Abstract
Background
Between August and December 2007, a mass poisoning due to adulterated marijuana was uncovered in the area of Leipzig, Germany.
Methods
Retrospective reports of patients with lead poisoning who were treated at Leipzig University Hospital. Analysis of data from the local health office, where marijuana consumers could have their blood lead concentration determined.
Results
At Leipzig University Hospital, 35 patients (7 female; age 24.2 ± 4.4 years) had to be treated for lead poisoning (blood lead levels 1063.3 ± 864.0 µg/L). Five hundred ninety-seven marijuana consumers (439 men, 158 women; age 26.9 ± 4.8 years) had their blood lead levels measured at the local health office. Among them, 27.3% had lead levels above the HBM-II threshold, 12.2% had concentrations that required monitoring, and 60.5% had levels below the HBM-I threshold.
Conclusion
Drug consumption should be considered in otherwise unexplained anemia and abdominal colic. Several hundred people suffered lead poisoning presumably resulting from the desire of drug dealers to maximize profits.
Keywords: lead poisoning, anemia, colic, drug abuse, marijuana
Acute and chronic lead poisoning is a rare diagnosis in today’s clinical practice (1, e1). The reasons include the strict legal regulations on chemicals, the environment, and the workplace, and particularly the fact that all old lead pipes for domestic water have been replaced.
Common sources of lead intoxication these days include lead-based paints in old houses, uncontrolled recycling procedures, car batteries, cosmetics, or toys (1–3). In the new German states, lead intoxications due to antiquated lead pipes were seen before reunification. Pathobiographical analysis of brilliant artists such as van Gogh, Goya, or Rembrandt give rise to the suspicion that their illnesses—such as for example Goya’s deafness—were caused by high concentrations of lead in their paints (e2, e3). It is also possible that Beethoven’s nemesis was his fondness for sweet wines—which had often been "refined" with lead acetate (e4).
In Leipzig, a series of lead intoxications occurred at the end of 2007 that were due to a hitherto unknown source of exposure: marijuana that had been adulterated with lead. Several people had to be treated for acute lead intoxication in the hospitals around the Leipzig area (4). The state attorney is bringing a case for dangerous bodily harm against persons unknown, and the European police authority Europol was consulted.
The authors report about the patients who were treated at Leipzig University Hospital and about the results of a screening program initiated by the local health office, in whose context marijuana consumers were able to have the lead concentrations in their blood measured.
Methods
The authors analyzed retrospectively the data of the patients with lead poisoning who had been treated at Leipzig University Hospital and data from the health office. They performed a literature search in Medline, using the search terms "lead and poisoning," "intoxication," "therapy," "exposure," cannabis," "marijuana," "drugs."
Results
Data from Leipzig University Hospital
From August 2007 to February 2008, 35 patients (including 7 women) were treated for lead poisoning at Leipzig University Hospital. All patients were marijuana users. Five patients additionally reported using alcohol or further drugs, such as cocaine, amphetamines, or heroin. All patients were young adults or teenagers (24.2 ± 4.4 years, range 16–33 years). The patients who received treatment had blood lead concentrations of 175–4570 µg/L (mean 1063.3 ± 864.0 µg/L, reference value <90 µg/L for men and <70 µg/L for women). Atomic absorption spectrometry was used, and elementary lead was found in one specimen. The average number of joints consumed per week in the cohort was 7. The blood lead concentrations did not correlate with the amount consumed. This is presumably due particularly to the different degrees of contamination of the marijuana. It was not possible to determine the lead concentration per gram of marijuana as purchasing the drug is a punishable offence.
Almost half the patients presented initially to the accident and emergency department. The following symptoms of lead intoxication were noted:
Acute colic (n = 19)
Hypochromic anemia and basophilic stippling (n = 23; four patients required transfusions)
Lead seam along the dental margin (n = 11)
Peripheral neuropathy (n = 4)
Encephalopathy ranging from headache to somnolence (n = 8)
Nausea and vomiting (n = 20)
Chronic fatigue and exhaustion (n = 35)
Loss of appetite and weight loss (n = 17)
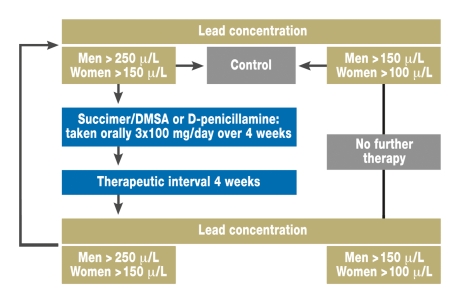
Figure 1 shows the therapeutic scheme used in the patients at Leipzig University Hospital.
