Abstract
Apoptosis is a well-characterized pathway to cell death, yet how it is related to other forms of cell death such as necrosis, and possibly also autophagic cell death has not been entirely clear. Difficulties arise because necrotic cell death is poorly characterized at the molecular level, and also because autophagy is primarily a survival pathway that has been associated with cell death induction in some circumstances. A common theme appears to be now emerging where autophagy promotes survival of apoptosis-defective cells, and inhibition of the autophagy survival function in this setting represents a means to divert cells into a necrotic cell fate. In cells denied the ability to commit suicide by apoptosis, and that are also unable to access the autophagy survival mechanism to sustain homeostasis, necrosis is the default activity. This was most recently illustrated with the discovery that the caspase and apoptosis inhibitor, zVAD, also inhibits a lysosomal protease, and thereby autophagy, and it is this dual inhibition that is responsible for induction of necrotic cell death.1 This radically alters the interpretation of earlier findings reporting induction of autophagic cell death by zVAD,2 instead, suggests that autophagy functions to promote cell survival.
Keywords: autophagy, autophagic cell death, apoptosis, necrosis, mTOR, zVAD, Cathepsin B
Autophagy is a cellular self-catabolic process where cellular components are engulfed and trafficked to lysosomes for proteolytic degradation.3 The main functional role of autophagy is to recycle cellular components to sustain metabolism during nutrient deprivation and to prevent the accumulation of damaged, toxic proteins and organelles during stress. In most situations, autophagy promotes survival to stress and starvation, but there is also evidence that if left unabated or if over stimulated, autophagy can progress to cell death. This is often referred to as autophagic cell death. Although evidence for physiological autophagic cell death in mammals is weak, it does play a role in the developmental degradation of salivary glands in Drosophila morphogenesis.4
There are multiple forms of cell death, and the most well characterized is apoptosis, which is a defined genetic pathway leading to rapid cell execution.5 Cells also die by necrosis, which is less well characterized but results in cell lysis due to physical trauma or metabolic insufficiency.6 Necrotic cell death is a more apparent route when apoptosis is defective, presumably due to limiting the options by which a cell has to die.7 There are reports of cell death with characteristics distinct from apoptosis, necrosis and autopagy, and even hybrid forms of cell death, but the underlying processes are largely unknown.8
Autophagy is also a common stress response and the mere presence of autophagosomes in dying cells is attributed, often erroneously, to autophagic cell death. A commonly utilized apoptosis inhibitor, zVAD, which blocks caspase protease activity required for apoptosis, is reported to induce autophagic cell death in some circumstances.2 Since excessive apoptosis is associated with some degenerative conditions, caspase inhibitors are thought to be of therapeutic value,9 but not so if they instead promote autophagic cell death. Wu and colleagues1 shed some light on this issue with their observation that zVAD inhibits caspases but also the lysosomal protease Cathepsin D. Thus, zVAD inhibits both apoptosis and flux through the autophagy pathway by preventing lysosomal degradation of autophagosome contents. The consequence of zVAD inhibition of both cell death by apoptosis and cell survival by autophagy is induction of necrotic cell death. This refutes a published example of autophagic cell death and supports the more mainstream role of autophagy in promoting cell survival.
Autophagy is a Dynamic Process
When investigating autophagy it is important to consider that it is a dynamic process regulated by autophagosome formation and trafficking to, and fusion with, lysosomes.10 Methods for measuring and quantifying autophagy often rely on monitoring the formation or presence of autophagosomes by surveillance for autophagosome markers (either morphologically and/or biochemically).11 An increase in autophagosomes, however, can result from either an increase of autophagy flux, and/or a blockage of autophagosome maturation (lysosomal fusion and degradation). Thus, autophagy inhibition can result in reduced autophagosome formation, as is the case with down regulation of upstream regulators Beclin 1 or Atg5, or alternatively can result in the massive accumulation of autophagosomes, as is the case with chloroquine or bafilomycin that block autophagy downstream of autophagosome formation at the level of lysosomes. Therefore, it is important to discern and dissect the dynamic process of autophagosome formation, allowing the correct interpretation of autophagy induction or inhibition.11 As the proteolytic inhibitory activity of zVAD encompasses caspases and apparently also a lysosomal protease, the accumulation of autophagosomes induced by zVAD results from inhibition of autophagic flux rather that induction of autophagic cell death.1
Autophagosomes Do Not Necessarily Mean Autophagic Cell Death
More caution and scrutiny is required for the declaration of a role for autophagy in cell death, as the presence of autophagic markers in dying cells are not always suggestive of autophagic cell death. Autophagic cell death should be defined as meeting specific criteria. First, there should indeed be the presence of autophagic features in dying cells. Second, there should be the absence of apoptotic and necrotic hallmarks. Third, induction of autophagy by starvation and rapamycin should be able to enhance cell death. Fourth, suppression of autophagy by multiple, independent means should rescue cell death. This can be accomplished by Atg and Beclin 1 knockdown or preferentially by genetic deletion, and with chemical inhibitors such as 3-MA and chloroquine. While RNAi is a powerful tool, off-target effects need to be ruled out by knocking down multiple targets with both positive and negative controls, and additionally supporting these findings with independent evidence. Fifth, validation of a functional role for autophagy in cell death in vivo and in a physiologically relevant situation should be the penultimate goal wherever possible. We have an amazing arsenal of technology at our fingertips due to sophisticated advances in molecular biology and biochemistry that allow us to decipher molecular mechanisms that regulate biological processes relevant for sustaining health and preventing and curing disease. These same tools also have the potential to generate equally amazing in vitro artifacts that have no physiological basis in vivo. As the field progresses and our understanding of the mechanisms regulating autophagy increases, additional markers and assays will aid in our ability to identify the different steps in the autophagy process and to define the circumstances where autophagy leads to survival or cell death.
