Abstract
Primary open-angle glaucoma is recognized as a disease of aging, and studies show a relationship between aging and trabecular meshwork (TM) cell density. Human TM cell division occurs primarily in the anterior, non-filtering region. A commonly used glaucoma treatment, laser trabeculoplasty (LTP), triggers and increases cell division, as well as cell migration of these anterior TM cells. These freshly-divided migrating cells repopulate the burned laser sites, suggesting that they are stem cells. Several studies concerning this putative TM stem cell will be discussed.
Keywords: trabecular meshwork, glaucoma, stem cells, progenitor cells, regeneration
1. Introduction
Regenerative studies of tissue regrowth originated in the late 17th century with investigations on the sliced polyp, Hydra. Studies with earthworms, snails, and salamanders also produced regrowth, but a specialized cell population that proliferated after injury was not found in adult animals until 1892. This cell population was suspected to be responsible for the regenerative capacities of many invertebrates, and these specialized cells were further identified in freshwater planarians in 1904 (Birnbaum and Sanchez Alvarado, 2008).
Regenerative studies with invertebrates have sparked interest in similar studies to treat loss and dysfunction in vertebrates. Regenerative therapies with similar specialized cells, stem cells, may potentially improve outcomes for damaged and diseased tissues. The therapeutic potential of stem cells is now well-appreciated, and understanding stem cell characteristics and types will speed progress in regenerative medicine. Stem cells can replace differentiated cells that are lost due to insults or injuries, thereby facilitating and advancing innovative disease treatments. They can provide trophic support to maintain homeostasis, and provide a reservoir of replacements throughout an organism’s life (Yamaguchi et al., 1990; Yu and Silva, 2008). Tissue regeneration can occur through transplantation of embryonic or adult stem cell populations (Morrison et al., 1997). Tissues maintain adult stem cells, or induce stem cell potential in differentiated cells. Growth, homeostasis, and repair processes are also affected by stem cells (Jones and Wager, 2008). Even the central nervous system (CNS), long accepted as incapable of regeneration, shows promise for plasticity and regeneration in adult tissue (Ohta et al., 2008).
In regenerative medicine, it will be important to establish criteria for what cell types can be classified as stem cells. The very definition of a stem cell, with the attendant notions of lineage restriction and the permanency of the differentiated cell state, is in a state of flux, and is now being revised (Lovell-Badge, 2001; Seaberg and van der Kooy, 2003). Adult mammalian tissues often possess adult stem cells and progenitor cells. Although there is some overlap, the distinction between the two types of cells has been blurred in recent years, and some suggest that these differences should be more rigorously emphasized instead to test specific hypotheses in stem cell biology (Seaberg and van der Kooy, 2003). Neural stem and progenitor cell types appear to be distinguishable from each other on the basis of Notch pathway signaling, which is thought to maintain stem cell characteristics, and also by functional traits (Hitoshi et al., 2002; Yanagi et al., 2006). These functional traits, found in both stem and progenitor cells, permit self-renewal and differentiation, but stem cells have a higher capacity for self-renewal in vivo and in vitro, and have the ability to give rise to progeny that are committed to differentiation. The progenitor cells, in general, have a more limited capacity for self-renewal, less self-renewal potential in vitro, and do not have the complex cellular interactions of stem cells (Dor and Melton, 2004; Ohta et al., 2008).
2. Stem cell properties
Indefinite self-renewal is a crucial property of stem cells. Both symmetric and asymmetric divisions are observed (Morrison et al., 1997). Symmetric division produces two copies of the parent stem cell, while asymmetric division produces a copy of the parent stem cell, and a daughter cell committed to differentiating along a specific lineage. The parent tissue stem cell is generally found in a specialized area, crypt, or niche (Jahagirdar and Verfaillie, 2005; Yu and Silva, 2008). Symmetric division, which is more common in mammalian systems, allows the stem cell pool to be regulated by factors that control the probability of self-renewing versus differentiation (Morrison et al., 1997). Stem cells typically cycle slowly, being in a mitotically quiescent form most of the time (Hall and Watt, 1989; Potten and Loeffler, 1990; Morrison et al., 1997).