Figure 1.
Simplified therapeutic scheme for lead intoxication (adapted from the guidelines of the German Society for Occupational Medicine and Environmental Medicine (Deutsche Gesellschaft für Arbeitsmedizin und Umweltmedizin e.V., DGAUM), AWMF online, June 2005
Health office data
The local health office offered anonymous blood testing for lead to marijuana users until end-August 2008. Each test cost 22 euros; participants had to pay for themselves. Altogether 597 specimens were examined (439 men, 158 women; age 26.9 ± 4.8 years). The oldest user was 56 years old and the youngest 15 years. According to the human biomonitoring value II (HBM-II) of 250 µg/L for men and 150 µg/L for premenopausal/fertile women, 27.3% had lead intoxication that necessitated treatment, 12.2% had lead concentrations that required monitoring, and 60.5% had concentrations below the HBM-I threshold (<150 µg/L or <100 µg/L) above which treatment would have been necessary. The health office had no reports of fatalities due to lead intoxication or of pregnancies in women with raised lead concentrations.
Pharmacokinetics
Lead compounds or dusts are mostly absorbed via inhalation (about 70% to 100%) or ingestion (about 5% to 20%) (2, 5). Lung absorption is effective (>90%) if the particle size is below 1 µm. This size is reached in lead fumes during the burning of a cigarette or joint, at temperatures above 1200 degrees centigrade (e5, e6). Absorption in the gastrointestinal tract depends on the physical and chemical attributes (metallic, anorganic, or organic lead) and other cationic foods (Zn2+, Fe2+, Ca2+). Metallic lead is slowly absorbed through the gastrointestinal tract and the skin, whereas tetraethyl lead (antiknock agent) is lipophilic and rapidly enters through the skin. If metallic lead or barely soluble lead salts are taken in only once, a toxic effect will be noticeable only in high concentrations. However, even tiny quantities, taken in over time, build up concentrations in the body and are excreted only very slowly.
In the blood, lead is bound mainly to erythrocyte proteins (99%, only 1% circulates freely). This proportion can be exchanged rapidly. Within 100 days the lead concentrates in organs such as the liver, kidneys, brain, lungs and the spleen, in the aorta, teeth, and the skeleton (6). Lead in the body is mainly stored in the bones instead of calcium phosphate (90%). Its half-life in blood is 30 days (5). Lead stored in the bones is eliminated with a terminal half-life of up to 37 years (5, 7). Because of the redistribution kinetics, the time of exposure cannot be determined with certainty. Metallic lead is excreted through bile (15%), through the kidneys (75%), and through the skin and its associated structures (hair, nails; 10%). Organic lead alkyl compounds are dealkylated via hepatic cytochrome P450–dependent mono-oxygenases, a process during which highly neurotoxic metabolites are formed (6).
Symptoms
Lead has a high affinity to sulfhydryl groups and interacts with different enzyme systems and intracellular calcium channels. The result is disruption of heme synthesis, neuronal and renal damage, and osteoblast dysfunction. Lead poisoning typically manifests with acute abdominal colic, anemia, renal tubulopathy, peripheral neuropathy, or encephalopathy (2). In (small) children, a risk of intellectual developmental deficits is present (6, 8). The box summarizes the symptoms and signs of lead poisoning.
Box. Symptoms and signs of lead intoxication (from [2, 6]).
