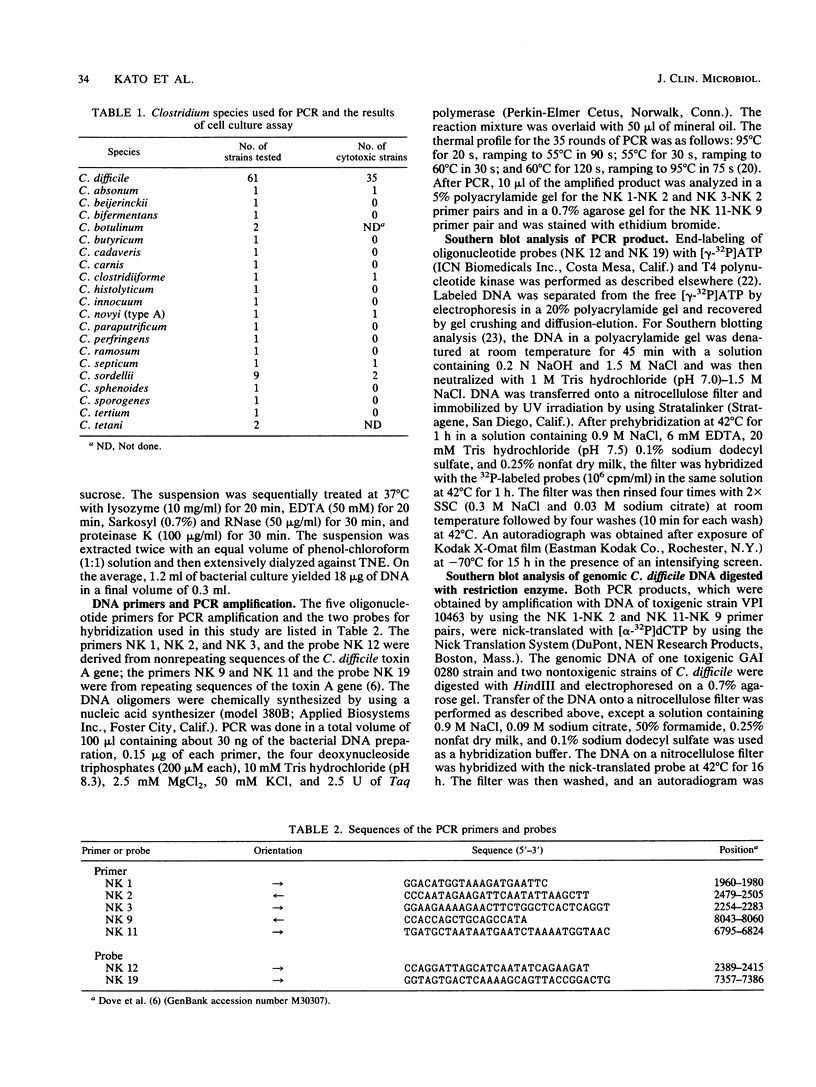
Abstract
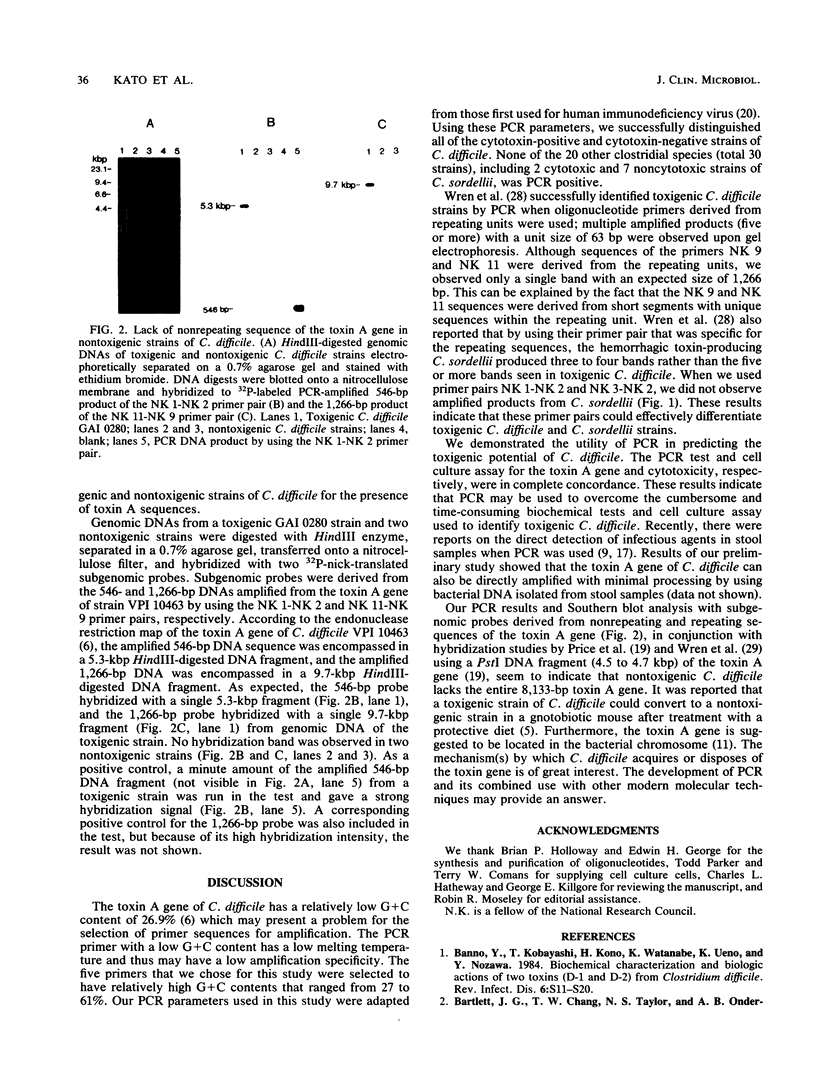
Toxigenic strains of Clostridium difficile are causative agents of pseudomembranous colitis and antimicrobial agent-associated diarrhea and colitis. The toxigenicity is routinely assayed by using highly sensitive cell cultures. We used a simple and rapid polymerase chain reaction (PCR) assay to differentiate toxigenic and nontoxigenic strains of C. difficile. Two sets of oligonucleotide primer pairs derived from nonrepeating sequences of the toxin A gene were used to amplify 546- and 252-bp DNA fragments. A primer pair derived from repeating sequences of the toxin A gene was used to amplify a 1,266-bp DNA product. Amplified products were visualized by polyacrylamide gel electrophoresis followed by ethidium bromide staining. All 35 cytotoxic strains of C. difficile tested generated the expected amplified DNA. In contrast, none of the 26 noncytotoxic strains tested gave positive results. Although the toxins of C. difficile have been demonstrated to cross-react serologically with the toxins of Clostridium sordellii, we did not detect any amplified DNA in two cytotoxic strains or seven noncytotoxic strains of C. sordellii. PCR was negative in all 30 strains of 20 other Clostridium species. Southern hybridization of HindIII-digested genomic DNA by use of subgenomic probes showed a single hybridization band in toxigenic strains but not in nontoxigenic strains. PCR appears to be a sensitive and specific assay for the rapid identification of toxigenic C. difficile. Nontoxigenic C. difficile appeared to lack the C. difficile toxin A gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Kobayashi T., Kono H., Watanabe K., Ueno K., Nozawa Y. Biochemical characterization and biologic actions of two toxins (D-1 and D-2) from Clostridium difficile. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S11–S20. doi: 10.1093/clinids/6.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- Bolton R. P., Tait S. K., Dear P. R., Losowsky M. S. Asymptomatic neonatal colonisation by Clostridium difficile. Arch Dis Child. 1984 May;59(5):466–472. doi: 10.1136/adc.59.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello S. P., Barclay F. E., Reed P. J., Welch A. R., Brown J. D., Burdon D. W. Analysis of latex agglutination test for Clostridium difficile toxin A (D-1) and differentiation between C difficile toxins A and B and latex reactive protein. J Clin Pathol. 1987 May;40(5):573–580. doi: 10.1136/jcp.40.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Muller M. C. Emergence in gnotobiotic mice of nontoxinogenic clones of Clostridium difficile from a toxinogenic one. Infect Immun. 