literature search failed to disclose a single documented case. As opioids for moderate to severe pain, tramadol and tilidine, for severe to very severe pain especially morphine and, if this is poorly tolerated by certain individuals, buprenorphine, hydromorphone, and oxycodone are used. Despite scant scientific evidence, piritramide is widely used in Germany (Recommendation level for postoperative use of opioids [also as patient-controlled analgesia, PCA] depending on the operation: A to C) (5).
Tramadol: Tramadol has not only an opioid receptor mediated action but also serotonergic and adrenergic mechanisms of action (11). After a saturation dose, a continuous long-term infusion is more suitable because of its better tolerability. Especially the combination with metamizole has proved successful particularly for visceral pain. Tramadol is not subject to the German Federal Narcotics Act.
Tilidine/naloxone: Tilidine/naloxone can be used orally in older children in the later postoperative course. Tilidine is not subject to the provisions of the German Federal Narcotics Act.
Morphine: Morphine is the opioid of choice for pediatric use. Starting doses are listed in table 5. The safety and efficacy of continuous intravenous administration of opioids for pain management are well established for all age groups (e1).
Table 5. Opioid starting doses for opioid-naive children and adolescents.
| Dosage route | Usual starting dose for children of body weight >10 kg and age >6 months | ||
| Opioids for severe and very severe pain (WHO III) | |||
| Buprenorphine | |||
| Intravenous | Loading dose | 0.003 mg/kg | (max. 0.15 mg) every 6 h |
| PCA loading dose | 0.001 mg/kg | (max. 0.06 mg) | |
| SDI | 0.0005 mg/kg/h | (max. 0.03 mg/h) | |
| Sublingual | 0.004 mg/kg | (max. 0.2 mg) every 8 h | |
| Hydromorphone | |||
| Intravenous | Loading dose | 0.01 mg/kg | (max. 0.5 mg) every 3 h |
| PCA loading dose | 0.004 mg/kg | (max. 0.2 mg) | |
| SDI | 0.005 mg/kg/h | (max. 0.2 mg/h) | |
| Oral | Immediate release | 0.03 mg/kg | (max. 1.3 mg) every 4 h |
| Sustained release | 0.06 mg/kg | (max. 4 mg) every 8 h | |
| Morphine *1 | |||
| Intravenous/ subcutaneous | Loading dose | 0.05 mg/kg | (max. 3 mg) every 3 h |
| PCA loading dose | 0.02 mg/kg | (max. 2 mg) | |
| SDI | 0.02 mg/kg/h | (max. 0.5 mg/h) | |
| Oral | Immediate release | 0.2 mg/kg | (max. 5 mg) every 4 h |
| Sustained release | 0.4 mg/kg | (max. 10 mg) every 8 h | |
| Oxycodone | |||
| Intravenous/ subcutaneous | Loading dose | 0.04 mg/kg | (max. 2 mg) every 4 h |
| PCA loading dose | 0.02 mg/kg | (max. 1.3 mg) | |
| SDI | 0.02 mg/kg/h | (max. 0.5 mg/h) | |
| Oral | Immediate release | 0.1 mg/kg | (max. 5 mg) every 4 h |
| Sustained release | 0.2 mg/kg | (max. 10 mg) every 8 h | |
| Opioids for moderate and severe pain (a dose of 10 mg/kg/day or 600 mg/day should not be exceeded) | |||
| Tramadol | |||
| Intravenous | Loading dose | 1 mg/kg | (max. 50 mg) every 4 h |
| PCA loading dose | 0.3 mg/kg | (max. 10 mg) | |
| SDI | 0.3 mg/kg/h | (max. 10 mg/h) | |
| Oral | Immediate release | 1 mg/kg | (max. 50 mg) every 4 h |
| Sustained release | 2 mg/kg | (max. 100 mg) every 8 h | |
| Tilidine/Naloxone | |||
| Oral | Immediate release | 1 mg/kg | (max. 50 mg) every 4 h |
| Sustained release | 2 mg/kg | (max. 100 mg) every 8 h | |
For infants <6 months and children of body weight <10 kg or in children with cerebral impairment, the starting doses should be reduced by two-thirds to one third of the dose shown here. Follow-on doses should always be titrated slowly depending on response. Max., maximum single dose at the start of opioid therapy in older children, adolescents, and young adults; SDI, slow drip infusion; for SDI the maximum dose per hour is stated; *1 parenteral piritramide is dosed like morphine. Modified from Cancer Pain Relief and Palliative Care in Children (WHO 1998 [11])
Morphine PCA: Patient-controlled analgesia (PCA) can be used in children aged six years or more (5, e14). Postoperatively, a basic infusion of 4 µg/kg/h morphine is not associated with an increased rate of adverse effects (e23). Pediatric opioid PCA requires special logistics and regular monitoring of vital signs. Loading doses are shown in table 5, the lockout time – loading dose demand does not result in a loading dose but is recorded by the PCA pump – is usually ten minutes.
Tramadol.
Tramadol is very well tolerated as a long-term infusion in pediatric use.
Piritramide: Because of its acidic pH, piritramide should not be given together with other drugs through the same intravenous line. The dosage corresponds to that of morphine (table 5).
Pethidine: The use of pethidine is no longer recommended because of its long half-life and the seizure threshold lowering metabolite norpethidine. Norpethidine can cumulate during prolonged pethidine use and postoperatively (11, e24, e25).