-
General (blood concentration >100 µg/L)
Abdominal colic
Anemia, basophilic stippling
Lead seam (“Burton’s line“)
Loss of appetite and weight loss
Hypertension
Neuropathy:
Neural conduction delay
Hyperreflexia
Tremor, muscle weakness; especially extensor weakness ("drop hand")
Paresthesias
Encephalopathy:
Headaches
Seizures
Delirium
Concentration deficit
Memory dysfunction
Irritability
Raised intracranial pressure
Renal insufficiency
Infertility
-
In children
Reversible aminoaciduria
Disturbed fine motor control
Retarded growth
Delayed speech development/learning deficit
Changed behavior/hyperactivity
IQ deficit
Abdominal cramps
Anemia
Lead inhibits enzymes of hemoglobin synthesis (δ-ALA dehydrogenase, coprogenase, and ferrochelatase). The transformation of δ-aminolevulinic acid (ALA) into porphobilinogen is blocked, as are the formation of protoporphyrin and the incorporation of iron into the protoporphyrin ring. Owing to the ineffective heme synthesis, raised concentrations of porphyrins can be shown in urine. A possible differential diagnosis is porphyria, which is also accompanied by abdominal colic.
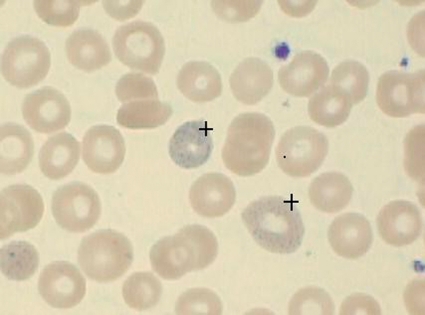
The maturation of the erythrocyte lines is inhibited as a result of the inhibited activity of pyrimidine 5'-nucleotidase, which eventually causes normocytic hypochromic anemia with a shortened survival time of the erythrocytes, especially because of hemolysis, and reticulocytosis (9). The typical basophile stippling of the erythrocytes is the result of sedimentation of denatured ribosomal DNA and of mitochondrial fragments (8, 10) (figure 2).
Figure 2.
Basophilic stippling of erythrocytes
Neuropathy
Lead competes for binding sites for cations such as calcium, zinc, or iron. Some of the toxic effects can be explained with its ability to substitute calcium. This influences mitochondrial function (inhibition of cell breathing) and neuronal signal transmission. The result: an increased release of neurotransmitters. The raised ALA concentration, which is a weak agonist of the gamma-aminobutyric acid (GABA) receptor, presynaptically inhibits the release of GABA (9). Conspicuous behaviors in lead intoxication or porphyria may be caused by this.
Depending on the extent and duration of exposure, lead can interfere with all systems of the central and peripheral nervous system. It has a toxic effect on immature astrocytes and, among others, invades the astroglia and neurons via voltage dependent calcium channels. Lead also affects myelin formation, which is essential for an intact blood-brain barrier (e7). An increased permeability for proteins such as albumin results in edema with raised intracranial pressure and encephalopathy. Toddlers are particularly at risk of permanent damage, owing to immaturity of synaptogenesis, cell migration, or glial cell growth (6).
Early symptoms of lead intoxication include irritability, headache, and impaired concentration. Subsequent symptoms include memory loss and cognitive impairments and disturbed fine motor control. In children, conspicuous behaviors—impulsiveness, attention deficit, reduced play activity, and loss in IQ—have been described (11). The peripheral neuropathy mostly manifests as a weakness in the extensor muscle in the upper limb, which in serious cases may present as the classic "drop hand."
Nephropathy
Lead-induced nephropathy manifests as proximal tubular damage, glomerular sclerosis, or interstitial fibrosis, and even renal failure. The extent of lead exposure does not correlate with the extent of nephrotoxic damage, which results in an increase in renal retention parameters in lowered glomerular filtration rate (GFR), proteinuria, aminoaciduria, glycosuria, or transport defects for organic anions (12, e8). The vitamin D hypovitaminosis caused by renal insufficiency is increased by the lead related inhibition of the transformation of 25-OH vitamin D into active 1,25-OH vitamin D (e9).
Reproduction
In women, lead is mobilized from the skeletal stores to an increased degree during pregnancy and breast feeding by means of hormonal remodeling processes. Lead penetrates the placental barrier. Fetotoxic effects result in premature births or miscarriages, malformation, developmental delays, and neurocognitive deficits, as a result of the effects on immature neuronal structures (13, 14). In men, lead intoxication can result in hypospermia, teratospermia, or infertility (6).