1988 Jun;56(6):1500–1504. doi: 10.1128/iai.56.6.1500-1504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove C. H., Wang S. Z., Price S. B., Phelps C. J., Lyerly D. M., Wilkins T. D., Johnson J. L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990 Feb;58(2):480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Fairweather N. F., Lyness V. A., Pickard D. J., Allen G., Thomson R. O. Cloning, nucleotide sequencing, and expression of tetanus toxin fragment C in Escherichia coli. J Bacteriol. 1986 Jan;165(1):21–27. doi: 10.1128/jb.165.1.21-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatelli J. C., Gingeras T. R., Richman D. D. Nucleic acid amplification in vitro: detection of sequences with low copy numbers and application to diagnosis of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 1989 Apr;2(2):217–226. doi: 10.1128/cmr.2.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy D. C., Nauman R. K., Paxton H. Detection of Borrelia burgdorferi using the polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1089–1093. doi: 10.1128/jcm.28.6.1089-1093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Rogers M. F., Ou C. Y., Rayfield M., Thomas P. A., Schoenbaum E. E., Abrams E., Krasinski K., Selwyn P. A., Moore J., Kaul A. Use of the polymerase chain reaction for early detection of the proviral sequences of human immunodeficiency virus in infants born to seropositive mothers. New York City Collaborative Study of Maternal HIV Transmission and Montefiore Medical Center HIV Perinatal Transmission Study Group. N Engl J Med. 1989 Jun 22;320(25):1649–1654. doi: 10.1056/NEJM198906223202503. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. C., Ruane P. J., Rosenblatt J. E., Lyerly D. M., Gleaves C. A., Smith T. F., Pierce P. F., Jr, Wilkins T. D. Comparison of culture, cytotoxicity assays, and enzyme-linked immunosorbent assay for toxin A and toxin B in the diagnosis of Clostridium difficile-related enteric disease. Diagn Microbiol Infect Dis. 1986 May;5(1):61–69. doi: 10.1016/0732-8893(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Menge S. K., Matsen J. M. Identification of toxigenic Clostridium difficile by counterimmunoelectrophoresis. J Clin Microbiol. 1980 May;11(5):470–473. doi: 10.1128/jcm.11.5.470-473.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren B. W., Clayton C. L., Castledine N. B., Tabaqchali S. Identification of toxigenic Clostridium difficile strains by using a toxin A gene-specific probe. J Clin Microbiol. 1990 Aug;28(8):1808–1812. doi: 10.1128/jcm.28.8.1808-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren B., Clayton C., Tabaqchali S. Rapid identification of toxigenic Clostridium difficile by polymerase chain reaction. Lancet. 1990 Feb 17;335(8686):423–423. doi: 10.1016/0140-6736(90)90267-9. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Fung J. C. Evaluation of the usefulness of counterimmunoelectrophoresis for diagnosis of Clostridium difficile-associated colitis in clinical specimens. J Clin Microbiol. 1983 Apr;17(4):610–613. doi: 10.1128/jcm.17.4.610-613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]