Regional anesthesia and analgesia
In children, regional anesthesia should be carried out under deep sedation or general anesthesia (table 6). The Pediatric Anesthesia Working Group of the German Society of Anesthesiology draws attention to the fact that routine clotting analyses are not required in healthy children before applying regional anesthesia. Ropivacaine appears to be the local anesthetic of first choice for epidural/caudal analgesia because of its low cardiotoxicity, low incidence of motor blocks, and favorable pharmacokinetics on continuous administration (5, e26). In children, respiratory and sedation monitoring should be practiced for at least 24 h after epidural opioid administration.
Chronic and recurrent pain
Headache is one of the commonest pediatric health problems (12, e2). In Germany an estimated 1 000 000 school days are missed every year because of headache.
Migraine: 5% to 10% of all 7- to 15-year-olds suffer from migraine (in most cases without aura). In the last 20 years the prevalence of migraine among 7-year-olds has risen from 1.9% to 5.2% (e27). The diagnostic criteria of the International Headache Society (IHS) define migraine without aura as follows (13).
At least five attacks fulfil the following criteria
Duration of attack 4 h (children 1 h) to 72 h
-
Headache fulfils at least two of the following criteria
Unilateral location (children also: bilateral frontal/temporal)
Pulsating
Moderate to severe pain intensity
Exacerbation on physical activity
-
Headache is accompanied by at least one of the following symptoms
Nausea and/or vomiting
Photophobia and phonophobia.
Morphine.
Morphine is the most thoroughly studied potent opioid in pediatrics; and can be safely used at any age (subject to compliance with age-appropriate weight based dosage and monitoring standards).
Use of opioids.
Opioids are always titrated according to effect. The starting dose is based on the child’s age and comorbidities.
Establishment of opioids.
Safety and efficacy of continuous intravenous administration of opioids for pain management are well established for all age groups.
Tension-type headache: Up to 25% of all children aged 7 to 15 years complain of episodic tension-type headache. In contrast to migraine, the pain attack may last from 30 minutes to 7 days and is of lower intensity (13). 5% of the children complain of nausea, 15% of photophobia/phonophobia respectively during the headache phase (e28, e29).
Diagnostic procedures for headache
Recurrent headache in childhood can usually be diagnosed on the basis of the medical history and physical examination (14). Electroencephalography (EEG) is only recommended if associated symptoms suggest the presence of a cerebral convulsive disorder (14). A cranial MRI (magnetic resonance imaging) scan is only indicated in exceptional cases (ebox 3).
e-Box 3. Indication for cranial MRI*1.
Cranial MRI is indicated for recurrent headache in the following cases:
Neurological evaluation shows abnormalities
Simultaneous presence of cerebral seizures
Changes in headache intensity, frequency or characteristics
Morning headache
Nausea/vomiting on awakening
Nausea between migraine attacks
Nocturnal headache
Sudden severe, first-onset headache
Other additional signs of illness (e.g. changes in personality) suggesting another neurological condition
*1 modified from Lewis et al. (14)
Regional anesthesia.
Regional anesthesia should only be performed under deep sedation or general anesthesia.
Pharmacological therapeutic approaches for acute migraine attacks: Treatment of migraine attacks is stratified according to severity, duration, and accompanying symptoms (12). Single-agent preparations are to be preferred and administered in sufficiently high dosages. Ibuprofen is superior to paracetamol; a direct comparison shows that complete pain relief after two hours is achieved twice as frequently with ibuprofen (15). For attacks of headache that are already initially very severe, rapidly progressive, or accompanied by numerous other symptoms and if the primary ibuprofen therapy is ineffective, sumatriptan nasal spray should be used, which is approved in Germany for use in children aged 12 years or more (dose: 10 mg intranasal) (8) (recommendation level A, [8, 12, 16, e30–e33]). Acetylsalicylic acid should only be used from age 12 years onwards because of the risk of Reye’s syndrome (e34).
Migraine prophylaxis: The efficacy of multimodal outpatient treatment programs (including elements from cognitive behavioral therapy, relaxation techniques, hypnosis, biofeedback, and endurance training) has been convincingly demonstrated (recommendation level A) (1, 17, 18).
Children and headache.
Headache in childhood is very common. Migraine attacks are often shorter than in adults and may often be bilateral. The differential diagnosis of childhood headache should be made by a pediatrician.
Purely clinical experience shows that a regular life style with sufficient sleep, a balanced diet and adequate fluid intake is helpful. Pharmacological prophylaxis in childhood should only be employed if attack therapy is inadequate and the non-pharmacological prophylactic measures fail (12). Migraine prophylaxis is performed for a period of three to six months keeping a migraine diary and is then interrupted to assess the spontaneous course. The medications are generally titrated upwards from an initial low level and finally tapered out. The most commonly used substances are the beta blocker metoprolol and the calcium channel blocker flunarizine (no beta blockers in patients with asthma) and the anticonvulsant topiramate (recommendation level D, because of conflicting study results [12, 19, e35–e41]).
Treatment options in tension-type headache: In most cases this headache can be treated using non-pharmacological strategies which have to be learned by the child in group or in one-to-one sessions, such as muscle relaxation by the Jacobson method as well as cognitive-behavioral strategies and biofeedback. TENS (transcutaneous electrical nerve stimulation) has proved helpful in open-label studies.
Chronic abdominal pain
The prevalence of chronic abdominal pain among children of school age is 10% to 25% (e42). Apley defines typical chronically recurrent abdominal pain as abdominal pain that occurs episodically at least once a month for at least three months in succession and impairs the child’s normal activities (20). At present, chronic abdominal pain is classified according to the Rome III criteria (21) (ebox 4). In more than half of the cases the functional abdominal pain is already primarily associated with other forms of pain such as headache and chest pain.
e-Box 4. ROME III criteria for classification of recurrent abdominal pain in childhood (e64)*1.
