Abstract
Objective
The regulation of apoptosis of intestinal mucosal cells is important in maintenance of normal intestinal physiology.
Summary
Sphingosine-1-phosphate (S1P) has been shown to play a critical role in cellular protection to otherwise lethal stimuli in several nonintestinal tissues.
Methods
The current study determines whether S1P protected normal intestinal epithelial cells (IECs) from apoptosis and whether Akt activation was the central pathway for this effect.
Results
S1P demonstrated significantly reduced levels of apoptosis induced by tumor necrosis factor-alpha (TNF-α)/cycloheximide (CHX). S1P induced increased levels of phosphorylated Akt and increased Akt activity, but did not affect total amounts of Akt. This activation of Akt was associated with decreased levels of both caspase-3 protein levels and of caspase-3 activity. Inactivation of Akt by treatment with the PI3K chemical inhibitor LY294002 or by overexpression of the dominant negative mutant of Akt (DNMAkt) prevented the protective effect of S1P on apoptosis. Additionally, silencing of the S1P-1 receptor by specific siRNA demonstrated a lesser decrease in apoptosis to S1P exposure.
Conclusion
These results indicate that S1P protects intestinal epithelial cells from apoptosis via an Akt-dependent pathway.
Keywords: Sphingosine, Apoptosis, Akt signaling, Intestinal epithelium
Introduction
Maintenance of gastrointestinal mucosal integrity is vital for many intestinal functions, including digestion of food; absorption and secretion of nutrients, fluids, and electrolytes; and expulsion of waste and bacteria. Both surgical intervention and critical illness will lead to injury of the intestinal tract, particularly at the gastrointestinal (GI) mucosal layer. Here the epithelial cells must be capable of maintaining their integrity, and do this through the interplay of multiple active processes including cell proliferation, migration, differentiation, and apoptosis [1]. Under normal physiological conditions, cells rapidly replicate in the intestinal crypts and migrate towards the villus tips as they differentiate. To help control the number of enterocytes, apoptosis also occurs in both crypt and villus to maintain a critical balance between newly replicating cells and senescent cells; dysregulation of this process in either direction can have serious consequences. It has been shown that the majority of cellular loss at the villus tips of the small intestine and at the colonic luminal surface is via apoptosis, and not via simple exfoliation [2, 3]. The entirety of this process is carefully regulated but the exact mechanisms involved in this process are not well understood.
The bioactive sphingolipid sphingosine-1-phosphate (S1P) has been shown in recent years to act as a key regulator of cell proliferation and apoptosis. S1P is ubiquitous in cells and is produced intracellularly, though its broad spectrum of effects occur both intracellularly and extracellularly via specific cell surface receptors [4]. This strong lipid mediator has been demonstrated to regulate a number of cellular processes, including cytoskeletal rearrangements, angiogenesis, cell proliferation, and cellular locomotion [5-7]. Interestingly, S1P has been reported to be abundant in intestinal tissue, and that several of the endothelial differentiation gene (EDG) receptor subtypes (the cell surface receptors for S1P, also called the S1P receptors) are expressed on intestinal epithelial cells [8-10]. Neither the basal levels of the EDG receptors nor the effects of S1P upon the intestinal epithelial cells have been reported. Furthermore, S1P's role in apoptosis has also been well studied in several cellular systems, and has been found in several cellular systems [11, 12] to be a strong suppressor of apoptosis, but the role of S1P in the GI system is unknown. Greater knowledge pertaining to regulators of apoptosis would be particularly important in the GI tract, due to the potential to directly affect disease processes that rely on cell survival in the face of proapoptotic stimuli, such as malabsorptive syndromes and colorectal cancers.
Recent evidence implicates a role for the serine–threonine kinase Akt in these interactions. Akt, also known as protein kinase B [13], is the cellular homologue of the oncogene v-Akt and has been found to play a critical role in the regulation of apoptosis and cell cycle progression [14, 15]. It has been found to promote cell survival in a variety of cell types, including epithelial cells [15, 16], endothelial cells [17, 18], and neurons [19], and its activation results from a number of external stimuli via receptor-mediated signaling, including several growth factors including platelet-derived growth factor, nerve growth factor, and insulin among many others [20]. Activated Akt has been shown to phosphorylate several downstream targets, culminating in an alteration in the apoptotic threshold of the cell in question [21, 22].
The current study tests the hypothesis that S1P protects intestinal epithelial cells from apoptosis by activating Akt kinase. First, we showed that S1P protected differentiated IEC cells from apoptotic stimuli in association with its ability to upregulate PI3K and Akt kinase activity. We then showed that this protective effect of S1P was specifically reduced by chemical blockade of PI3K or through loss of Akt activity in a DNMAkt stable cell line. Finally, we demonstrated basal expression of the EDG receptor proteins and that the S1P effect on the intestinal cells was inhibited through silencing these receptors by specific transfections of EDG receptor siRNA.