3. Types of stem cells
3.1. Embryonic stem cells
When fertilized eggs and early blastomeres develop into fetuses, they are said to be totipotent; they can give rise to all cell types and to an individual organism (Do et al., 2006). At the blastocyst stage, ESCs, which derive from the inner cell mass, are clearly distinguished (Fulka et al., 2008). The ESCs of the inner cell mass are pluripotent, and can give rise to all cell types of an organism, but not to an entire organism. Cultured ex vivo, ESCs propagate as a homogeneous, uncommitted cell population for an almost unlimited period of time without losing their pluripotency or stable karyotype (Prelle et al., 2002; Yu and Silva, 2008). ESCs have the greatest differentiation plasticity and the most potential for tumor formation (Prelle et al., 2002; Brevini et al., 2008; Dunn, 2008). Human ESC cells were initially established from embryos (Thomson et al., 1998) and early fetuses (Shamblott and Gearhart, 1998; Prelle et al., 2002). However, human ESCs have not currently been used clinically, although efforts have been made to approve their use (Choumerianou et al., 2008).
3.2. Adult stem cells (somatic stem cells)
3.2.1. Possible uses of adult stem cells
Although they are less flexible than ESCs, multipotent stem cells have an unexpected plasticity; they can develop into several cell types (Prelle et al., 2002). Their identification and ex vivo expansion can be difficult, but they have a relatively unlimited capacity for self-renewal (Herrera et al., 2006; Schaffler and Buchler, 2007; Mimeault and Batra, 2008).
There are a variety of multipotent adult stem or progenitor cells for cell replacement strategies and tissue engineering to treat disease in a number of organs and tissues (Mimeault and Batra, 2008). Bone marrow-, umbilical cord-, adipose tissue-, skin- and amniotic fluid-derived mesenchymal stem cells may be acceptable alternatives to ESC in many cases. Bone marrow-derived stem cells have been used with some success in the clinic for bone, cartilage, spinal cord, cardiac and bladder regeneration (Korbling and Estrov, 2003; Bajada et al., 2008). Clinically, phase II and phase III studies for ischemic heart disease are in process (Choumerianou et al., 2008). Neuronal stem cells have great potential in cell-based therapy in brain and spinal cord injuries, and in disease. In other studies of multipotent cells, fetal dopaminergic cells have been investigated for use in Parkinson’s Disease (Freed et al., 2001), mesenchymal cells have been examined for use in osteogenesis imperfecta (LeBlanc et al., 2005), and replacement cells for type I diabetes (Goss et al., 2002) have recently been investigated. Many other studies are in progress.
Tissues often have multipotent stem cells, which can self-renew and generate specialized cell types within the tissue of origin (Prelle et al., 2002). Tissue-specific stem cells differentiate into the cells of that tissue. Heart-specific stem cells, for example, can differentiate into cardiomyocytes, smooth muscle, and vascular endothelial cells. Tissue-specific stem cells are less likely to trigger immune rejection, but isolation, ex vivo expansion, and availability may limit their use (Choumerianou et al., 2008).
3.2.2. Multipotent bone marrow stem cells
Hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) are two subtypes of multipotent bone marrow stem cells. They are attracted from distant sites after injury and aid in tissue repair (Lavker et al., 2004; Mimeault and Batra, 2008). They are self-renewing, inhabit the bone marrow, and may be used for autologous transplantation. MSC transplantation avoids immune rejection and formation of teratomas, with few ethical or political concerns (Kan et al., 2007). Commercial cell culture media facilitate their easy differentiation into chondrogenic, osteogenic, and adipogenic cells ex vivo.
3.2.3. Reprogramming somatic stem cells
Because of the ethical controversies associated with embryonic stem cells, pluripotent cells generated without destroying embryos are attractive, and may avoid immunorejection problems found with ESCs. Pluripotent stem cells may be generated by reprogramming adult somatic or germ cells by several methods, each with its own advantages and disadvantages.