Gastrointestinal tract
The typical signs of lead poisoning include abdominal colic and analgesic-resistant abdominal pain, whose pathogenesis can be explained with effects on the visceral muscles from lead competing with calcium on the ion channels (6).
Cardiovascular effects
Meta-analyses have shown a statistical association between the degree of lead intoxication in the blood and the risk of hypertension (15, 16, e10). Data from NHANES II (US National Health and Nutrition Evaluation Surveys), a longitudinal study of the general population (n = 4292), have shown that cardiovascular mortality was 39% higher if the lead concentration in the blood reached 200 to 290 µg/L than if it was <100 µg/L (relative risk 1.39; 95% confidence interval [CI] 1.01 to 1.91) (17). The relative risk of cardiovascular mortality was 1.59 (95% CI 1.28 to 1.98) for lead concentrations above 100 µg/L compared with concentrations <50 µg/L (NHANES III, n = 9757) (18).
Carcinogenesis
The International Agency for Research on Cancer classified anorganic lead compounds as likely to be carcinogenic in 2005 (e11). Data from epidemiological studies and meta-analyses have assessed the relative risk for tumor development for high grade exposure in the workplace at 1.11 (95% CI 1.05 to 1.17) for all tumors. Especially stomach and lung cancers and tumors of the deferent urinary tract seemed more common than in the control group (19).
Diagnosis
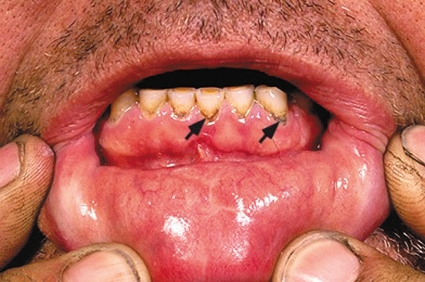
Lead poisoning can be diagnosed from the development of a lead seam along the gingivodental margin (figure 3). The dark blue–purplish black seam results from a reaction of the lead with sulfur ions that are formed by bacteria in the oral cavity and that form deposits as lead sulfide along the dental-gingival margin (20, 21). A lead seam does not correlate with the amount of lead in the blood, but is a reliable indicator for longer term exposure to lead.
Figure 3.
Lead seam along gingivodental margin
The blood lead level is measured in EDTA whole blood by using atomic absorption spectrometry (detection threshold 10 µg/L). The upper reference value in women is 70 µg/L and in men, 90 µg/L (22). The following threshold values for lead concentrations in the blood are considered normal in the general population without work-related exposure (human biomonitoring values, 2002): HBM I <100 µg/L (fertile women, children) and 150 µg/lL (men, women aged >45 years), and HBM II >150 µg/L and 250 µg/L (22). The whole blood concentration is the most commonly used biomarker for lead exposure, but only acute exposure from the preceding 35 days can be measured. Because lead is eliminated to variable extents through urine and the redistribution kinetics described earlier, lead measurement in urine is a qualitative measurement and does not provide valid information on the exposure quantity.
Treatment
The first step entails interrupting the patient’s exposure. Chelator therapy increases renal excretion, which would otherwise take months or years, 25- to 30-fold and is indicated if lead concentrations are in excess of 400 µg/L or if symptoms of lead intoxication are present (14). Sufficient diuresis needs to be established, which may necessitate giving diuretics where required. Chelators bind lead reversibly and remove it from the organism via renal excretion of water-soluble complexes. Because of the redistribution of lead from the organ stores into the blood, repeat measurements may be required after 4 weeks, potentially followed by a repeated therapeutic cycle (23). Because of delivery problems, the recommended treatment with 2,3-dimercaptopropane-1-sulfonate (DMPS) (oral 3×100 mg/day) could not be administered. The authors’ therapeutic regimen entailed initially a 4 week period in which patients received 3×100 mg/day of dimercaptosuccinic acid (DMSA/Succimer). Succimer has to be imported, which may delay the start of therapy.
