Abstract
Introduction
The term cancer of unknown primary site (CUP) syndrome is used to describe malignancies in which a complete diagnostic work-up detects metastases in the absence of an identifiable primary tumor.
Methods
Based on a selective literature review, national and international guidelines, and the experience of the "Arbeitskreis CUP-Syndrom der Arbeitsgemeinschaft Internistische Onkologie der Deutschen Krebsgesellschaft" (CUP Syndrome Committee of the Medical Oncology Joint Working Group of the German Cancer Society), developments in the diagnosis and treatment of CUP syndrome are reported.
Results
Most patients diagnosed with CUP have an unfavorable prognosis, with a life expectancy of less than 12 months. Nevertheless, it is important to identify subsets of patients in whom specific treatment offers the chance of long-term survival or even full recovery.
Discussion
Only rigorous further development of diagnostic tools and treatment protocols will enable an improvement of the poor prognosis of patients with CUP syndrome. Specific molecular treatment strategies have shown promising results.
Keywords: CUP syndrome, diagnosis, treatment success, molecular medicine, metastasis
The term cancer of unknown primary site (CUP) syndrome is for malignancies in which a complete diagnostic work-up detects only metastases, but no primary tumor. The presentation of the CUP syndrome is histopathologically and clinically heterogenous, with several common biological characteristics, and requires a specific diagnostic and therapeutic procedure. The clinical course is characterized by a short medical history, with nonspecific symptoms, advanced metastasis at the time of diagnosis, atypical pattern of metastasis, and an unfavorable prognosis in the majority of cases.
The CUP syndrome is comparatively frequent, corresponding to 3% to 5% of all malignancies. It occurs somewhat more frequently in men than in women. It is among the 10 most frequent malignancies in Europe. The mortality rate in Germany in 1997 is given as 4.5 per 100 000 deaths for women and 7.1 per 100 000 deaths for men.
Little is known of the etiology and pathogenesis of this illness. It is generally assumed that the metastases have a growth advantage over the primary tumor. Alternatives which have been considered, particularly for head and neck tumors, are that there is immunological regression of the primary tumor coupled to progressive metastasis, or that there is malignant transformation of scattered epithelial cells at the site of metastasis, without a primary tumor (1). Although the metastases rapidly become symptomatic, the primary tumor mostly remains undiscovered throughout the course of the disease. Even when modern radiological and endoscopic procedures are used systematically, the primary tumor is only identified in 10% to 20% of CUP patients in the course of their disease (2, 3). It is often clinically difficult to distinguish between primary tumor and metastasis, particularly when multiple tumors are identifiable in the lung or liver and when the histological and immunohistological findings are ambiguous.
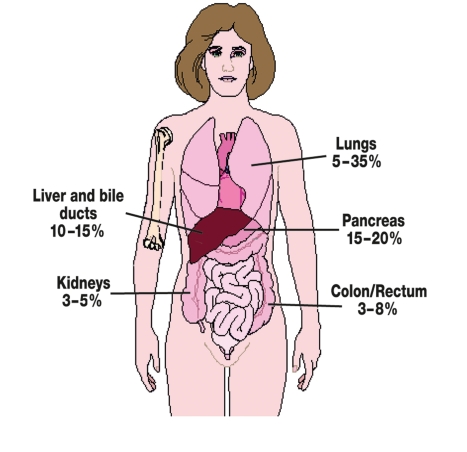
The primary tumor is identified post mortem in 50% to 75% of cases. Post-mortem studies identify the lung or the pancreas as the site of the primary tumor in about half of the cases (3, e1). The primary tumor is more rarely found in the liver, bile ducts, colon, rectum, or kidneys (figure 1).
Figure 1.
Identified primary tumors in patients with CUP syndrome. The frequencies in the illustration are based on historical data (6, 7) and have been compiled from several dissection studies
Large retrospective studies give the median survival of patients with the CUP syndrome as 3 to 6 months. These studies found that the one year survival rate was under 20% (e2–e4). On the other hand, some more recent prospective studies with selected patients have found a median survival of 6 to 13 months, with a one year survival rate of between 25% and 53% (4–7).
The authors would like to report on progress in the diagnosis and treatment of patients with the CUP syndrome. They would like to emphasize that, even though the prognosis is still very poor, it is very important to identify patients with specific subgroups of the CUP syndrome, who can be given specific treatment, with the option of long-term survival or even cure. This review article is based on a selection of scientific articles identified with Medline, using the terms "cancer of unknown primary," "CUP," and "occult primary cancer." We have concentrated on studies with modern diagnostic procedures and on randomized clinical trials. Single case reports have been excluded.