-
Functional dyspepsia
The following criteria must be fulfilled at least once per week for at least two months:
Persistent or recurrent pain centered in upper abdomen (above umbilicus)
Not relieved by defecation or associated with change in form or frequency of bowel action (this means that no irritable bowel syndrome is present)
No signs of inflammatory, anatomical, metabolic or neoplastic process that would explain the patient’s symptoms
-
Irritable bowel syndrome
The following criteria must be fulfilled at least once per week for at least two months:
-
Abdominal discomfort (an unpleasant sensation not described as pain) or painassociated for 25% of the time or more with two or more of the following criteria:
Improvement with defecation
Change in frequency of stool
Change in form or appearance of stool
No signs of inflammatory, anatomical, metabolic or neoplastic process that would explain the patient’s symptoms
-
-
Abdominal migraine
All of the following criteria must be fulfilled at least twice in the last 12 months:
Paroxysmal episodes of intense periumbilical pain lasting 1 or more hours
Healthy in between for weeks or months
Pain interferes with normal activities
-
Pain associated with two or more of:
Loss of appetite
Nausea
Vomiting
Headache
Photophobia
f. Pallor
No signs of inflammatory, anatomical, metabolic or neoplastic process that wouldexplain the patient’s symptoms
-
Functional abdominal pain
The following criteria must be fulfilled at least once per week for at least two months:
Episodic or continuous abdominal pain
Insufficient criteria for other functional gastrointestinal disorders
No signs of inflammatory, anatomical, metabolic or neoplastic process that wouldexplain the patient’s symptoms
-
Functional abdominal pain syndrome of childhood
The criteria for functional abdominal pain of childhood must be fulfilled for at least 25% of the time and include at least one of the following points:
Interferes with normal activities
Additional somatic symptoms (headache, limb pain, sleep difficulty)
*1 modified from Raquin A, Di Lorenzo C, Forbes D et al.: Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2006; 130: 1527–37
Children with functional abdominal pain show characteristic features (e42):
Periumbilical (45%) or epigastric pain (40%) lasting for less than one hour in two thirds of the children and almost always for less than three hours
Child and parents can almost never name measures that shorten the pain event
Pain is frequently associated with autonomic nervous symptoms such as pallor and nausea
Physical examination reveals no abnormalities.
Caution is advised if one of the warning signs mentioned in the box occur (box).
Box. Alarm signs for the presence of another disease than abdominal pain are:
Persisting pain in the right upper or right lower quadrant
Pain that rouses the child from sleep
Pain duration >12 h
Distinctly periodic course
Nocturnal diarrhea
Persisting vomiting
Dysphagia
Gastrointestinal blood loss
Perianal diseases
Unwanted weight loss
Slowing of longitudinal growth
Delayed puberty
Fever of indeterminable etiology
Arthritis
Family history of inflammatory bowel diseases, celiac disease or peptic ulceration
Medication with acetylsalycilic acid (ASA).
Due to the risk of Reye’s syndrome, ASA should generally only be used from age 12 years onwards.
Somatic, mental, and social "abnormalities": Many of the children affected show "abnormalities" such as disorders of gastrointestinal motility, carbohydrate malabsorption or positive Helicobacter pylori tests. The presence of positive organic findings, however, has no influence on the long-term course (22). According to the ESPGHAN (European Society for Pediatric Gastroenterology, Hepatology, and Nutrition) Consensus Statement, there is no validated relationship between the Helicobacter infection and chronic recurrent abdominal pain (22). Children with abdominal pain and non-ulcerative gastritis do not benefit symptomatically from HP eradication. Many psychosocial "abnormalities" have been proclaimed for these children and their families. The study results are contradictory, especially in cases where not healthy but chronically ill children and their families were used as reference group.
Bio-psycho-social model
After excluding other diseases by means of medical history and physical examination, the pediatrician can continue searching for "the" cause or interpret the functional pain within the context of a bio-psycho-social model (23, e43): a child’s vulnerability in terms of functional abdominal pain is an interplay between organic dysfunction (in these cases especially of the gastrointestinal tract) and psychological factors. Once functional abdominal pain has developed, the recurrent pain experience increases the child’s vulnerability, and this all the more, the more clearly the pain is perceived and intensified by the environment. Stressors can be psychosocial or biological in nature (e.g. lactose loading).
Treatment
Treatment of migraine attacks.
The treatment of migraine attacks is stratified according to pain and course. Non-pharmacological strategies for migraine prophylaxis are very well validated and effective over years. Pharmacological prophylaxis of migraine plays a secondary role.
In several studies on the long term course over 5 to 30 years, the children who formerly suffered functional abdominal symptoms were now suffering considerably more frequently from chronic abdominal pain, other somatic symptoms, and psychosocial disorders (e44). No significantly positive therapeutic results were obtained for functional abdominal symptoms on introducing lactose-free diet or increasing nutritional dietary fiber (24). The course of functional abdominal symptoms can be favorably influenced by strategies such as comprehensive education about the maintenance of chronic pain and learning appropriate pain related coping strategies. Cognitive behavioral therapy combined with training measures for parents are also promising (recommendation level A) (1, e44). Other forms of chronic abdominal pain also benefit from pharmacological therapeutic approaches such as attack therapy with ibuprofen for abdominal migraine.