Induced pluripotent stem (iPS) cells have been established by stably transfecting mouse and human fibroblasts with the transcription factors Oct-3/4, Sox2, Klf4, and c-Myc, which are expressed at high levels in ESCs. These transfected cells can differentiate into all three germ layer-derived cells and are syngeneic (Yuasa and Fukada, 2008). They closely resemble ESCs, but the reprogramming efficiency is low (Huangfu et al., 2008). A new method of reprogramming using a histone deacetylase inhibitor, valproic acid, avoids transfection of the oncogenes Klf4 and c-Myc (Yamanaka, 2007; Huangfu et al., 2008; Okabayashi and Asashima, 2008). Human fibroblasts may then be reprogrammed with only two transcription factors, Oct4 and Sox2. This inhibitor thereby facilitates reprogramming efficiency, safety, and practicality. These two factor-induced human iPS cells are similar to human ESCs in pluripotency, global gene expression profiles, and epigenetic states.
Pluripotent cells may also be obtained by transferring a nucleus from a somatic cell into an enucleated egg by Somatic Cell Nuclear Transfer (SCNT). The resulting blastocyst will have the same genetic material as the donor nucleus. This technically demanding procedure produced the first clone of an adult mammal, Dolly the sheep, and has since been repeated for a cat, cow, horse, mule, monkey, and dog (Yamanaka, 2007; Choumerianou et al., 2008).
4. Stem cell niches
Wolf and Trentin noted in the 1960s that stem cells were destined to differentiate upon leaving the bone marrow microenvironment (Wolf and Trentin, 1968). Since then, surface markers for individual stem cells have been identified, and gene knockouts have established essential environmental components for stem cell survival in the bone marrow microenvironment (Powell, 2005). The niche sites that stem cells occupy are composed of other types of cells and extracellular matrix (ECM) (Watt and Hogan, 2000; Spradling et al., 2001; Lin, 2002; Fuchs et al., 2004; Li and Xie, 2005; Scadden, 2006). Other niche cells have roles in secretion and organization of the ECM, and in balancing self-renewal with differentiation (Fuchs et al., 2004). ECM components, like laminin, influence self-renewal and differentiation by directly interacting with the housed stem cells via cell or membrane contacts, or indirectly by growth factors, e.g. BMP-4 (Lovell-Badge, 2001). Once a cell commits to a differentiation pathway, it initiates a journey leading away from the stem cell niche. Injuries can trigger a stem cell to migrate to an environment that initiates differentiation (Jahagirdar and Verfaillie, 2005).
Successful expansion of bone marrow multipotent cells ex vivo for therapeutic use may require specific three-dimensional stem cell niche units (Prelle et al., 2002; Wilson and Trumpp, 2006). For growth of some stem cells, modifications of artificial stem cell niches for expansion may be necessary to provide cells with the proper growth factors and other signals found in vivo in the stem cell niche. Understanding how different individual stem cells interact with their own microenvironments in adult mammalian tissues may be necessary for further exploitation of this potential resource (Lensch et al., 2006).
5. Trabecular meshwork and stem cells
5.1. TM insert cells as replacement cells
Is there an individual stem cell for the trabecular meshwork (TM)? Does it have its own microniche? Can it be used as a replacement cell for age or glaucoma-related reduced TM cellularity? Approximately 30 years ago, Raviola identified a population of unusual cells at the anterior end of the TM, just beneath Schwalbe’s line (SL) (Raviola, 1982). These cells formed a discontinuous cord, oriented circumferentially at the corneal periphery and deep to the corneal endothelial lining of the anterior chamber (Fig. 1). They were different from those of the filtering meshwork, with inclusions that morphologically resembled whorled multi-lamellar bodies of type II alveolar epithelial cells of the lung. However, stem cell characteristics were not attributed to these cells (Raviola, 1982).
Fig. 1.
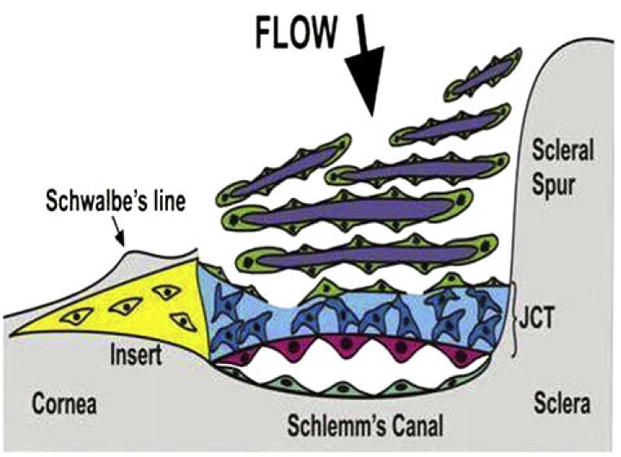
Schematic of position of the TM insert region (in yellow) inferior to Schwalbe’s line and anterior to the juxtacanalicular region of the TM (JCT) and Schlemm’s Canal.
