Abstract
Introduction
Peri- or postpartum cardiomyopathy (PPCM) is a rare, life-threatening heart disease of unclear origin and is characterized by heart failure of sudden onset between the final weeks of pregnancy and 6 months after delivery.
Methods
Selective literature search in the databases of the National Center for Biotechnology Information based on the key words "peri- and postpartum cardiomyopathy," "pregnancy“ and "heart failure" and additional information from the authors’ personal experience.
Results
PPCM is often not diagnosed until late in its course, because its clinical manifestations are highly variable and a heart disease may not be suspected at first. Frequent presenting symptoms of PPCM, such as prostration, shortness of breath on mild exertion, and coughing, are often initially misinterpreted as evidence of pneumonia or as physiological accompaniments of pregnancy and delivery. The clinical picture of PPCM corresponds to a dilated cardiomyopathy (DCM) with signs of severe heart failure. Therefore, treatment with ACE inhibitors, diuretics, aldosterone antagonists, and beta-blockers is required. Recent research findings suggest a possible new approach to the treatment of PPCM with bromocriptine, which inhibits the release of prolactin, a lactation-promoting hormone. To date, only the treatment of heart failure in PPCM is evidence-based, while all other treatments are "level C," i.e., based on expert opinion only.
Conclusion
The early diagnosis and interdisciplinary management of PPCM can often lead to substantial recovery from heart failure and cardiomyopathy.
Keywords: postpartum disorder, cardiomyopathy, heart failure, pregnancy, diagnosis
Peripartum or postpartum cardiomyopathy (PPCM) is a serious disease of poorly understood etiology. It is characterized by rapid onset heart failure during the final weeks of pregnancy or up to 6 months postpartum. The clinical picture of PPCM has the appearance of a dilated cardiomyopathy (DCM), but differs from other forms of DCM in its rapid development. Indeed apparently healthy women can develop sufficiently severe cardiac failure to require heart transplantation. Around 80% of symptomatic patients recover, although fewer than 30% achieve complete recovery with normalization of left ventricular function and chamber size (8).
A central element in the diagnosis of PPCM is a rapid onset of systolic dysfunction (left ventricular ejection fraction of less than 45%) with left ventricular enlargement. The phenotype of a dilated cardiomyopathy develops in close proximity to child birth (last month of pregnancy up to 6 months postpartum, figures 1a, 2a and b). In the authors’ clinical experience, the first symptoms are often dyspnea, cough, leg edema and generalized lassitude, sometimes accompanied by peripheral arterial thromboembolism (box). Restricted left ventricular pump function may be associated with severe mitral regurgitation secondary to left ventricular dilatation. The physiological adaptations to pregnancy and birth are also associated with prothrombotic tendency (4), meaning that the risk of left ventricular thrombus formation and peripheral arterial emboli is increased in PPCM patients with an ejection fraction of less than 35%. The risk of cardiac arrhythmias and sudden cardiac death is also increased in women with PPCM (8). ECG and chest radiography are of secondary importance due to their poor specificity and limited diagnostic use (12). NT-proBNP (N-terminal pro-Brain Natriuretic Peptide) as a typical marker for severe heart failure is usually markedly raised in PPCM patients.
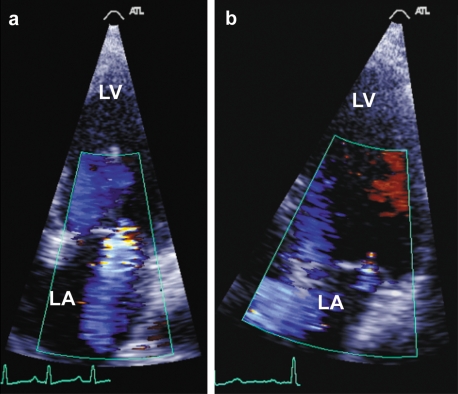
Figure 1.
Echocardiogram in a patient with
a) severe mitral regurgitation with acute PPCM 3 weeks post cesarean section, and
b) normalization of the appearances 5 months after treatment with bromocriptine.
The ejection fraction on the echocardiogram measured 17% in the acute phase and 57% after 5 months. Further findings for this patient are shown in (6).
LV, left ventricle; LA, left atrium.
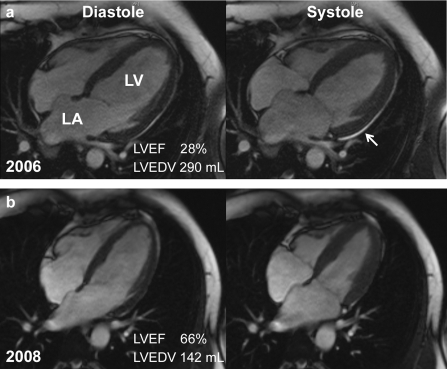
Figure 2.