Somatoform pain disorders
Somatoform pain disorders are dominated by persistent and intolerable pain which cannot be explained in terms of physiological processes (e45). The prevalence of somatoform pain disorder in childhood is 2% to 3% (200 000 affected children in Germany) (e46). For extreme pain-related life impairment, high school absenteeism, concurrent emotional problems or if outpatient therapeutic interventions fail, inpatient multimodal pain therapy is recommended (recommendation level B, evidence level of studies: 3 and 4a [e46, e47]).
Conclusion
Functional abdominal pain.
Children with functional abdominal pain show characteristic features. More than half of those affected have other types of pain such as headache and chest pain. Chronic abdominal pain can rarely be explained in monocausal terms.
Somatoform pain disorders.
Cognitive-behavioral therapy combined with training for parents is promising.
For severe life impairment, inpatient multimodal pain therapy is recommended.
Children with acute pain are entitled to adequate, age-appropriate pain evaluation and treatment. Extremely severe acute and recurrent or chronic pain compromises their development. Nationwide health care structures providing adequate postoperative acute pain therapy and multimodal treatment programs for chronic pain are lacking in Germany.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1:
Pain associated with acute otitis media is common. In what range does the medium pain intensity lie on a scale of 0 to 10 (0 = no pain, 10 = maximal pain)?
2–3
3–4
5–6
7–8
9–10
Question 2:
Which of the following principles is important for preventing the development of a pain memory?
Antibiotic treatment should be initiated for immediate pain relief in acute diseases such as otitis media.
Postoperatively, especially after bone fractures, older children (above 10 years) need analgesics more often than young children.
Postoperatively, pharmacological pain treatment is needed only for infants after painful locally limited procedures.
Sufficient pain prevention is necessary for a single, very painful procedure in children of every age.
Pain prevention before medical procedures is only necessary for children aged two years or more, since younger patients no longer remember the pain.
Question 3:
What is the gold standard of qualitative and quantitative pain measurement for school age children?
Verbal self-reporting
Independent observer
Electromyography
Neurological examination
Sensitivity test
Question 4:
What is the procedure for postoperative pain measurement?
Postoperative pain in children should usually be assessed by asking the parents because children cannot accurately judge their pain themselves.
Postoperative pain measurement should be done once daily.
In children up to four years of age, systematic observation of pain related behavior is unnecessary to assess their pain.
In children who cannot express themselves in German, an independent assessment of their pain is also appropriate over four years of age.
A systematic pain assessment is impossible in sedated patients.
Question 5:
From what age onwards at the earliest should a face scale for self-assessment of pain intensity be integrated into pediatric care, taking into account the child’s developmental status and previous experience?
From two years of age onwards
From four years of age onwards
From five years of age onwards
From three years of age onwards
From seven years of age onwards
Question 6:
Which drug is recommended in international guidelines to treat acute attacks of childhood migraine?
Dihydroergotamine
Ergotamine
Ibuprofen
Tramadol
Metoprolol
Question 7:
Which regional anesthesiological technique can be used in an extensive abdominal surgical procedure in children as part of balanced perioperative analgesia?
Caudal anesthesia
Peridural anesthesia
Brachial plexus blockade
Subpubic penile block
Intrathecal opioids
Question 8:
Migraine attacks are generally triggered by normal everyday stress. What should be remembered when treating migraine attacks?
Pharmacological prophylaxis is necessary for children suffering from migraine.
Pharmacological attack therapy should only be started if dietary interventions alone have failed
Besides pharmacological attack therapy, cognitive-behavioral training and endurance sport are important.
Paracetamol is superior to ibuprofen in the oral treatment of migraine attacks.
Pharmacological treatment is superfluous for attacks lasting less than two hours.
Question 9:
How should opioids be administered in newborns, infants, and children with pre-existing cerebral impairment?
Treatment should be started with a low initial dose and further doses should be titrated according to response.
The starting dose depends on the patient’s height and further doses should be based on the child’s age.
Treatment should be started with a high initial dose and continued with a maintenance dose.
The starting dose corresponds to the usual starting dose for children (body weight >10 kg, age >6 months) and the intervals between the opioid doses should be short in this patient population.
The starting dose corresponds to the usual starting dose for children (body weight >10 kg, age >6 months) and is continuously increased.
Question 10:
What applies for the bio-psycho-social model for functional abdominal pain in childhood?
In functional abdominal pain, an inherited or acquired vulnerability interacts with external stressors.
Excessive anxiety of parents, a Helicobacter pylori infection or aversion to school are the commonest causes of functional abdominal pain in children.
If functional abdominal pain has developed, the pain experience decreases the child’s vulnerability.
Lactose loading is the key factor in the development of functional abdominal pain.
Cognitive-behavioral therapy cannot influence the course.
Further Information.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Chambers of Physicians of the German federal states (Länder). CME points of the Chambers of Physicians can be acquired only through the Internet by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website:
Participants in the CME program can manage their CME points with their 15-digit "uniform CME number" (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the www.aerzteblatt.de website under "meine Daten" ("my data"), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in volume 37/2008. The CME unit "Urticaria: It’s History-Based Diagnosis and Etiologically Oriented treatment“ (volume 25/2008) can be accessed until 1 August 2008.
For volume 33/2008 we plan to offer the topic "The Diagnosis and Treatmene of Primary Osteoporosis According to Current Guidelines."
Solutions to the CME questionnaire in volume 21/2008:
Ukena D, Fishman L, Niebling W-B; Bronchial Asthma: Diagnosis and Long-Term Treatment in Adults:
1/a, 2/b, 3/b, 4/a, 5/d, 6/b, 7/d, 8/c, 9/a, 10/c
Acknowledgments
Translated from the original German by mt-g.