Basic diagnosis
The basic diagnostic strategy in CUP syndrome does not have the objective of identifying the primary tumor by using all available methods. It is more important to distinguish localized from disseminated disease and thus to identify potentially curable and therapy-sensitive tumor entities. This must be achieved as rapidly as possible and with a minimum of stress to the patient (2, 3, 8, 9). Routine diagnostic procedures include a detailed medical history, a thorough medical examination, basic laboratory diagnosis, CT investigation of the neck, chest, abdomen and pelvis, a gynecological investigation for women, and a tumor biopsy, which is primarily used to confirm the tumor diagnosis (box). The further diagnostic and therapeutic procedure is greatly influenced by the histopathological and immunohistological findings, together with the anatomical localization of the tumors (9). Aside from basic laboratory parameters, some selected tumor markers should be determined. These should be markers which are known to be useful for deciding treatment strategies. The following tumor markers may directly indicate the primary tumor: AFP (hepatocellular carcinoma, germ cell tumors), beta-hCG (germ cell tumors) and PSA (prostate carcinoma). If the histological diagnosis is a differentiated neuroendocrine tumor, calcitonin can indicate a medullar thyroid gland carcinoma. Other tumor markers, such as CEA, CA 125, CA 19-9, or Ca 15-3, are of low specificity and can only be used to follow the course of the illness.
Box. Basic diagnostic strategy for patients with CUP syndrome.
Medical history, physical examination, including testicular palpation for men and breast examination for women
Histology and cytology with immunohistology
CT neck, chest, abdomen and pelvis
Women: gynecological investigation
Routine laboratory, PSA (men >40 years), AFP, beta-hCG
Additional diagnostic procedures depending on working diagnosis, if this is of therapeutic consequence
AFP, alpha-fetoprotein; beta-hCG, beta–human choriogonadotropin; CT, computed tomography; PSA, prostate-specific antigen
Histology and immunohistology
Tumor biopsy or cytology of malignant effusions is part of the essential diagnostic workup of CUP syndrome, to confirm the diagnosis of malignancy and to guide further diagnostic steps. As the overall prognosis of these patients is poor, the procedure for taking the biopsy should expose the patients to as little stress as possible and be as noninvasive as possible. It must nevertheless provide enough material to allow extensive immunohistological investigation.
Adenocarcinoma (50% to 70%), undifferentiated carcinomas (20% to 30%), squamous cell carcinomas (5% to 8%) and undifferentiated tumors (2% to 3%) may be distinguished histologically (e1–e3, 8). If cervical lymph node metastases are not considered, the proportion of squamous cell carcinomas sinks to under 5%. Tumors with neuroendocrine differentiation, including small cell carcinomas, may be relatively rare (2% to 4%), but deserve special attention, as they are sensitive to chemotherapy (10).
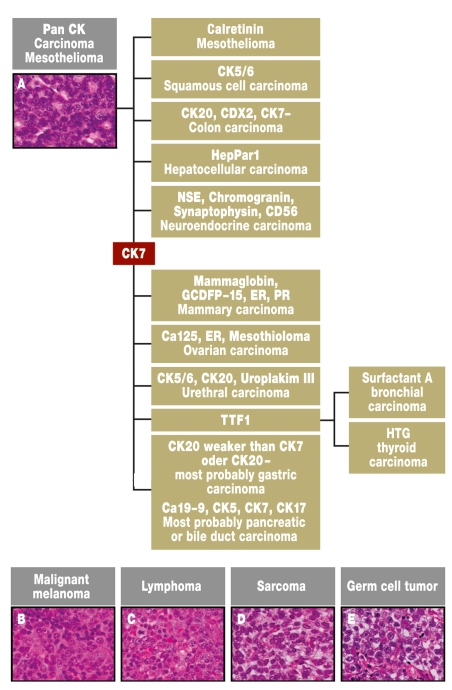
Immunohistology is of special importance for the classification of metastases when the primary tumor is unknown (9, 11, 12). This can restrict the number of possible primary tumors, as well as exclude tumor entities with well defined histology, such as lymphomas, sarcomas, or melanomas. To avoid excessive diagnostic work, a panel of immunological stains should be worked through which permits the identification of defined tumor entities (figure 2). The initial positive stains can suggest possible diagnoses, which can be followed up with other stains. Even though the primary tumor can often not be identified with epithelial tumors, it is often feasible to reduce the number of possible primary tumors.
Figure 2.
Algorithm for the histological and immunohistological investigation of tumor manifestations with unknown primary tumor. The probable diagnosis is gradually approached using the immunohistological profile. There are additional marker profiles for the subgroups of specific tumors, but these will not be discussed in this review. The diagnoses are given in regular typeface, the immunohistological markers are given in bold (e18).
PET and PET/CT diagnosis
Positron emission tomography (PET) is a nuclear medical procedure which can be used for patients with the CUP syndrome to localize the primary tumor or previously unrecognized metastases. There is also an indication to use this method if there is localized manifestation of the tumor and if additional metastatic colonization must be excluded before potentially curative treatment strategies are started.