Around this time, decreases in TM cellularity were observed to occur with both age and glaucoma (Alvarado et al., 1981, 1984; Grierson and Howes, 1987; Tschumper et al., 1990; Liton et al., 2005). Similar age-dependent cellular losses in the TM were found in canines with congenital glaucoma (Samuelson et al., 2001). Mechanical stresses, oxidative challenges, or other insults and injuries also may contribute to cell death or dysfunction in TM (Oertel et al., 2000; Gonzalez et al., 2006).
Although time, disease, and various insults decrease TM cellularity, one clinical glaucoma treatment caused increased cellularity. In 1989, a study that subjected human anterior segments in organ culture to laser trabeculoplasty found that TM cell division increased (Acott et al., 1989). Laser-treated explants showed a 4-fold increase in cell division over non-treated controls. More than 60% of the cell division localized to the anterior, non-filtering region of the TM. This region was designated the insert area, reflecting where the TM appears to insert into the cornea beneath Schwalbe’s line, and was suggested to contain “stem-like cells”. Normal, unstimulated cell division was also observed predominantly in this insert region. Sixty percent of tritiated thymidine labeled cells migrated out of the insert region to repopulate laser burn sites within one week. This study suggested that the insert cells serve as a source for TM cell renewal, indicating that they may be stem cells. Rigorous analysis of this possibility remains to be accomplished. Others obtained similar results on TM cell division subsequent to using argon or Nd: YAG lasers in human or monkey eyes, although the insert area in these studies was not examined in detail (Grierson et al., 1983; Alexander and Grierson, 1989; Alexander et al., 1989; Dueker et al., 1990).
More recently, studies showed that a small number of cells escape replicative senescence in primary cultures of HTM cells (Challa et al., 2003). The expression profile of these cells, known as the novel cells, was compared with that of normal human TM cells (HTM), Schlemm’s Canal (SC) cells, or fibroblasts by microarray. This novel cell type showed a different pattern of gene expression, with a high expression of ankyrin G, which is absent in SC cells, and is very weakly expressed in HTM cell cultures. Human milk fat globule-1 protein (HMFG-1 or breast epithelial antigen) was also highly expressed in the novel cell, but not in fibroblasts. Other genes, including chitinase-3-like-1 (also known as YKL-40, cartilage glycoprotein-39, HC-gp39, and CHI3L1), tissue inhibitor of metalloproteinases-3 (TIMP-3), and interstitial collagenase, had lower expression levels in the novel cell than in TM, SC, or fibroblasts, respectively. These gene expression differences further established the novel cell as different from other TM or local cells, and were consistent with differences in morphology, ultrastructure, and growth patterns.
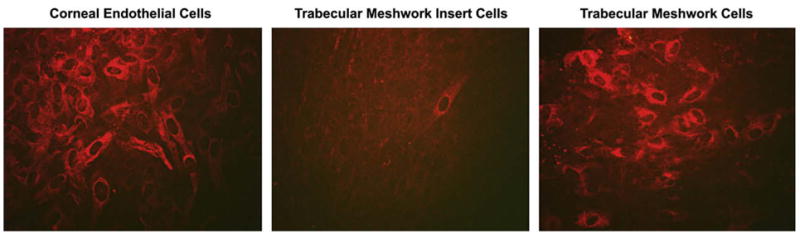
Since that time, our immunohistochemistry observations (Kelley et al., in preparation, Fig. 2), show differences in corneal endothelium, TM insert cells, and TM cells with CHI3L1, reaffirming the above-mentioned microarray studies. HMFG-1 also showed differences between the non-filtering TM insert cells and filtering HTM cells, with a distinctly increased amount of HMFG-1 expression in TM insert cells (Kelley et al., in preparation, Fig. 3). The similarities between the unique cell described by Raviola, the insert cell, and the novel cell strongly suggest that these cells are all the same cell type, which appears to have stem-like properties.