Footnotes
Conflict of interest statement
Mr. Zernikow received lecture fees, remuneration for consultations, and financial support for conference visits from the following companies: AstraZeneca, Aventis, Boots Healthcare, Bristol-Myers Squibb, Cephalon, Grünenthal, Janssen Cilag, Mundipharma, Pfizer, and Reckitt Benckiser.
Ms. Hechler declares that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Eccleston C, Morley S, Williams AC, Yorke L, Mastroyannopoulou K. Systematic review of randomised controlled trials of psychological therapy for chronic pain in children and adolescents, with a subset of meta-analysis of pain relief. Pain. 2002;99:157–165. doi: 10.1016/s0304-3959(02)00072-6. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 3.Christensen FM, Mortensen O. Long-term prognosis in children with recurrent abdominal pain. Arch Dis Child. 1975;50:110–114. doi: 10.1136/adc.50.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LS, Garber J., Van Slyke DA, Greene JW. Long-term health outcomes in patients with recurrent abdominal pain. J Pediatr Psychol. 1995;20:233–245. doi: 10.1093/jpepsy/20.2.233. [DOI] [PubMed] [Google Scholar]
- 5.Association of Paediatrics Anaesthetists. Good Practise in Postoperative and Procedural Pain. www.apagbi.org.uk. 2007.
- 6.Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth. 2000;10:303–318. doi: 10.1046/j.1460-9592.2000.00530.x. [DOI] [PubMed] [Google Scholar]
- 7.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale - Revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 8.von Baeyer CL. Children’s self-report of pain intensity: Scale selection, limitations and interpretations. Pain Res Manag. 2006;11:157–162. doi: 10.1155/2006/197616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardwell M, Siviter G, Smith A. Non-steroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy (Review) Cochrane Database Syst Rev. 2005;2005 doi: 10.1002/14651858.CD003591.pub2. Art. No. CD003591- DOI10.1002/14651858.CD003591.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Meyer O, Geadicke G, Salama A. Demonstration of drug-dependent antibodies in two patients with neutrophenia and successful treatment with granulocyte-colony-stimulation factor. Transfusion. 1999;39:527–530. doi: 10.1046/j.1537-2995.1999.39050527.x. [DOI] [PubMed] [Google Scholar]
- 11.Zernikow B, Friedrichsdorf S, Wamsler C, Michel E. Schmerztherapie und palliative Versorgung krebskranker Kinder (Deutsche Ausgabe der WHO-Empfehlungen „Cancer pain relief and palliative care in children“) Datteln, Vestische Kinderklinik Datteln- Universität Witten/Herdecke. 2002 [Google Scholar]
- 12.Evers S, Pothmann R, Überall MA, Naumann E, Gerber W-D. Therapie idiopathischer Kopfschmerzen im Kindesalter. Empfehlungen der Deutschen Migräne- und Kopfschmerzgesellschaft. Schmerz. 2002;16:48–56. doi: 10.1007/s004820100073. [DOI] [PubMed] [Google Scholar]
- 13.Kopfschmerzklassifikationskomitee der International Headache Society. Die internationale Klassifikation von Kopfschmerzerkrankungen. Nervenheilkunde. 2003;22:531–670. [Google Scholar]
- 14.Lewis DW, Ashwal S, Dahl G, et al. Practice parameter: Evaluation of children and adolescents with recurrent headaches: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;59:490–498. doi: 10.1212/wnl.59.4.490. [DOI] [PubMed] [Google Scholar]
- 15.Hamalainen ML, Hoppu K, Valkeila E, Santavuori P. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology. 1997;48:103–107. doi: 10.1212/wnl.48.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Silver S, Gano D, Gerretsen P. Acute treatment of paediatric migraine: A meta-analysis of efficacy. Journal of Paediatrics and Child Health. 2008;44:3–9. doi: 10.1111/j.1440-1754.2007.01206.x. [DOI] [PubMed] [Google Scholar]
- 17.Hermann C, Kim M, Blanchard EB. Behavioral and prophylactic pharmacological intervention studies of pediatric migraine: An exploratory meta-analysis. Pain. 1995;60:239–256. doi: 10.1016/0304-3959(94)00210-6. [DOI] [PubMed] [Google Scholar]
- 18.Trautmann E, Lackschewitz H, Kroner-Herwig B. Psychological treatment of recurrent headache in children and adolescents - a meta-analysis. Cephalalgia. 2006;26:1411–1426. doi: 10.1111/j.1468-2982.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- 19.Damen L, Bruijn J, Verhagen AP, et al. Prophylactic treatment of migraine in children. Part 2. A systematic review of pharmacological trials. Cephalalgia. 2006;26(5):497–505. doi: 10.1111/j.1468-2982.2005.01047.x. [DOI] [PubMed] [Google Scholar]
- 20.Apley H, Hale B. Children with recurrent abdominal pain; a field survey of 1000 school children. Arch Dis Child. 1958;33:165–170. doi: 10.1136/adc.33.168.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drossman DA. The functional gastrointestinal disorders and the Rome III process. In: Drossman DA, Corazziari E, Delvaux M, Spiller R, Talley NJ, Thompson WG, et al., editors. Rome III: The functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates; 2006. pp. 1–30. [Google Scholar]