The role of PET in the CUP syndrome depends on whether the patient has cervical metastasis (predominantly squamous cell carcinoma) or extracervical metastases (predominantly adenocarcinoma). Although PET is established in head and neck CUP, its use in extracervical CUP is still controversial.
The use of PET in the extracervical CUP syndrome has recently been subject to a meta-analysis, including 221 patients from 10 studies (13). Most of these studies had small numbers of patients. Many patients had only one metastasis localization, the preceding basic diagnostic procedure was not standardized and often consisted of only a medical history, a physical examination, a chest X-ray, and abdominal ultrasound. In 41% of patients, a primary tumor was identified which had not been found in the basic diagnostic procedures. In 59% of patients, the primary tumor was in the lung. In 37% of patients, previously unidentified metastases were found. The sensitivity of PET in these studies was 91.9%; the specificity was 81.9%. One problem was the high rate of false positive findings in the lower digestive tract (58%) and this limits the usefulness of PET diagnosis below the diaphragm.
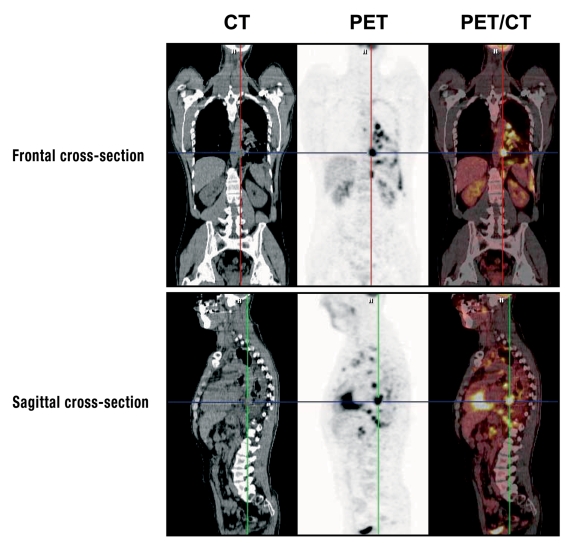
Combined PET/CT systems have been available for some years. These combine the high spatial resolution and detailed anatomical images of CT with the highly sensitive metabolic information of PET (figure 3). Another advantage of the PET/CT hybrid technique is that it takes less time than using PET and CT separately. In three published case series with a total of 91 patients, the primary tumor was identified in 40% of cases with the hybrid technique (e5–e7). If PET/CT is compared with PET alone, it is concluded that it is not clear that there are any more correct positive results, but that the proportion of false positives is reduced (e5, e7).
Figure 3.
Investigation of a patient with CUP syndrome using the PET/CT hybrid technique. The images are from a 42-year old male patient with a history of smoking, who first visited his GP with swelling of the cervical lymph nodes and left hematothorax. Extirpation of a cervical lymph node gave the histological diagnosis of squamous cell carcinoma. The tumor cells were TTF1-positive in immunohistology. The PET/CT investigation found multiple mediastinal lymph node metastases—right intraclavicular and paraaortic at the level of the celiac trunk—, also suspected pleural carcinosis. The present findings indicate a primary tumor in the left lung, even though this could not be directly identified. With the kind approval of Professor Uwe Haberkorn MD, Department of Nuclear Medicine, Heidelberg University
Although there have been some encouraging results with PET and PET/CT investigations in small series of patients with the CUP syndrome, it must be clearly stated that there is currently no consensus on the use of these methods in extracervical CUP syndrome and that these methods require further validation in clinical studies.
Genomic analyses and gene expression studies with microarrays
In contrast to most other malignancies, there are only few data on the expression and mutation status of tumor suppressor genes and oncogenes in CUP syndrome. The reason for this is that most studies have been performed on small and highly heterogenous groups of patients.
Early data from Hedley et al. showed that cell populations with aberrant DNA content can be detected by flow cytometry in about 70% of patients with adenocarcinomas with an unknown primary tumor. This value is similar to that in patients with adenocarcinomas of known origin (e8). Two independent studies have used comparative genomic hybridization (CGH) in patients with the CUP syndrome. Apart from increases in 7q22, no specific chromosomal aberrations were found (e9, e10). Detection of an isochromosome 12 (i[12p]) is characteristic of germ cell tumors and is a predictive marker for response to platinum chemotherapy. In a study on 40 poorly differentiated CUP tumors (14), i(12p) could be detected in 30% of cases; this correlated with response to platinum chemotherapy (75% versus 18%, p = 0.002).
The frequency of p53 mutations is apparently lower in the CUP syndrome (26%) than in other malignancies, in which the frequency is mostly >50% (e11). This is however with the reservation that only a few CUP tumors have been examined and that mutation analysis was restricted to exons 5–9 of the p53 gene—those most affected by mutations.