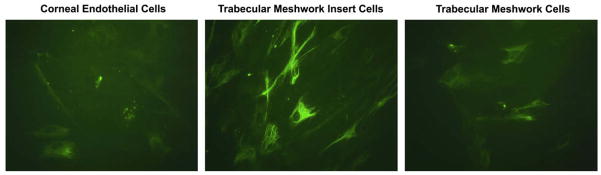
Fig. 2.
YKL-40 immunostaining in cultured mature corneal endothelium, TM insert and TM cells.
Fig. 3.
HMFG-1 immunostaining in cultured mature corneal endothelium, TM insert cells, and TM cells.
5.2. Isolation of TM insert or progenitor cells
Currently, several approaches to isolation of TM adult stem or progenitor cells seem feasible. One method to separate TM insert cells is by surgical dissection of the TM insert area with a high resolution surgical microscope. The insert cells comprise approximately 2–5% of the total TM cell population; hence, this is a difficult surgical technique (Kelley et. al., in preparation).
To isolate undifferentiated TM progenitor cells, production of free-floating neurospheres from HTM cultures may be a suitable method (Chipperfield et al., 2005; Moe et al., 2005; Gonzalez et al., 2006). HTM primary cells cultured in serum-free media on non-adhesive substrates will form spherical clusters of cells, and these may contain multipotent progenitor cells. Microarray analysis of neurospheres showed high expression of two TM markers, matrix Gla protein (MGP) and CHI3L1, which indicated that these free-floating spheres originated from HTM cells. Nestin, a marker for neural precursor cells, and leukemia inhibitory factor, a gene involved in maintenance of undifferentiated progenitor cells, were found in high amounts as well. The undifferentiated cells in the neurospheres cultures appeared morphologically identical to mature TM cells, and the overall gene expression profile of the spheres was similar to those previously reported for TM cell monolayers. These undifferentiated progenitors, however, had a greater resistance to trypsin digestion than normal HTM. Production of these cultured neurospheres may allow collection of possible TM stem/progenitor cells. The neurospheres can be expanded in vitro for 3 months and then stored with cryopreservation in serum-free media supplemented with 10% DMSO (Gonzalez et al., 2006).
To optimize and facilitate procuring TM insert cells, we have initiated molecular marker studies, using microarray to compare mature filtering TM cells with non-filtering TM insert cells. These studies (Kelley et al., in preparation) are in progress and have found some differences between TM insert cells and mature TM cells, as did previous microarray studies reported in the novel cell type and in neurospheres (Challa et al., 2003; Gonzalez et al., 2006). We are also using TM markers established by others searching for a corneal and limbal stem cell population (Whikehart et al., 2005). Their studies showed that DNA replication, nestin, alkaline phosphatase, and telomerase were all found in both the TM and TM insert region (McGowan et al., 2007). Telomerase, an enzyme involved in maintaining telomere lengths, is a marker of transient amplifying cells and stem/progenitor cells (Ulaner and Giudice, 1997; Amit et al., 2000). After wounding the cornea, additional markers were found in the TM and TM insert region, including two potential markers for stem cells, Oct-3/4 and Wnt-1, and two differentiation markers, Pax-6 and Sox2 (McGowan et al., 2007).
6. Summary
Reprogramming of adult somatic or germ cells to become induced pluripotent cells or somatic nuclear transfer cells resolves immunorejection problems found with ESCs. Generation of induced pluripotent status may side-step some of the ethical controversies found with ESCs, but may not satisfy all critics. Although these pluripotent cells may be patient-specific, the overall level of safety with these therapies, fusion with cells other than ESCs, and other factors must be determined for reprogramming methods to be used clinically. In the trabecular meshwork, the putative stem cells investigated as the Schwalbe’s Line cell, as the novel non-senescent cell type, or as the TM insert cell, may well be the same, undifferentiated cell type. This putative adult stem/progenitor cell might be expanded ex vivo to replace missing or nonfunctional TM cells in glaucomatous patients, thereby preventing vision loss, and alleviating the need for lasers, drugs, and compliance. Additionally, to devise safer, more reliable therapies to trigger division and migration of these cells to places of cellular loss in the TM would be very valuable. In substituting for repetitive and expensive laser treatments, stem cell applications could hopefully provide a lasting solution for glaucomatous patients.