Cardiac magnetic resonance imaging (MRI) of the same PPCM patient
a) In the acute phase (diastole and systole) markedly dilated left ventricle with severely compromised function, dilated left atrium and small pericardial effusion (arrow: The atrial septum is distorted in the acute phase which is accompanied by severe mitral regurgitation and raised left atrial pressure in right atrial filling)
b) during the course of bromocriptine treatment, left ventricular size and mass (diastole and systole) reduced markedly and systolic function improved.
LV, left ventricle; LA, left atrium; LVEF, left ventricular ejection fraction (normal range in MRI: 67 ± 5 %); LVEDV, left ventricular end diastolic volume (normal range in MRI for women: 96 ± 23 mL)
In addition, weight loss of >20 kg during treatment is detectable in the reduced thoracic wall thickness; further MRI findings for this patient are shown in (6)
Box. Symptoms and signs which, in the postpartum period, suggest PPCM.
Lassitude and exhaustion
Dyspnea
Paroxysmal nocturnal dyspnea
Wet crepitations over the lung fields
Shadowing on the chest X-ray
Leg edema
Nocturia
Palpitations or missed beats
Newly arising repolarization abnormalities on the ECG
Arrhythmias on the ECG
Systolic murmur
Impaired left ventricular function
New-onset secondary mitral regurgitation
Arterial emboli
Cerebral emboli
Our research on the subject of PPCM involved a search of the databases of the National Center for Biotechnology Information (NCBI) using the search terms "peri- and postpartum cardiomyopathy", "pregnancy" and "heart failure" with no time restriction. Since no randomized controlled trials exist to date, the authors own clinical experience has been included.
Epidemiology and risk factors
The incidence of PPCM is quoted as 1 : 3500 to 1 : 1400 for the USA and Europe, 1 : 1000 for South Africa and 1 in 299 for Haiti (8). Since the course of the illness is similar in all cases, it is assumed that the same disease is being described across the different regions (8).
A higher incidence is noted in black African women. This, together with the high incidence among the black population of Haiti and Africa, point to possible genetic factors which increase the risk of PPCM, at least in these regions (8). Additional potential risk factors include preeclampsia, hypertension, tocolytic medication, smoking, multiple pregnancy, teenage pregnancy, and pregnancy in older women. Nonetheless a quarter to a third of all PPCM patients are young, apparently healthy primigravida or primiparous women (8).
Assuming an incidence of 1 : 3500 to 1 : 1400 births would yield an expected incidence of up to 300 patients per year in Germany, with severe, critical cardiac failure in around 30. However, in the year 2007 alone 17 cases of newly diagnosed PPCM were reported to our center alone, which suggests that the true incidence is higher.
There are no prospective studies of PPCM to date, and there is not statistical documentation of the disease in Germany. A systematic analysis of the incidence and potential risk factors and prognostic markers could lead to improved interdisciplinary communication and a higher level of awareness of this clinical condition. The goal must be to identify PPCM across the board, and offer them optimal treatment.
This applies equally to cardiologists, gynecologists, respiratory physicians, nephrologists and primary care physicians, any of whom may be the first point of presentation for these women.
Long term prognosis
A study of 100 patients from South Africa reported a 15% mortality rate for PPCM. In 23% left ventricular (LV) function returned to normal after 6 months (8, 9). A study from Haiti also quoted a mortality rate of 15% and reported eventual normalization in 31% of PPCM patients (3, 8). A recently published study reports on 100 patients, of whom 67% were white Americans with an initial left ventricular ejection fraction of 29 ± 11%. In 54% LV function improved, and the maternal mortality was 9% (2). These data, which are summarized in the Lancet (8), show that despite optimal heart failure treatment, no clinical improvement in pump function was observed in 30% to 40% of PPCM patients, and terminal cardiac failure supervened in 9% to 23%.
Diagnosis of PPCM
The diagnosis of PPCM is often made late. This results from the very variable clinical presentations of affected women, and from the fact that potential cardiac causes are overlooked in previously healthy young women. In the authors’ experience, the first symptoms are often dyspnea and cough, which are often interpreted as signs of pneumonia or as a physiological consequence of pregnancy and birth. Significantly increased shadowing is commonly seen on chest X-ray, which is interpreted as an infiltrate. Often further investigation is deferred until one or more antibiotics have proved ineffective. Other symptoms of PPCM such as leg edema, possible subjective cardiac arrhythmias, or even stroke or peripheral emboli are not associated with PPCM but are thought to be consequences of the postpartum readjustment phase.