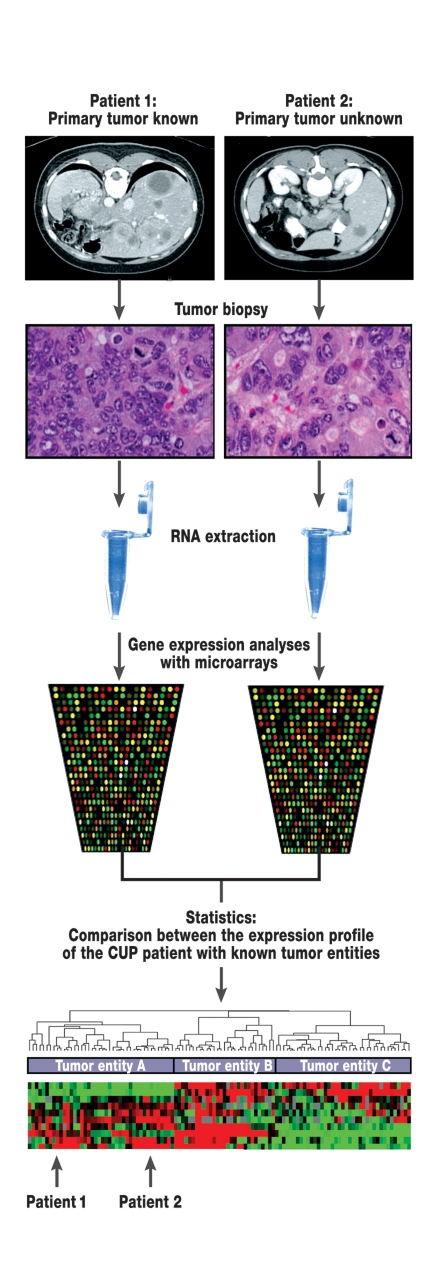
The main objective of gene expression analysis in the CUP syndrome is to identify the site of the primary tumor. With this aim in mind, the RNA expression profile of CUP tumors has been compared with the profiles for tumors with known primaries. Thus Tothill et al. investigated whether tumors with known primaries can be classified with gene expression analysis using cDNA microarrays (15) (figure 4). They developed a classifier which allowed the correct classification of 229 tumor samples to 13 tumor entities with a precision of 89%. The classifier was then used to classify tumors in CUP patients to the organ of the primary tumor; this was successful in 11 of 13 cases. Several additional studies have also been recently published which indicate that primary tumor identification is possible with gene expression analysis (e12–e14).
Figure 4.
Principle of gene expression analyses with microarrays in patients with CUP syndrome. Biopsies are taken of metastases in patients with known primary tumors (patient 1) and tumor manifestations of CUP patients (patient 2). RNA is then extracted from tumor tissue. Using microarray technology, a specific gene expression profile can be prepared from the RNA from each tumor. With the help of statistical methods, a comparison can be made between the gene expression patterns in patients with CUP syndrome and patients with known primary tumors. In the present example, patient 1 would be assigned to the same group as patient 2. It is possible that studies of this sort may lead to changes in therapy.
The authors would nevertheless like to point out that both genomic investigations and gene expression analyses should be restricted to clinical studies, as they are highly expensive, take a great deal of time, and the current data is rather limited.
Treatment
There is no drug specifically approved for the treatment of the CUP syndrome. Identification of subgroups with favorable prognosis is of decisive importance for the therapy of patients with CUP syndrome (table 1). The first step is always to test whether a patient has one of these well characterized disease entities and then to plan the treatment accordingly. These CUP subgroups with favorable prognosis are mostly rare and a detailed description of the therapeutic procedure would go beyond the scope of this review article. For additional information, please refer to the guidelines of the German Society for Hematology and Oncology (http://www.dgho.de), the European Society of Medical Oncology (http://www.esmo.org), and the National Comprehensive Cancer Network (http://www.nccn.org).
Table 1. Tumor cell constellations with favorable prognosis. These affect about 20% to 30% of all patients with CUP syndrome and are treated with specific protocols.
| Tumor constellation | Clinical presentation and recommended additional diagnostic procedures | Recommended therapy |
| Axillary lymph node metastases of an adenocarcinoma in women |
|
|
| Peritoneal carcinosis of a serous papillar adenocarcinoma in women |
|
|
| Cervical lymph node metastases of squamous cell carcinoma |
|
|
| Inguinal lymph node metastases of squamous cell carcinoma |
|
|
| Undifferentiated neuroendocrine carcinoma |
|
|
| Poorly differentiated carcinoma with germ cell characteristics |
|
|
| Solitary metastasis |
|
|
AFP, alpha-fetoprotein; CHT, chemotherapy; beta-hCG, beta-human choriogonadotropin; PET, positron emission tomography; CT, computed tomography; ESMO, European Society of Medical Oncology; FIGO; Fédération Internationale de Gynécologie et Obstétrique
About 70% of all patients with CUP syndrome cannot be classified to one of the subgroups with a favorable prognosis. This large group exhibits the following characteristics: in histology, either adenocarcinoma or undifferentiated carcinoma, disseminated tumor growth, negative hormone receptor status, no exclusive peritoneal carcinosis. Local therapy (resection followed by radiation) is used for CUP with only one recognized metastasis, but is not applicable for this larger group. For study purposes, CUP patients in this larger group were taken together and given chemotherapy, which was usually palliative.