Acknowledgments
The authors thank Genevieve Long, Ph.D., for editorial assistance. Support was provided by NIH EY003279, EY008247, EY010572, Research to Prevent Blindness, Prevent Blindness America, and the Gustavus and Louise Pfeiffer Research Foundation.
References
- Acott TS, Samples JR, et al. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am J Ophthalmol. 1989;107:1–6. doi: 10.1016/0002-9394(89)90805-2. [DOI] [PubMed] [Google Scholar]
- Alexander RA, Grierson I. Morphological effects of argon laser trabeculoplasty upon the glaucomatous human meshwork. Eye. 1989;3 (Pt 6):719–726. doi: 10.1038/eye.1989.111. [DOI] [PubMed] [Google Scholar]
- Alexander RA, Grierson I, et al. The effect of argon laser trabeculoplasty upon the normal human trabecular meshwork. Graefes Arch Clin Exp Ophthalmol. 1989;227:72–77. doi: 10.1007/BF02169830. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, et al. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, et al. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727. [PubMed] [Google Scholar]
- Amit M, Carpenter MK, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Bajada S, Mazakova I, et al. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2:169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- Birnbaum KD, Sanchez Alvarado A. Slicing across kingdoms: regeneration in plants and animals. Cell. 2008;132:697–710. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevini TA, Pennarossa G, et al. Parthenogenesis as an approach to pluripotency: advantages and limitations involved. Stem Cell Rev. 2008;4:127–135. doi: 10.1007/s12015-008-9027-z. [DOI] [PubMed] [Google Scholar]
- Challa P, Gonzalez P, Liton PB, Caballero M, Epstein DL. Gene expression profile in a novel cell type in primary cultures of human trabecular meshwork. Invest Ophthalmol Vis Sci. 2003;44(Suppl) [Google Scholar]
- Chipperfield H, Cool SM, et al. Adult CNS explants as a source of neural progenitors. Brain Res Brain Res Protoc. 2005;14:146–153. doi: 10.1016/j.brainresprot.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Choumerianou DM, Dimitriou H, et al. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008;14:53–60. doi: 10.1089/teb.2007.0216. [DOI] [PubMed] [Google Scholar]
- Do JT, Han DW, et al. Reprogramming somatic gene activity by fusion with pluripotent cells. Stem Cell Rev. 2006;2:257–264. doi: 10.1007/BF02698052. [DOI] [PubMed] [Google Scholar]
- Dor Y, Melton DA. How important are adult stem cells for tissue maintenance? Cell Cycle. 2004;3:1104–1106. [PubMed] [Google Scholar]
- Dueker DK, Norberg M, et al. Stimulation of cell division by argon and Nd-YAG laser trabeculoplasty in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1990;31:115–124. [PubMed] [Google Scholar]
- Dunn JC. Tissue engineering and regenerative science in pediatrics. Pediatr Res. 2008;63:459–460. doi: 10.1203/PDR.0b013e3181739fbe. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, et al. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Fulka H, St John JC, et al. Chromatin in early mammalian embryos: achieving the pluripotent state. Differentiation. 2008;76:3–14. doi: 10.1111/j.1432-0436.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Epstein DL, et al. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp Eye Res. 2006;82:959–967. doi: 10.1016/j.exer.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss JA, Schock AP, et al. Achievement of insulin independence in three consecutive type-1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation. 2002;74:1761–1766. doi: 10.1097/00007890-200212270-00020. [DOI] [PubMed] [Google Scholar]
- Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye. 1987;1 (Pt 2):204–210. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- Grierson I, Marshall J, et al. Human trabecular meshwork in primary culture: a morphologic and autoradiographic study. Exp Eye Res. 1983;37:349–365. doi: 10.1016/0014-4835(83)90172-0. [DOI] [PubMed] [Google Scholar]
- Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bruno S, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cell. 2006;24:2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Osafune K, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- Jahagirdar BN, Verfaillie CM. Multipotent adult progenitor cell and stem cell plasticity. Stem Cell Rev. 2005;1:53–59. doi: 10.1385/SCR:1:1:053. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wager AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kan I, Melamed E, et al. Autotransplantation of bone marrow-derived stem cells as a therapy for neurodegenerative diseases. Handb Exp Pharmacol. 2007:219–242. doi: 10.1007/978-3-540-68976-8_10. [DOI] [PubMed] [Google Scholar]