- 22.Drumm B, Koletzko S, Oderda G. Helicobacter pylori infection in children: A consensus statement. European Paediatric Task Force on Helicobacter pylori. J Pediatr Gastroenterol Nutr. 2000;30:207–213. doi: 10.1097/00005176-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics Subcommittee on Chronic Abdominal Pain, North American Society for Pediatric Gastroenterology Hepatology and Nutrition. Chronic abdominal pain in children. Pediatrics. 2005;115:e370–e381. [Google Scholar]
- 24.Huertas-Ceballos A, MacArthur C, Logan S. Dietary interventions for recurrent abdominal pain (RAP) in childhood. Cochrane Database Syst Rev. 2002;(2) doi: 10.1002/14651858.CD003019. CD003019. DOI 10.1002/14651858.CD003019. [DOI] [PubMed] [Google Scholar]
- 25.Lesko SM, Louik C, Vezina RM, Mitchel AA. Asthma morbidity after the short-term use of ibuprofen in children. Pediatrics. 2002;109:E20–E23. doi: 10.1542/peds.109.2.e20. [DOI] [PubMed] [Google Scholar]
- e1.Howard RF. Current status of pain management in children. JAMA. 2003;290:2464–2469. doi: 10.1001/jama.290.18.2464. [DOI] [PubMed] [Google Scholar]
- e2.Roth-Isigkeit A. Zur Epidemiologie von anhaltenden und/oder wiederkehrenden Schmerzen bei Kindern. Monatsschr Kinderheilkd. 2006;154:741–754. [Google Scholar]
- e3.Feldman W. Evidence-based Pediatrics. Hamilton, London, Saint Louis: B.C. Decker Inc.; 2000. [Google Scholar]
- e4.Hayden GF, Schwartz RH. Characteristics of earache in children with acute otitis media. Am J Dis Child. 1985;1398:721–723. doi: 10.1001/archpedi.1985.02140090083037. [DOI] [PubMed] [Google Scholar]
- e5.Burke P, Bain J, Robinson D, Dunleavy J. Acute red ear in children: controlled trial of non-antibiotic treatment in general practice. Br Med J. 1991;303(6802):558–562. doi: 10.1136/bmj.303.6802.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108:221–229. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- e7.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-C): A preliminary validation. Pain. 2006;123:254–263. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- e8.Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: An experimental investigation. Pain. 2002;95:111–118. doi: 10.1016/s0304-3959(01)00385-2. [DOI] [PubMed] [Google Scholar]
- e9.Brna P, Dooley J, Gordon K, Dewan T. The prognosis of childhood headache: A 20-year follow-up. Arch Ped Adoles Med. 2005;159:1157–1160. doi: 10.1001/archpedi.159.12.1157. [DOI] [PubMed] [Google Scholar]
- e10.Jones GT, Silman AJ, Macfarlane GJ. Are common symptoms in childhood associated with chronic widespread body pain in adulthood? Results from the 1958 British Birth Cohort Study. Arthritis Rheum. 2007;55:1669–175. doi: 10.1002/art.22587. [DOI] [PubMed] [Google Scholar]
- e11.Hotopf M, Carr S, Mayou R, Wadsworth M, Wessely S. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. Br Med J. 1998;316(7139):1196–1200. doi: 10.1136/bmj.316.7139.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Walker LS, Guite JW, Duke M, Greene JW. Recurrent abdominal pain: A potential precursor of irritable bowel syndrome in adolescents and young adults. J Pediatr. 1998;132:1010–1015. doi: 10.1016/s0022-3476(98)70400-7. [DOI] [PubMed] [Google Scholar]
- e13.Campo JV, Di Lorenzo C, Chiapetta L, Bridge J, Colborn DK, Gartner JCJ, et al. Adult outcomes of pediatric recurrent abdominal pain: Do they just grow out of it? Pediatrics. 2001;108:E1. doi: 10.1542/peds.108.1.e1. [DOI] [PubMed] [Google Scholar]
- e14.Denecke H, Hünseler C. Messen und Erfassen von Schmerz. In: Zernikow B, editor. Schmerztherapie bei Kindern. Berlin, Heidelberg, New York: Springer; 2005. pp. 45–47. [Google Scholar]
- e15.Cohen LL, Lemanek K, Blount RL, Dahlquist LM, Lim CS, Palermo TM, et al. Evidence-based Assessment of Pediatric Pain. J Pediatr Psychol. 2007 Nov 17;:1–17. doi: 10.1093/jpepsy/jsm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347:1094–1113. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- e17.Labouvie H, Kusch M, Bode U. Psychologische Interventionen bei akuten Schmerzen im Kindesalter. In: Zernikow B, editor. Schmerztherapie bei Kindern. Berlin: Springer; 2005. pp. 132–142. [Google Scholar]
- e18.Lönnqvist PA, Morton NS. Postoperative analgesia in infants and children. Br J Anaesth. 2005;95:59–68. doi: 10.1093/bja/aei065. [DOI] [PubMed] [Google Scholar]
- e19.Mantzke US, Brambrink AM. Paracetamol im Kindesalter: Aktueller Wissensstand und Hinweise für einen rationalen Einsatz zur postoperativen Analgesie. Anaesthesist. 2002;51:735–746. doi: 10.1007/s00101-002-0359-9. [DOI] [PubMed] [Google Scholar]
- e20.Moiniche S, Romsing J, Dahl JB, Tramer MR. Nonsteroidal anti-inflammatory drugs and the risk of operative site bleeding after tonsillectomy: a quantitative systematic review. Anesth Analg. 2003;96:68–77. doi: 10.1097/00000539-200301000-00015. [DOI] [PubMed] [Google Scholar]