Table 2 summarizes all nine prospective randomized studies published on this subject (5–7, 16–20). The groups of patients and study designs are markedly heterogenous, so that it is difficult to compare the individual studies. The number of recruited patients was between 34 and 101, so that there is really no comparison with the much larger randomized clinical trials with other tumor entities, which included much larger groups of patients. In three studies, the efficacy of cisplatin regimens was compared with regimens not containing cisplatin (17–19). The response rate with cisplatin regimens was somewhat better (27% to 50%) than with regimens without cisplatin (14% to 42%). On the other hand, the toxicity of the cisplatin regimens was higher. Thus chemotherapy with combinations containing cisplatin is apparently superior to combinations not containing cisplatin in randomized studies on the CUP syndrome, even though this has not yet been formally proved.
Table 2. Prospective randomized studies on the treatment of patients with CUP syndrome.
| Study (reference) | Number of patients | Chemotherapy | Response rate (%) | Median PFS (months) | Median OS (months) |
| Palmeri et al., 2006 (7) | 66 | Cisplatin + gemcitabine + paclitaxel vs | 48.5 | 7.4 | 9.6 |
| cisplatin + gemcitabine + vinorelbin | 42.3 | 7.5 | 13.6 | ||
| Hübner et al., 2005 (6) | 92 | Carboplatin + paclitaxel vs | 23.8 | 7.0 | 11.0 |
| gemcitabine + vinorelbin | 20.0 | 3.2 | 6.1 | ||
| Culine et al., 2003 (5) | 80 | cisplatin + gemcitabine vs | 55 | 5 | 8 |
| cisplatin + irinotecan | 38 | 4 | 6 | ||
| Assersohn et al., 2003 (20) | 88 | 5-FU vs | 11.6 | 4.1 | 6.6 |
| 5-FU + mitomycin C | 20 (NS) | 3.6 (NS) | 4.7 (NS) | ||
| Dowell et al., 2001 (21) | 34 | Carboplatin + etoposide vs | 19 | NA | 6.5 |
| paclitaxel + 5-FU + folinic acid | 19 (NS) | 8.4 (NS) | |||
| Falkson et al., 1998 (19) | 84 | Cisplatin + epirubicin + mitomycin C vs | 50 | 4.5 | 9.4 |
| mitomycin C | 17 (p < 0.01) | 2.0 (p = 0.05) | 5.4 (p = 0.05) | ||
| Eagan et al., 1987 (18) | 55 | Cisplatin + doxorubicin + mitomycin C vs | 27 | NA | 4.6 |
| doxorubicin + mitomycin C | 14 (NS) | 5.5 (NS) | |||
| Milliken et al., 1987 (17) | 101 | Cisplatin + vinblastin + bleomycin vs | 32 | NA | 6.2 |
| doxorubicin + mitomycin C | 42 | 4.5 (NS) | |||
| Woods et al., 1980 (16) | 47 | cyclophosphamide + methotrexate + 5-FU vs | 5 | NA | 1.7 |
| doxorubicin + mitomycin C | 36 (p < 0.01) | 4.5 (NS) |
CUP, carcinoma of unknown primary site; 5-FU, 5-fluorouracil; NA, not available; NS, not statistically significant; PFS, progression free survival; OS, overall survival
Phase II studies in recent years confirm the therapeutic value of platinum derivatives and of the newer substances, such as taxanes, gemcitabine, and irinotecan (5–7, 21). These regimens led to median survival times of up to 13.6 months (7). In spite of the difficulties in interpreting these results, the consequence has been that most oncology centers now regard regimens containing platinum as standard therapy for the treatment of CUP patients. Most research groups prefer combinations of two substances over threefold combinations, as the tolerability of the former is better and it has not been shown that the threefold combinations are superior. Dose escalation, including high-dose therapy with autologous blood stem cell transplantation, and dose-dense chemotherapy protocols supported by hematopoietic growth factors have not led to any improvement in the therapeutic results either (e15, e16).