- Kelley MJ, Ryan EP, et al. Trabecular Meshwork Stem Cell Characterization. IOVS. 2009 in preparation. [Google Scholar]
- Korbling M, Estrov Z. Adult stem cells for tissue repair – a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Tseng SC, et al. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- LeBlanc K, Gotherstrom C, et al. Fetal mesenchymal stem cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- Lensch MW, Daheron L, et al. Pluripotent stem cells and their niches. Stem Cell Rev. 2006;2:185–201. doi: 10.1007/s12015-006-0047-2. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- Liton PB, Challa P, et al. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell-Badge R. The future for stem cell research. Nature. 2001;414:88–91. doi: 10.1038/35102150. [DOI] [PubMed] [Google Scholar]
- McGowan SL, Edelhauser HF, et al. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007;13:1984–2000. [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe MC, Varghese M, et al. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 2005;128:2189–2199. doi: 10.1093/brain/awh574. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, et al. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Oertel MF, May CA, et al. Alpha-B-crystallin expression in tissues derived from different species in different age groups. Ophthalmologica. 2000;214:13–23. doi: 10.1159/000027469. [DOI] [PubMed] [Google Scholar]
- Ohta K, Ito A, et al. Neuronal stem/progenitor cells in the vertebrate eye. Dev Growth Differ. 2008;50:253–259. doi: 10.1111/j.1440-169X.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- Okabayashi K, Asashima M. Regenerative medicine: history and perspectives. Nippon Rinsho. 2008;66:825–830. [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Powell K. Stem-cell niches: it’s the ecology, stupid! Nature. 2005;435:268–270. doi: 10.1038/435268a. [DOI] [PubMed] [Google Scholar]
- Prelle K, Zink N, et al. Pluripotent stem cells–model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002;31:169–186. doi: 10.1046/j.1439-0264.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- Raviola G. Schwalbe line’s cells: a new cell type in the trabecular meshwork of Macaca mulatta. Invest Ophthalmol Vis Sci. 1982;22:45–56. [PubMed] [Google Scholar]
- Samuelson D, Plummer C, et al. Schwalbe line’s cell in the normal and glaucomatous dog. Vet Ophthalmol. 2001;4:47–53. doi: 10.1046/j.1463-5224.2001.00142.x. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based therapies. Stem Cell. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Gearhart J. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, et al. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tschumper RC, Johnson DH, et al. Glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Curr Eye Res. 1990;9:363–369. doi: 10.3109/02713689008999624. [DOI] [PubMed] [Google Scholar]
- Ulaner GA, Giudice LC. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod. 1997;3:769–773. doi: 10.1093/molehr/3.9.769. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Whikehart DR, Parikh CH, et al. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816–824. [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Wolf NS, Trentin JJ. Hemopoietic colony studies.V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968;127:205–214. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, et al. Negative regulation of transforming growth factor-b by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Yanagi Y, Inoue Y, et al. Properties of growth and molecular profiles of rat progenitor cells from ciliary epithelium. Exp Eye Res. 2006;82:471–478. doi: 10.1016/j.exer.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Yu D, Silva GA. Stem cell sources and therapeutic approaches for central nervous system and neural retinal disorders. Neurosurg Focus. 2008;24:E11. doi: 10.3171/FOC/2008/24/3-4/E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa S, Fukada K. Recent advances in cardiovascular regenerative medicine: the induced pluripotent stem cell era. Expert Rev Cardiovascul Ther. 2008;6:803–810. doi: 10.1586/14779072.6.6.803. [DOI] [PubMed] [Google Scholar]