Calcium EDTA and penicillamine are effective chelators; however, penicillamine has a problematic side effect spectrum. Antioxidants, such as vitamin C, can have a synergistic effect with chelator treatment (24). In tetraethyl lead (antiknock agent), chelator therapy is almost completely ineffective. Medical charcoal is the treatment of choice in this setting. The options for symptomatic treatment of abdominal cramps are limited because these symptoms do not respond well to relaxants or analgesics. In patients with pronounced anemia, transfusion may become necessary.
No randomized controlled trials have been conducted for lead intoxication. The therapy should follow the suggestions made by the specialist societies (25). A formal reference value—a threshold level of lead in the blood from which therapy is indicated—does not exist. Experts recommend starting chelator treatment if concentrations exceed 400 µg/L (14). The authors proceeded according to the scheme shown in figure 1 in their own inpatients.
Conclusions
The cases of lead intoxication in young adults described in this article give reason to include the misuse of marijuana or other recreational drugs among possible causes of anemia and abdominal colic. Lead-tainted marijuana has thus far not been described as a source of exposure (4), but case reports exist about adulterations of opium, heroin, methamphetamines, or cocaine with lead compounds (e12–e21). In the US state of Oregon, a case was reported in 1988 of mass intoxication with lead adulterated amphetamines (e22) that bore similarities to the recent Leipzig incident. The origin and purpose of the lead in marijuana in the Leipzig scenario has not been criminalistically explained and is not known to the public. Because of its high specific weight (11.3 g/cm3; compared with confectioners’ sugar at 0.8 g/cm3), lead is particularly useful for driving up profits, which was the hypothetical reason for the contaminated marijuana. According to oral communications from the drug advisory center and the health office, no lead-tainted marijuana is now available.
It is of note that a relatively high proportion of users (6.5%) who had their blood lead levels measured by the health authorities were younger than 18 years. This raises the suspicion that drug misuse starts early, in the teenage years. A targeted education campaign about drug consumption and prevention should be started for young teenagers.
Treatment can effectively be administered by using a chelator—under the condition that the patient henceforth abstains from misusing recreational drugs. However, it needs to be clarified in advance whether health insurers will cover the—not insubstantial—costs.
Acknowledgments
Particular thanks are due to Ms S. Lein, addiction officer of the City of Leipzig; Dr. Möller from the Leipzig Health Office; Dr. M. Windgassen, KfH (Kuratorium für Dialyse und Nierentransplantation e.V.) Renal Center in Grimma; Dr. M. Wiedmann, formerly Leipzig University Hospital; Professor B. Ruf, St Georg Hospital in Leipzig; the police force of the City of Leipzig; and all doctors and patients who participated in the investigation and treatment.
Translated from the original German by Dr. Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Suplido ML, Ong CN. Lead exposure among small-scale battery recyclers, automobile radiator mechanics, and their children in manila. The Philippines Environ Res. 2000;82:231–238. doi: 10.1006/enrs.1999.4024. [DOI] [PubMed] [Google Scholar]
- 2.Patrick L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev. 2006;11:2–22. [PubMed] [Google Scholar]
- 3.Hogervorst J, Plusquin M, Vangronsveld J, et al. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res. 2007;103:30–37. doi: 10.1016/j.envres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Busse F, Omidi L, Timper K, Leichtle A, Windgassen M, Kluge E, Stumvoll M. Lead poisoning due to adulterated marijuana. N Engl J Med. 2008;358:1641–1642. doi: 10.1056/NEJMc0707784. [DOI] [PubMed] [Google Scholar]