Both the CUP Syndrome Committee of the Medical Oncology Joint Working Group (CUP-AIO) of the German Cancer Society and the American Minnie Pearl Cancer Research Network prefer carboplatin to cisplatin, as the tolerability is better. In several phase II studies in recent years, paclitaxel has turned out to be a suitable combination partner for carboplatin (4, 6, 22, 23). On the other hand, there have been no randomized comparisons which have demonstrated that paclitaxel is superior to other substances in combination with carboplatin. Combination therapy with carboplatin and paclitaxel gives a response rate of 30% to 40% and 2-year survival rates of 20% to 25% in the primary treatment of patients with CUP syndrome.
In summary, the dual combination of a platinum and a taxane derivative can now be regarded as standard first line therapy for the treatment of patients in good general condition suffering from CUP syndrome (histology: adenocarcinoma or undifferentiated carcinoma). As an alternative for patients in poor general condition, monotherapy with gemcitabine can be considered.
Attempts at specific molecular therapy
Immunohistochemical studies (24, e17) have shown that, just as with other tumor entities, various oncogenes and growth factor receptors are overexpressed in tumor cells from CUP patients. For example, Massard et al. (24) have recently demonstrated by immunohistochemistry that the epidermal growth factor receptor (EGFR) is expressed in 66% of all CUP tumors, whereas Her2/neu was only expressed in 4% of tumors. Only 10% of the biopsies were positive for c-kit.
The data from a phase II study have recently been published, in which patients with the CUP syndrome (most of whom had been previously treated) were treated with a combination of the EGFR inhibitor erlotinib and the antiangiogenetic VEGF antibody bevacizumab (25). According to the RECIST criteria (response evaluation criteria in solid tumors), 5 of 48 patients (10%) achieved partial remission. In addition, 29 of 48 patients (61%) exhibited temporary stabilization of their illness, corresponding to lack of progression within 8 weeks of therapy. The therapy was comparatively well tolerated and led to a mean overall survival of 7.4 months. This result is superior to many frequently used second-line chemotherapies for the CUP syndrome, so that it appears that the combination of erlotinib and bevacizumab is active.
In summary, we can say that the prognosis for the CUP syndrome is still poor and that we will only be able to improve it by consistently developing both diagnostic and therapeutic strategies. This necessitates that patients with CUP syndrome, exactly like patients with other malignancies, are treated within controlled clinical studies. These must be accompanied by a supporting scientific program to supply the urgently needed data to improve our understanding of the pathophysiology of this neglected disease at a molecular level.
Acknowledgments
This manuscript is largely based on discussions related to the CUP Syndrome Committee of the Medical Oncology Joint Working Group (AIO). In particular, we wish to thank Dr. Albrecht Kretschmar and Professor Michael Stahl for their contributions to the discussion.
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Footnotes
Dr. Folprecht has received fees and financial support for research from Merck, Sanofi-Aventis and Pfizer and fees from Lilly and Takeda. The other authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Califano J, Westra WH, Koch W, et al. Unknown primary head and neck squamous cell carcinoma: molecular identification of the site of origin. J Natl Cancer Inst. 1999;91:599–604. doi: 10.1093/jnci/91.7.599. [DOI] [PubMed] [Google Scholar]
- 2.Varadhachary GR, Abbruzzese JL, Lenzi R. Diagnostic strategies for unknown primary cancer. Cancer. 2004;100:1776–1785. doi: 10.1002/cncr.20202. [DOI] [PubMed] [Google Scholar]
- 3.Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094–2103. doi: 10.1200/JCO.1995.13.8.2094. [DOI] [PubMed] [Google Scholar]
- 4.Greco FA, Burris HA, Litchy S, et al. Gemcitabine, carboplatin, and paclitaxel for patients with carcinoma of unknown primary site: a Minnie Pearl Cancer Research Network study. J Clin Oncol. 2002;20:1651–1656. doi: 10.1200/JCO.2002.20.6.1651. [DOI] [PubMed] [Google Scholar]