In the authors’ experience the delay in reaching a correct diagnosis ranged from weeks to months in around 30% of cases, which agrees with the findings of other authors (5, 6, 8, 12). Misinterpretation of the clinical picture and delayed diagnosis and treatment of heart failure can have detrimental consequences, and observational data suggest that potential specific treatments are only effective if started early (5, 6, 8, 12).
Who makes the diagnosis of PPCM?
Patients with PPCM most commonly present to gynecologists or primary care physicians. Where pneumonia is suspected a referral to a respiratory physician is often made. It would be desirable, however, for patients presenting postpartum with signs of cardiac failure such as shortness of breath, edema or general lassitude, or with peripheral emboli or cardiac arrhythmias, to receive an urgent echocardiogram to exclude PPCM. By way of illustration a few cases are outlined as follows:
A 33-year-old, previously fit woman collapses at home two weeks after an uncomplicated cesarean section. She complains of an inability to lie flat since the delivery due to shortness of breath, and has attributed this to allergy.
A 40-year-old woman requires resuscitation during a cesarean section and requires intensive care. Her cardiac function is severely compromised.
A 25-year-old patient complains of exhaustion and shortness of breath 6 weeks after giving birth. Chest X-ray shows shadowing but no inflammatory markers are found on blood testing.
A woman of black African origin consults her primary care physician one week postpartum because of a flu-like infection. Virology is positive, and the ECG suspicious, leading to the suspicion of myocarditis. However, the myocardial biopsy is inconclusive.
In all four cases, a history, examination and echocardiography lead ultimately to the diagnosis of PPCM.
Treatment of PPCM
The clinical course of PPCM resembles that of a dilated cardiomyopathy with the typical signs of severe cardiac failure. Treatment for cardiac failure is therefore indicated, in accordance with the German Cardiological Society’s guidelines with ACE inhibitors, diuretics, aldosterone antagonists and, where the patient is hemodynamically stable, with beta-blockers. These can be used because the patient is no longer pregnant and because PPCM patients should not breastfeed.
It has been suggested that immunological processes may play a role in the pathophysiology of PPCM. A small, nonrandomized pilot study suggests that immunoglobulin treatment may be effective in PPCM (1). Another pilot study suggested that the serum level of the pro-inflammatory cytokine tumor necrosis factor (TNF) is raised, and that treatment with pentoxifyllin, an inhibitor of TNF production, may have a beneficial effect on recovery from PPCM (11).
Recent research suggests a possible new approach to the treatment of PPCM. Based on experimental studies on transgenic mice lacking the transcription factor STAT3 in cardiac muscle, the authors were able to demonstrate that a lack of antioxidant enzymes such as manganese sodium dismutase (MnSOD) leads to increased free oxygen radical production in the heart postpartum. This leads to higher oxidative stress, and in turn to a fatal cleavage of prolactin into a proapoptotic and antiangiogenetic 16-kDa subform. Prolactin ist a hormone which is produced in the anterior pituitary gland, predominantly in pregnancy and during breastfeeding. It is released cyclically in large quantities, and leads to the growth of the ductal system of the breast, milk production, and the involution of the uterus after birth. Prolactin can be cleaved into 16-kDa prolactin form, which has been associated with PPCM. The 16-kDa prolactin destroys the endothelium and damages in particular the microcirculation in the myocardium, reducing the metabolic activity of cardiac muscle cells (figure 3). This leads to a significantly reduced pump function, and to the clinical manifestations of PPCM in the mouse model (5). Investigations have therefore focussed on whether the pharmacological inhibition of prolactin secretion via the dopamine D2 receptor agonist bromocriptine prevents the development of PPCM in the animal model. This was clearly the case. Hence bromocriptine, a medication which has been used for many years to inhibit lactation, can prevent PPCM in transgenic mice (figure 3) (5).
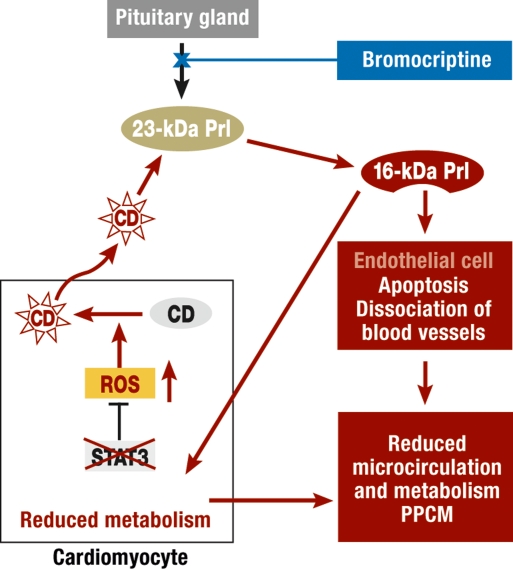
Figure 3.