- 5.Schrenk D. Blei. In: Seeger R, Neumann HG, editors. Giftlexikon. Vol. 514. Stuttgart: Deutscher Apotheker Verlag; 2005. [Google Scholar]
- 6.Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115:455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piomelli S. Childhood lead poisoning. Pediatr Clin North Am. 2002;49:1285–1304. doi: 10.1016/s0031-3955(02)00097-4. vii. [DOI] [PubMed] [Google Scholar]
- 9.Warren MJ, Cooper JB, Wood SP, Shoolingin-Jordan PM. Lead poisoning, haem synthesis and 5-aminolaevulinic acid dehydratase. Trends Biochem Sci. 1998;23:217–221. doi: 10.1016/s0968-0004(98)01219-5. [DOI] [PubMed] [Google Scholar]
- 10.Valentine JL, Baloh RW, Browdy BL, et al. Subclinical effects of chronic increased lead absorption - a prospective study. Part IV. Evaluation of heme synthesis effects. J Occup Med. 1982;24:120–125. [PubMed] [Google Scholar]
- 11.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsden PA. Increased body lead burden - cause or consequence of chronic renal insufficiency? N Engl J Med. 2003;348:345–347. doi: 10.1056/NEJMe020164. [DOI] [PubMed] [Google Scholar]
- 13.Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Med Sci Monit. 2005;11:RA329–RA336. [PubMed] [Google Scholar]
- 14.Kosnett MJ, Wedeen RP, Rothenberg SJ, et al. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463–471. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staessen JA, Bulpitt CJ, Fagard R, Lauwerys RR, Roels H, Thijs L, Amery A. Hypertension caused by low-level lead exposure: Myth or fact? J Cardiovasc Risk. 1994;1:87–97. [PubMed] [Google Scholar]
- 16.Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An Epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. J Hum Hypertens. 2002;16:123–131. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- 17.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–2449. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 18.Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006;114:1538–1541. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu H, Boffetta P. Cancer and occupational exposure to inorganic lead compounds: a meta-analysis of published data. Occup Environ Med. 1995;52:73–81. doi: 10.1136/oem.52.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce JM. Burton’s line in lead poisoning. Eur Neurol. 2007;57:118–119. doi: 10.1159/000098100. [DOI] [PubMed] [Google Scholar]
- 21.Burton H. On a remarkable effect on the human gums produced by the absorption of lead. Med Chir Trans. 1840;23:63–79. doi: 10.1177/095952874002300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HBM-Kommission. Aktualisierung der Referenzwerte für Blei, Cadmium und Quecksilber im Blut und im Urin von Erwachsenen. Bundesgesundheitsbl - Gesundheitsforsch - Gesundheitsschutz. 2003;46:1112–1113. [Google Scholar]
- 23.Gracia RC, Snodgrass WR. Lead toxicity and chelation therapy. Am J Health Syst Pharm. 2007;64:45–53. doi: 10.2146/ajhp060175. [DOI] [PubMed] [Google Scholar]
- 24.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11:114–127. [PubMed] [Google Scholar]
- 25.DGAUM, Gesellschaft für Arbeitsmedizin und Umweltmedizin e. V.: Leitlinien der Deutschen Gesellschaft für Arbeitsmedizin und Umweltmedizin e.V. (DGAUM). AWMF online: http://leitlinien.net/2005. [Google Scholar]
- e1.Bellinger DC, Bellinger AM. Childhood lead poisoning: the torturous path from science to policy. J Clin Invest. 2006;116:853–857. doi: 10.1172/JCI28232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Montes Santiago J. Goya, Fortuny, Van Gogh, Portinari: lead poisoning in painters across three centuries. Rev Clin Esp. 2006;206:30–32. doi: 10.1016/s0014-2565(06)72707-2. [DOI] [PubMed] [Google Scholar]