- 5.Culine S, Lortholary A, Voigt JJ, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study-trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01) J Clin Oncol. 2003;21:3479–3482. doi: 10.1200/JCO.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 6.Hübner G, Steinbach S, Kohne CH, et al. Paclitaxel/carboplatin versus gemcitabine/vinorelbine in patients with adeno- or undifferentiated carcinoma of unknown primary (CUP) - a randomized prospective phase-II-trial. Proc Am Soc Clin Oncol. 2005;23 doi: 10.1038/sj.bjc.6604818. (Abstract 4089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmeri S, Lorusso V, Palmeri L, et al. Cisplatin and gemcitabine with either vinorelbine or paclitaxel in the treatment of carcinomas of unknown primary site: results of an Italian multicenter, randomized, phase II study. Cancer. 2006;107:2898–2905. doi: 10.1002/cncr.22379. [DOI] [PubMed] [Google Scholar]
- 8.Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5:3403–3410. [PubMed] [Google Scholar]
- 9.Bugat R, Bataillard A, Lesimple T, et al. Summary of the standards, options and recommendations for the management of patients with carcinoma of unknown primary site (2002) Br J Cancer. 2003;89(Suppl 1):59–66. doi: 10.1038/sj.bjc.6601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hainsworth JD, Spigel DR, Litchy S, Greco FA. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol. 2006;24:3548–3554. doi: 10.1200/JCO.2005.05.0575. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann O, Fietze E, Dietel M. Immunohistochemical diagnosis in cancer metastasis of unknown primary tumor. Pathologie. 2002;23:183–197. doi: 10.1007/s00292-001-0496-y. [DOI] [PubMed] [Google Scholar]
- 12.Hossfeld DK, Wittekind C. Metastasen bei unbekanntem Primärtumor: Das CUP-Syndrom. Dtsch Arztebl. 2005;102:A904–A907. [Google Scholar]
- 13.Sève P, Billotey C, Broussolle C, Dumontet C, Mackey JR. The role of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography in disseminated carcinoma of unknown primary site. Cancer. 2007;109:292–299. doi: 10.1002/cncr.22410. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Rodriguez E, Reuter VE, Bosl GJ, Mazumdar M, Chaganti RS. Molecular and cytogenetic studies in the diagnosis of patients with poorly differentiated carcinomas of unknown primary site. J Clin Oncol. 1995;13:274–282. doi: 10.1200/JCO.1995.13.1.274. [DOI] [PubMed] [Google Scholar]
- 15.Tothill RW, Kowalczyk A, Rischin D, et al. An expression-based site of origin diagnostic method designed for clinical application to cancer of unknown origin. Cancer Res. 2005;65:4031–4040. doi: 10.1158/0008-5472.CAN-04-3617. [DOI] [PubMed] [Google Scholar]
- 16.Woods RL, Fox RM, Tattersall MH, Levi JA, Brodie GN. Metastatic adenocarcinomas of unknown primary site: a randomized study of two combination-chemotherapy regimens. N Engl J Med. 1980;303:87–89. doi: 10.1056/NEJM198007103030205. [DOI] [PubMed] [Google Scholar]
- 17.Milliken ST, Tattersall MH, Woods RL, et al. Metastatic adenocarcinoma of unknown primary site: a randomized study of two combination chemotherapy regimens. Eur J Cancer Clin Oncol. 1987;23:1645–1648. doi: 10.1016/0277-5379(87)90443-3. [DOI] [PubMed] [Google Scholar]
- 18.Eagan RT, Therneau TM, Rubin J, Long HJ, Schutt AJ. Lack of value for cisplatin added to mitomycin-doxorubicin combination chemotherapy for carcinoma of unknown primary site: a randomized trial. Am J Clin Oncol. 1987;10:82–85. doi: 10.1097/00000421-198702000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Falkson CI, Cohen GL. Mitomycin C, epirubicin and cisplatin versus mitomycin C alone as therapy for carcinoma of unknown primary origin. Oncology. 1998;55:116–121. doi: 10.1159/000011845. [DOI] [PubMed] [Google Scholar]
- 20.Assersohn L, Norman AR, Cunningham D, et al. A randomized study of protracted venous infusion of 5-fluorouracil (5- FU) with or without bolus mitomycin C (MMC) in patients with carcinoma of unknown primary. Eu J Cancer. 2003;39:1121–1128. doi: 10.1016/s0959-8049(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 21.Dowell JE, Garrett AM, Shyr Y, Johnson DH, Hande KR. A randomized phase II trial in patients with carcinoma of an unknown primary site. Cancer. 2001;91:592–597. doi: 10.1002/1097-0142(20010201)91:3<592::aid-cncr1039>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Briasoulis E, Kalofonos H, Bafaloukos D, et al. Carboplatin plus paclitaxel in unknown primary carcinoma: a phase II Hellenic Cooperative Oncology Group Study. J Clin Oncol. 2000;18:3101–3107. doi: 10.1200/JCO.2000.18.17.3101. [DOI] [PubMed] [Google Scholar]
- 23.Greco FA, Rodriguez GI, Shaffer DW, et al. Carcinoma of unknown primary site: sequential treatment with paclitaxel/carboplatin/etoposide and gemcitabine/irinotecan: a Minnie Pearl Cancer Research Network phase II trial. Oncologist. 2004;9:644–652. doi: 10.1634/theoncologist.9-6-644. [DOI] [PubMed] [Google Scholar]