Schematic representation of prolactin (Prl) release (23-kDa Prl) from the pituitary gland and prolactin cleavage in the pathological situation of PPCM in STAT3 KO mice. The lack of STAT3 in cardiac muscle leads to a reduction in synthesis of the antioxidative enzyme MnSOD, and a corresponding increase in free oxygen radicals (ROS). This causes a release of the protease cathepsin D from lysosomes (CD, black: lysosomal inactive form, red: active form released from lysosomes), which cleaves normal prolactin (23-kDa prolactin) into the biologically active, anti- angiogenic and pro-apoptotic 16 kDa sized prolactin fragment (16-kDa Prl). 16-kDa prolactin destroys arterioles and capillaries in the myocardium, acts as a vasoconstrictor, and reduces myocardial metabolism and cardiomyocytic contractility (5)
There is evidence from the blood of patients with acute PPCM that the pathological mechanism described above may be relevant here. Hence the serum levels of oxidized low-density lipoprotein (oxLDL), as indicator of oxidative stress, and activity of the prolactin cleaving enzyme cathepsin D, were both raised relative to healthy breastfeeding women (5). Raised levels of 16-kDa prolactin were also found in 3 of 5 sera from PPCM patients, but in no healthy women (5). Based on the animal studies and parallels in these serological analyses, the authors are investigating in a nonrandomized pilot study whether bromocriptine beneficially affects the clinical course of recurrent PPCM in women undergoing a subsequent pregnancy following PPCM. This is important because these women have a high risk of persistent disease with poor prognosis (5, 6). This approach matches the preventive strategy employed in the animal studies.
This study was conducted in South Africa because the incidence of PPCM is high there. In Germany, where PPCM is rare, there are almost no patients who become pregnant again against medical advice. All 12 patients in the pilot study received standard heart failure treatments, 6 patients received bromocriptine treatment in addition. Prolactin levels fell, as expected, in the bromocriptine group, whereas in the group who breastfed, the prolactin level remained high, and the cleavage of prolactin into toxic 16-kDa prolactin remained possible.
Three patients (50%) died within 4 months of terminal heart failure in the non-bromocriptine group. In the 3 surviving patients, recurrent left ventricular dysfunction arose after 3 months. None of the bromocriptine-treated group died, and cardiac function improved in all cases (table) (5). Hence the administration of bromocriptine in patients with a history of PPCM was able to prevent recurrence in a subsequent pregnancy. No serious side effects were observed, and none would be expected in the dosages used, based on existing data for bromocriptine (5–7, 13). This South African pilot study shows that bromocriptine can prevent the development of PPCM in women at high risk. However, the more important question in terms of the number of patients is whether bromocriptine is beneficial in patients with acute PPCM. In Germany we have data for 6 patients who have undergone a trial of treatment with bromocriptine, who were suffering from acute PPCM with signs of severe heart failure (NYHA III to IV, New York Heart Association–classification III to IV: symptoms on minimal exertion or at rest) and severely restricted pump function (left ventricular ejection fraction [EF] of between 12% and 30%).
Table. Results of the pilot study with bromocriptine (nonrandomized, nonmasked): All 12 patients received standard heart failure treatment postpartum (ACE inhibitors and a beta-blocker), 6 patients additionally received bromocriptine.
| With bromocriptine | Without bromocriptine | ||||
| Total patients | n = 12 | n = 6 | n = 6 | ||
| Other medications: | Beta-bockers | Yes | 100% | Yes | 100% |
| ACE-inhibitors | Yes | 100% | Yes | 100% | |
| Diuretics | Yes | 100% | Yes | 100% | |
| Deaths | Bromocriptine | Yes | 100% | No | 100% |
| 0 | 0% | 3 | 50% | ||
| *Heart failure (NYHA) | 1 month prepartum | 1.8 ± 0.9 | 1.4 ± 0.5 | ||
| Heart failure (NYHA) | 3 months postpartum | 1.0 ± 0 | 2.3 ± 0.6 | ||
These data are quoted from the "supplemental data" cited from Hilfiker-Kleiner et al. Cell 2007 (5);
*Heart failure grade 1 corresponds to NYHA I (normal); NYHA 4 corresponds to NYHA IV (severest heart failure)
All 6 patients showed significant echocardiographic improvements in pump function over a 6-month period (increase in EF from 15% to 44%, median basal EF 24%, median 6-month EF 51%, n = 6). The echocardiographic and MRI findings for one of these patients are shown in figures 1 and 2. Further clinical details of two of these cases have already been published (6).