- e21.Krishna S, Hughes LF, Lin SY. Postoperative hemorrhage with nonsteroidal anti-inflammatory drug use after tonsillectomy: a meta-analysis. Arch Otolaryngol Head Neck Surg. 2003;129:1086–1089. doi: 10.1001/archotol.129.10.1086. [DOI] [PubMed] [Google Scholar]
- e22.Jeyakumar A, Brickman TM, Williamson ME, Hirose K, Krakovitz P, Whittemore K, et al. Nonsteroidal anti-inflammatory drugs and postoperative bleeding following adenosillectomy in pediatric patients. Arch Otolaryngol Head Neck Surg. 2008;134:24–27. doi: 10.1001/archoto.2007.11. [DOI] [PubMed] [Google Scholar]
- e23.Doyle E, Robinson D, Morton NS. Comparison of patient-controlled analgesia with and without a background infusion after lower abdominal surgery in children. Br J Anaesth. 1993;71:670–673. doi: 10.1093/bja/71.5.670. [DOI] [PubMed] [Google Scholar]
- e24.Morton NS. Management of postoperative pain in children. Arch Dis Child Educ Pract Online. 2007;92:ep14–ep19. doi: 10.1136/adc.2004.070888. [DOI] [PubMed] [Google Scholar]
- e25.American Academy of Pediatrics. The Assessment and Management of Acute Pain in Infants, Children and Adolescents. Pediatrics. 2001;108:793–797. doi: 10.1542/peds.108.3.793. [DOI] [PubMed] [Google Scholar]
- e26.Mazoit J, Dalens BJ. Ropivacaine in infants and children. Curr Opin Anaesthesiol. 2003;16:305–307. doi: 10.1097/00001503-200306000-00010. [DOI] [PubMed] [Google Scholar]
- e27.Sillanpää M, Anttila P. Increasing prevalence of headache in 7-year-old schoolchildren. Headache. 1996;36:466–470. doi: 10.1046/j.1526-4610.1996.3608466.x. [DOI] [PubMed] [Google Scholar]
- e28.Rossi LN, Vajani S, Cortinovis I, Spreafico F, Menegazzo L. Analysis of the International Classification of Headache Disorders for diagnosis of migraine and tension-type headache in children. Dev Med Child Neurol. 2008;50:305–310. doi: 10.1111/j.1469-8749.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- e29.Özge A, Bugdayci R, Sasmaz T, Kaleagasi H, Kurt O, Karakelle A, et al. The sensitivity and specificity of the case definition criteria in diagnosis of headache: a school-based epidemiological study of 5562 children in Mersin. Cephalalgia. 2002;22:791–798. doi: 10.1046/j.1468-2982.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- e30.Überall M, Wenzel D. Intranasal sumatriptan for the acute treatment of migraine in children. Neurology. 1999;52:1507–1510. doi: 10.1212/wnl.52.7.1507. [DOI] [PubMed] [Google Scholar]
- e31.Winner P, Rothner AD, Saper J, Nett R, Asgharnejad M, Laurenza A, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics. 2000;165:989–997. doi: 10.1542/peds.106.5.989. [DOI] [PubMed] [Google Scholar]
- e32.Ahonen K, Hämäläinen ML, Rantala H, Hoppu K. Nasal sumatriptan is effective in treatment of migraine attacks in children: A randomized trial. Neurology. 2004;62:883–887. doi: 10.1212/01.wnl.0000115105.05966.a7. [DOI] [PubMed] [Google Scholar]
- e33.Winner P, Rothner AD, Wooten JD, Webster C, Ames M. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache. 2006;46:212–222. doi: 10.1111/j.1526-4610.2006.00339.x. [DOI] [PubMed] [Google Scholar]
- e34.Tfelt-Hansen P. Triptans vs Other Drugs for Acute Migraine. Are There Differences in Efficacy? A Comment. Headache. 2008;48:601–605. doi: 10.1111/j.1526-4610.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- e35.Winner P, Pearlman EM, Linder SL, Jordan DM, Fisher AC, Hulihan J. Topiramate Pediatric Migraine Study Investigators. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache. 2005;45:1304–1312. doi: 10.1111/j.1526-4610.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- e36.Winner P, Gendolla A, Stayer C, Wang S, Yuen E, Battisti WP, et al. Topiramate for migraine prevention in adolescents: a pooled analysis of efficacy and safety. Headache. 2006;46:1503–1510. doi: 10.1111/j.1526-4610.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- e37.Sorge F, De Simone R, Marano E, Nolano M, Orefice G, Carrieri P. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled, crossover study. Cephalalgia. 1988;8:1–6. doi: 10.1046/j.1468-2982.1988.0801001.x. [DOI] [PubMed] [Google Scholar]
- e38.Sorge F, Marano E. Flunarizine v. placebo in childhood migraine. A double-blind study. Cephalalgia. 1985;5(Suppl 2):145–148. doi: 10.1177/03331024850050S227. [DOI] [PubMed] [Google Scholar]
- e39.Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol Scand. 1984;50:109–115. doi: 10.1111/j.1600-0404.1974.tb01350.x. [DOI] [PubMed] [Google Scholar]
- e40.Forsythe WI, Gilles D, Sills MA. Propanolol („Inderal“) in the treatment of childhood migraine. Development Medicine & Child Neurology. 1984;26:737–741. doi: 10.1111/j.1469-8749.1984.tb08166.x. [DOI] [PubMed] [Google Scholar]
- e41.Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics. 1987;79:593–597. [PubMed] [Google Scholar]
- e42.Berger T, Damschen U. Rezidivierende Bauchschmerzen. In: Zernikow B, editor. Schmerztherapie bei Kindern. Heidelberg: Springer; 2005. [Google Scholar]