- 24.Massard C, Voigt JJ, Laplanche A, et al. Carcinoma of an unknown primary: are EGF receptor, Her-2/neu, and c-Kit tyrosine kinases potential targets for therapy? Br J Cancer. 2007;97:857–861. doi: 10.1038/sj.bjc.6603942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hainsworth JD, Spigel DR, Farley C, Thompson DS, Shipley DL, Greco FA. Minnie Pearl Cancer Research Network: Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: the Minnie Pearl Cancer Research Network. J Clin Oncol. 2007;25:1747–1752. doi: 10.1200/JCO.2006.09.3047. [DOI] [PubMed] [Google Scholar]
- e1.Le Chevalier T, Cvitkovic E, Caille P, et al. Early metastatic cancer of unknown primary origin at presentation. A clinical study of 302 consecutive autopsied patients. Arch Intern Med. 1988;148:2035–2039. [PubMed] [Google Scholar]
- e2.Altman E, Cadman E. An analysis of 1539 patients with cancer of unknown primary site. Cancer. 1986;57:120–124. doi: 10.1002/1097-0142(19860101)57:1<120::aid-cncr2820570124>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- e3.van de Wouw AJ, Janssen-Heijnen ML, Coebergh JW, Hillen HF. Epidemiology of unknown primary tumors; incidence and population-based survival of 1285 patients in Southeast Netherlands, 1984-1992. Eur J Cancer. 2002;38:409–413. doi: 10.1016/s0959-8049(01)00378-1. [DOI] [PubMed] [Google Scholar]
- e4.Levi F, Te VC, Erler G, Randimbison L, La Vecchia C. Epidemiology of unknown primary tumors. Eur J Cancer. 2002;38:1810–1812. doi: 10.1016/s0959-8049(02)00135-1. [DOI] [PubMed] [Google Scholar]
- e5.Gutzeit A, Antoch G, Kühl H, et al. Unknown primary tumors: detection with dual-modality PET/CT - initial experience. Radiology. 2005;234:227–234. doi: 10.1148/radiol.2341031554. [DOI] [PubMed] [Google Scholar]
- e6.Nanni C, Rubello D, Castellucci P, et al. Role of 18F-FDG PET-CT imaging for the detection of an unknown primary tumor: preliminary results in 21 patients. Eur J Nucl Med Mol Imaging. 2005;32:589–592. doi: 10.1007/s00259-004-1734-3. [DOI] [PubMed] [Google Scholar]
- e7.Pelosi E, Pennone M, Deandreis D, Douroukas A, Mancini M, Bisi G. Role of whole body positron emission tomography/computed tomography scan with 18F-fluorodeoxyglucose in patients with biopsy proven tumor metastases from unknown primary site. Q J Nucl Med Mol Imaging. 2006;50:15–22. [PubMed] [Google Scholar]
- e8.Hedley DW, Leary JA, Kirsten F. Metastatic adenocarcinoma of unknown primary site: abnormalities of cellular DNA content and survival. Eur J Cancer Clin Oncol. 1985;21:185–189. doi: 10.1016/0277-5379(85)90171-3. [DOI] [PubMed] [Google Scholar]
- e9.Pantou D, Tsarouha H, Papadopoulou A, et al. Cytogenetic profile of unknown primary tumors: clues for their pathogenesis and clinical management. Neoplasia. 2003;5:23–31. doi: 10.1016/s1476-5586(03)80014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Petersen I, Hidalgo A, Petersen S, et al. Chromosomal imbalances in brain metastases of solid tumors. Brain Pathol. 2000;10:395–401. doi: 10.1111/j.1750-3639.2000.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Bar-Eli M, Abbruzzese JL, Lee-Jackson D, Frost P. p53 gene mutation spectrum in human unknown primary tumors. Anticancer Res. 1993;13:1619–1623. [PubMed] [Google Scholar]
- e12.Ma XJ, Patel R, Wang X, et al. Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med. 2006;130:465–473. doi: 10.5858/2006-130-465-MCOHCU. [DOI] [PubMed] [Google Scholar]
- e13.Rosenfeld N, Aharonov R, Meiri E, et al. Micro-RNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- e14.Bridgewater J, van Laar R, Floore A, Van’T Veer L. Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer. 2008;98:1425–1430. doi: 10.1038/sj.bjc.6604315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Culine S, Fabbro M, Ychou M, Romieu G, Cupissol D, Pujol H. Chemotherapy in carcinomas of unknown primary site: a high-dose intensity policy. Ann Oncol. 1999;10:569–575. doi: 10.1023/a:1026478009050. [DOI] [PubMed] [Google Scholar]
- e16.Culine S, Kramar A, Saghatchian M, et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 2002;20:4679–4683. doi: 10.1200/JCO.2002.04.019. [DOI] [PubMed] [Google Scholar]
- e17.Hainsworth JD, Lennington WJ, Greco FA. Overexpression of Her-2 in patients with poorly differentiated carcinoma or poorly differentiated adenocarcinoma of unknown primary site. J Clin Oncol. 2000;18:632–635. doi: 10.1200/JCO.2000.18.3.632. [DOI] [PubMed] [Google Scholar]
- e18.Morawietz L, Dietel M. Der unbekannte Primarius - eine diagnostische Herausforderung. Im Focus Onkologie. 2007;5:59–64. [Google Scholar]