Autophagy is a Survival Pathway That Can Inhibit Cell Death by apoptosis or necrosis
There is clear evidence from the phenotypes of mutant mice, and cells derived therefrom, that autophagy functions to sustain cell survival, particularly during stress.7,12–18 It is also clear that there is functional interaction between autophagy and cell death pathways.3,19 In response to metabolic stress, autophagy can delay cell death by apoptosis, and in apoptotic-defective cells, inactivation of the autophagy survival pathway promotes necrotic cell death in vitro and in tumors in vivo.7,13,18 This suggests that defects in apoptosis are overcome by induction of metabolic catastrophe, a death process where cells that cannot die by apoptosis and cannot access the autophagy survival pathway when metabolically stressed are forced to die by necrosis.20 While there are consequences to switching from apoptotic to necrotic cell death, this importantly demonstrates that necrosis can also be a genetically programmed pathway to cell death. This is similar to what is found in zVAD-treated cells where apoptotic caspases and lysosomal cathepsins are inhibited, resulting in blockade of both apoptosis and autophagosome maturation (Fig. 1).1 In support of these observations, the mTOR inhibitor rapamycin protects cells from zVAD-induced death, and inhibition of autophagy pharmacologically (with chloroquine or 3MA), or by siRNA, promotes cell death by zVAD (Fig. 1). Thus a consistent theme is emerging where necrotic cell fate can be dictated when cell death by apoptosis, and cell survival by autophagy, are simultaneously inhibited. These observations are fundamentally important, particularly to therapeutic schemes that involve modulation of apoptosis or autophagy.
Figure 1.
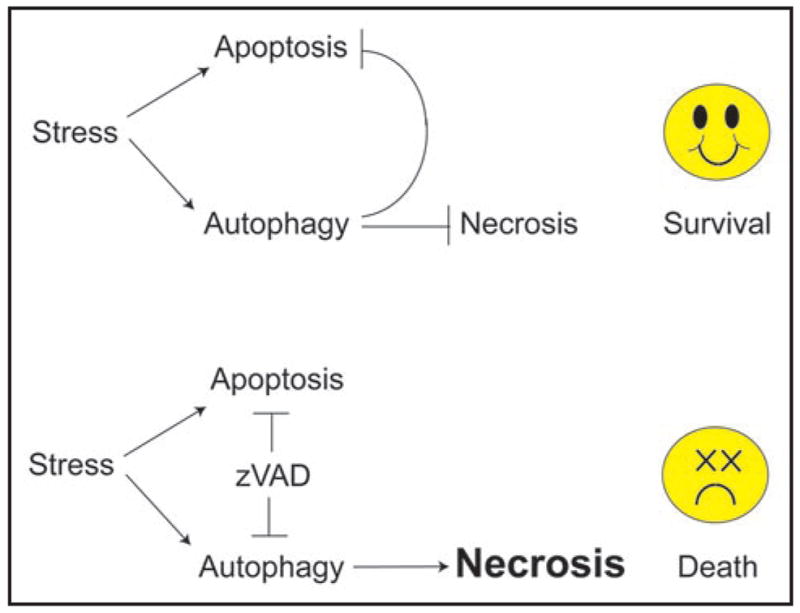
Inhibition of cell death by apoptosis, and concomitant inhibition of survival by autophagy is a formula for induction of necrotic cell death. See text for explanation.
References
- 1.Wu YT, Tan HL, Huang Q, et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy. 2008;4 doi: 10.4161/auto.5662. in press. [DOI] [PubMed] [Google Scholar]
- 2.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 3.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–48. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–96. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 7.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 9.Linton SD. Caspase inhibitors: a pharmaceutical industry perspective. Curr Top Med Chem. 2005;5:1697–717. doi: 10.2174/156802605775009720. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 11.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 13.Karantza-Wadsworth V, Patel S, Kravchuk O, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–35. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 17.Lum JJ, Bauer DE, Kong M, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Mathew R, Kongara S, Beaudoin B, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–7. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–83. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]