Conclusion
PPCM is a potentially life-threatening illness which usually arises shortly after delivery. Early diagnosis is prognostically important in these young women. Hence the possibility of PPCM should be kept in mind by the physician as a possible complication of the postpartum period, wherever there are symptoms of heart failure, but also in the face of nonspecific complaints. If the diagnosis is made early, there can be hope of improvement of cardiac function and normalization of ventricular size via specific treatment with bromocriptine.
PPCM should particularly be considered wherever the symptoms listed in the box are present. Women with these symptoms in the postpartum period (and up to 6 months postpartum) should receive urgent cardiological review including echocardiography. An ECG is desirable but it should be noted that a normal ECG does not exclude PPCM and is therefore not suited for diagnosis. In patients with severe heart failure at the NYHA stage III to IV level (symptoms on mild exertion or at rest) treatment should be undertaken in a larger center with facilities for mechanical support and if necessary cardiac transplantation.
Bromocriptine is licensed for the postpartum suppression of lactation, but not currently for the treatment of PPCM. The Hannover Medical School (MHH) is currently planning a randomized study to test the efficacy of bromocriptine as a new treatment agent for acute PPCM. Since PPCM is a rare disorder, it would be desirable to include as many patients as possible in the study. This represents an opportunity to test the efficacy of bromocriptine as treatment for a rare but life-threatening condition, to reinforce initial, highly promising results, and to establish a treatment based on the underlying pathology.
Key messages.
Postpartum cardiomyopathy (PPCM) is a rare but potentially life-threatening illness.
The incidence of this condition may be higher than hitherto assumed. The collection of national data would benefit patients and would increase awareness of PPCM.
PPCM is characterized by the nonspecific symptoms of heart failure occurring in the first few days and up to 6 months postpartum.
Early diagnosis and onset of treatment can be key in prognosis.
Classical heart failure treatment including ACE inhibitors, diuretics, aldosterone antagonists and, where the patient is hemodynamically stable, beta-blockers, is indicated. Breastfeeding should be discontinued prior to this. Bromocriptine is not only a useful treatment to ease the cessation of breastfeeding, but early observations suggest that its prolonged use has beneficial effects on the clinical course of PPCM.
Acknowledgments
We would like to thank the Leducq Foundation for supporting the PPCM Project.
Translated from the original German by Dr. Sandra Goldbeck-Wood.
Footnotes
Further information on PPCM can be found on the following German-language website: www99.mh-hannover.de/kliniken/kardiologie/index.html
Questions and information can be sent to: Schridde.Isolde@mh-hannover.de or Luehrssen.Karin@mh-hannover.de
Conflict of interest statement
The authors declare no conflict of interest in the terms of the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Bozkurt B, Villaneuva FS, Holubkov R, et al. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol. 1999;34:177–180. doi: 10.1016/s0735-1097(99)00161-8. [DOI] [PubMed] [Google Scholar]
- 2.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, Shotan A. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 3.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–1606. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M. Haemostasis and pregnancy. Thromb Haemost. 2006;95:401–413. doi: 10.1160/TH05-11-0753. [DOI] [PubMed] [Google Scholar]
- 5.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Hilfiker-Kleiner D, Meyer GP, Schieffer E, et al. Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol. 2007;50:2354–2355. doi: 10.1016/j.jacc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Imran SA, Ur E, Clarke DB. Managing prolactin-secreting adenomas during pregnancy. Canadian Family Physician. 2007;53:653–658. [PMC free article] [PubMed] [Google Scholar]
- 8.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–693. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 9.Sliwa K, Forster O, Libhaber E, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–446. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 10.Sliwa K, Norton GR, Kone N, et al. Impact of initiating carvedilol before angiotensin-converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure. J Am Coll Cardiol. 2004;44:1825–1830. doi: 10.1016/j.jacc.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 11.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2002;4:305–309. doi: 10.1016/s1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 12.Stepan H, Walther T, Pfeiffer D. Peripartum cardiomyopathy - the (un)known obstetrical cardiologic emergency situation. Z Kardiol. 2003;92:811–816. doi: 10.1007/s00392-003-0981-9. [DOI] [PubMed] [Google Scholar]
- 13.Witlin AG, Mattar F, Sibai BM. Postpartum stroke: a twenty-year experience. Am J Obstet Gynecol. 2000;183:83–88. doi: 10.1067/mob.2000.105427. [DOI] [PubMed] [Google Scholar]