- e43.Crushell E, Rowland M, Doherty M, Gormally S, Harty S, Bourke B, et al. Importance of parental conceptual model of illness in severe recurrent abdominal pain. Pediatrics. 2003;112:1368–1372. doi: 10.1542/peds.112.6.1368. [DOI] [PubMed] [Google Scholar]
- e44.Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics. 2003;111:e1–e11. doi: 10.1542/peds.111.1.e1. [DOI] [PubMed] [Google Scholar]
- e45.Remschmidt H, Schmidt M, Poustka F. Multiaxiales Klassifikationsschema für psychische Störungen des Kindes- und Jugendalters nach ICD-10 der WHO. Bern,: Hans Huber; 2006. [Google Scholar]
- e46.Dobe M, Damschen U, Reiffer-Wiesel B, Sauer C, Zernikow B. Dreiwöchige stationäre multimodale Schmerztherapie bei Kindern und Jugendlichen mit chronischen Schmerzen. Schmerz. 2006;20:51–60. doi: 10.1007/s00482-005-0457-0. [DOI] [PubMed] [Google Scholar]
- e47.Hechler T, Dobe M, Kosfelder J, et al. Effectiveness of a three-week multimodal inpatient pain treatment for children and adolescents suffering from chronic pain: Statistical and clinical significance. Clin J Pain. 2008 doi: 10.1097/AJP.0b013e318185c1c9. in Press. [DOI] [PubMed] [Google Scholar]
- e48.Halpern SM, Fitzpatrick R, Volans GN. Ibuprofen toxicity. A review of adverse reactions and overdose. Adverse Drug React Toxicol Rev. 1993;12:107–128. [PubMed] [Google Scholar]
- e49.Rainsford KD, Roberts SC, Brown S. Ibuprofen and paracetamol: Relative safety in non-prescription dosages. J Pharm Pharmacol. 1997;49:345–376. doi: 10.1111/j.2042-7158.1997.tb06809.x. [DOI] [PubMed] [Google Scholar]
- e50.Bradley RL, Ellis PE, Thomas P, Bellis H, Ireland AJ, Sandy JR. A randomized clinical trial comparing the efficacy of ibuprofen and paracetamol in the control of orthodontic pain. Am J Orthod Dentofacial Orthop. 2007;132:511–517. doi: 10.1016/j.ajodo.2006.12.009. [DOI] [PubMed] [Google Scholar]
- e51.Ali S, Klassen TP. Ibuprofen was more effective than codeine or acetaminophen for musculoskeletal pain in children. Evid Base Med. 2007;12:144. doi: 10.1136/ebm.12.5.144. [DOI] [PubMed] [Google Scholar]
- e52.Silver S, Gano D, Gerretsen P. Acute treatment of paediatric migraine: A meta-analysis of efficacy. J Paed Child Health. 2008 Sep 14;44:3–9. doi: 10.1111/j.1440-1754.2007.01206.x. Epub 2007. [DOI] [PubMed] [Google Scholar]
- e53.Ulinski T, Guigonis V, Dunan O, Bensman A. Acute renal failure after treatment with non-steroidal anti-inflammatory drugs. Eur J Pediatr. 2004;163:148–150. doi: 10.1007/s00431-003-1392-7. [DOI] [PubMed] [Google Scholar]
- e54.Stanford EA, Chambers CT, Craig KD. A normative analysis of the development of pain-related vocabulary in children. Pain. 2005;114:278–284. doi: 10.1016/j.pain.2004.12.029. [DOI] [PubMed] [Google Scholar]
- e55.Harbeck C, Peterson L. Elephants dancing in my head: A developmental approach to children’s concepts of specific pains. Child Dev. 1992;63:138–149. [PubMed] [Google Scholar]
- e56.Chen X. Growing up in a collectivistic culture: Socialization and socio-emotional development in Chinese children. In: Comunian AL, Gielen UP, editors. International perspective on human development. Lengerich, Italy: Pabst Science Publishers; 2000. pp. 331–353. [Google Scholar]
- e57.von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: Overview and implications for practice. J Pain. 2004;5:241–249. doi: 10.1016/j.jpain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- e58.Kokki H, Lintula H, Vanamo K, Heiskanen M, Eskelinen M. Oxycodone vs placebo in children with undifferentiated abdominal pain: a randomized, double-blind clinical trial of the effect of analgesia on diagnostic accuracy. Arch Pediatr Adolesc Med. 2005;159:320–325. doi: 10.1001/archpedi.159.4.320. [DOI] [PubMed] [Google Scholar]
- e59.Evers S. Controlled trials in pediatric migraine: Crossover versus parallel group. Curr Pain Headache Rep. 2007;11:241–244. doi: 10.1007/s11916-007-0197-1. [DOI] [PubMed] [Google Scholar]
- e60.Goodenough B, Kampel L, Champion GD, Laubreaux L, Nicholas MK, Ziegler JB, et al. An investigation of the placebo effect and age-related factors in the report of needle pain from venipuncture in children. Pain. 1997;72:383–391. doi: 10.1016/s0304-3959(97)00062-6. [DOI] [PubMed] [Google Scholar]
- e61.Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: A randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128:31–39. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- e62.Tait AR, Voepel-Lewis T, Malviya S. Factors that influence parents’ assessments of the risks and benefits of research involving their children. Pediatrics. 2004;113:727–732. doi: 10.1542/peds.113.4.727. [DOI] [PubMed] [Google Scholar]
- e63.Flor H, Hermann C. Schmerz. In: Flor H, Birbaumer N, Hahlweg K, editors. Grundlagen der Verhaltensmedizin. Göttingen: Hogrefe; 1999. pp. 249–330. [Google Scholar]
- e64.Raquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]