- e3.Friedman T, Westreich M, Lurie DJ, Golik A. Rembrandt - aging and sickness: a combined look by plastic surgeons, an art researcher and an internal medicine specialist. Isr Med Assoc J. 2007;9:67–71. [PubMed] [Google Scholar]
- e4.Reiter C. Beethovens Todesursachen und seine Locken - Eine forensisch-toxikologische Recherche. Wiener Beethoven-Gesellschaft. Mitteilungsblatt. 2008 [Google Scholar]
- e5.Rabinowitz PM, Siegel MD. Acute inhalation injury. Clin Chest Med. 2002;23:707–715. doi: 10.1016/s0272-5231(02)00025-4. [DOI] [PubMed] [Google Scholar]
- e6.Baker RA. Temperature distribution inside a burning cigarette. Nature. 1974;247 [Google Scholar]
- e7.Cheong JH, Bannon D, Olivi L, Kim Y, Bressler J. Different mechanisms mediate uptake of lead in a rat astroglial cell line. Toxicol Sci. 2004;77:334–340. doi: 10.1093/toxsci/kfh024. [DOI] [PubMed] [Google Scholar]
- e8.Staessen JA, Lauwerys RR, Buchet JP, et al. Impairment of renal function with increasing blood lead concentrations in the general population. The cadmibel study group. N Engl J Med. 1992;327:151–156. doi: 10.1056/NEJM199207163270303. [DOI] [PubMed] [Google Scholar]
- e9.Yu CC, Lin JL, Lin-Tan DT. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. 2004;15:1016–1022. doi: 10.1097/01.asn.0000118529.01681.4f. [DOI] [PubMed] [Google Scholar]
- e10.Schwartz J. Lead, blood pressure, and cardiovascular disease in men. Arch Environ Health. 1995;50:31–37. doi: 10.1080/00039896.1995.9955010. [DOI] [PubMed] [Google Scholar]
- e11.Rousseau MC, Parent ME, Nadon L, Latreille B, Siemiatycki J. Occupational exposure to lead compounds and risk of cancer among men: a population-based case-control study. Am J Epidemiol. 2007;166:1005–1014. doi: 10.1093/aje/kwm183. [DOI] [PubMed] [Google Scholar]
- e12.Masoodi M, Zali MR, Ehsani-Ardakani MJ. Abdominal pain due to lead-contaminated opium: a new source of inorganic lead poisoning in Iran. Arch Iran Med. 2006;9:72–75. [PubMed] [Google Scholar]
- e13.Beattie AD, Briggs JD, Canavan JS, Doyle D, Mullin PJ, Watson AA. Acute lead poisoning: five cases resulting from self-injection of lead and opium. Q J Med. 1975;44:275–284. [PubMed] [Google Scholar]
- e14.Fitzsimons EJ, Dagg JH. Lead poisoning in a drug addict; the intravenous injection of suppository extracts. Br J Clin Pract. 1982;36:284–285. [PubMed] [Google Scholar]
- e15.Algora M, Martin-Castillo A, Zabala P, Fernandez MN. Lead poisoning due to drug addiction: a new source of poisoning with clinical interest and important epidemiological consequences. An Med Interna. 1989;6:483–485. [PubMed] [Google Scholar]
- e16.D’Alessandro, Gandolfo L, Macri A, et al. An unusual mechanism of lead poisoning. Presentation of a case. Recenti Prog Med. 1989;80:140–141. [PubMed] [Google Scholar]
- e17.Antonini G, Palmieri G, Millefiorini E, Spagnoli LG, Millefiorini M. Lead poisoning during heroin addiction. Ital J Neurol Sci. 1989;10:105–108. doi: 10.1007/BF02333882. [DOI] [PubMed] [Google Scholar]
- e18.Teggi A, Lanzalone CM, De Rinaldis ML, et al. A case of lead poisoning in a drug addict. Observations on etiopathogenetic peculiarities. Clin Ter. 1988;124:223–236. [PubMed] [Google Scholar]
- e19.Manzano Molina J, Martin del Yerro JL, Fernandez Alvaro P, Scapa Martin A, Cano A, Fernandez Fuertes I. Lead poisoning and drug addiction. Rev Clin Esp. 1987;181:117–118. [PubMed] [Google Scholar]
- e20.Parras F, Patier JL, Ezpeleta C. Lead-contaminated heroin as a source of inorganic-lead intoxication. N Engl J Med. 1987;316 doi: 10.1056/NEJM198703193161217. [DOI] [PubMed] [Google Scholar]
- e21.Norton RL, Burton BT, McGirr J. Blood lead of intravenous drug users. J Toxicol Clin Toxicol. 1996;34:425–430. doi: 10.3109/15563659609013813. [DOI] [PubMed] [Google Scholar]
- e22.From the Centers for Disease Control: Lead poisoning associated with intravenous-methamphetamine use - Oregon, 1988. JAMA. 1990;263:797–798. [PubMed] [Google Scholar]