Methods
Materials
Disposable cultureware was purchased from Corning GlassWorks (Corning, NY). Tissue culture media and dialyzed fetal bovine serum (dFBS) were purchased from Invitrogen (Carlsbad, CA), reagents were obtained from Sigma Chemical (St. Louis, MO), and sphingosine-1-phosphate (S1P) was purchased from Sigma-Aldrich Co. Caspase-3 antibodies and all secondary antibodies conjugated to horseradish peroxidase were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against phosphorylated Akt (p-Akt at serine 473) and total Akt were purchased from Cell Signaling (Beverly, MA). The IEC-6 cell line was purchased from American Type Culture Collection (Rockville, MD).
Tissue Culture
These experiments utilized differentiated intestinal epithelial cells, stable Cdx2-transfected IEC-6 cells (Cdx-2). The Cdx-2 cells (IEC-Cdx2L1 cells; a kind gift of Dr. P. G. Traber, University of Pennsylvania, Philadelphia, PA) were developed and characterized by Suh and Traber [23]; their development and characterization has been described previously [24, 25]. They are nontumorigenic and have been shown [26] to retain characteristics of undifferentiated intestinal epithelial cells. They were maintained as subconfluent monolayers in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 10 μg/ml insulin, 50 μg/ml gentamicin, and isopropyl-beta-D-thiogalactopyranoside (IPTG, 4 mM). Flasks were incubated at 37°C in a humidified atmosphere of 90% air-10% CO2. Some experiments utilized IEC-6 cells, which are less differentiated and more representative of crypt cells. The IEC-6 cell line, as characterized by Quaroni et al. [27], originated from normal rat intestinal crypt cells. The cell lines were purchased at passage 13 and used between passages 15 and 20.
Apoptosis Protocol and Assessment of Morphology and Annexin V Staining
In several of the experiments, we assessed the responses of the intestinal epithelial cells to apoptotic conditions. To examine the apoptotic response of intestinal epithelial cells we utilized the TNF-α/CHX-induced apoptosis as a model. To determine the effect of S1P in protecting IECs from apoptotic stimuli, cells were exposed to TNF-α/CHX for 4 h with or without S1P exposure (2.5 mM) for 1 h prior to the apoptosis challenge. This dose of S1P, as presented in the “Results” section, was found to be the optimal dose in dose-response experiments for a range encompassing what has been reported by several authors in other cell systems [5, 28].
After various experimental treatments, cells were photographed with a Nikon inverted microscope before fixation. Annexin V staining of apoptosis was carried out by using a commercial apoptosis kit (Clontech Laboratories, Inc. Palo Alto, CA) and performed according to the protocol recommended by the manufacturer. Briefly, cells were rinsed with 1× binding buffer, and resuspended in 200 μl of 1× binding buffer. Five microliters of Annexin V was added on the slide and incubated at room temperature for 10 min in the dark. Annexin-stained cells were visualized and photographed under fluorescence microscope using a dual filter set for fluoroscein isothiocyanate (FITC) & rhodamine, and the percentage of “apoptotic” cells was determined.
Plasmid Construction and Transfection
The full-length cDNA of the dominant negative Akt mutant (DNMAkt), containing a Myc-His tag at its 3′ end and a substitution of methionine (ATG) for a lysine (AAG) at residue 179, was inserted into the Klenow-blunted NheI and PmeI sites of expression vector pUSEamp(+) (Upstate Biotechnology, Charlottesville, VA) with the cytomegalovirus promoter. The IEC-6 cells were transfected with the pUSEampDNAkt or pUSEamp(+) vectors containing no DNMAkt cDNA by using the Lipofectamine kit and performed as per the manufacturer protocol (Invitrogen, Carlsbad, CA). After the 3 h incubation, the transfection medium was replaced by the standard growth medium containing 5% FBS for 2 days before exposure to the selection medium. These transfected cells were selected for Akt integration by incubation with the selection medium containing 0.6 mg/ml G418, and clones resistant to the selection medium were isolated, cultured, and screened for DNMAkt expression by Western blot analysis using the specific anti-Myc antibody. The characterization studies were conducted and published earlier [15].
RNA Interference
The siRNA (small interfering RNA) were designed to cleave specifically the EDG1 (S1P1) mRNA (siEDG1); these, as well as the control (‘scrambled’) C-siRNA which had no specific homology to any one gene, were synthesized and purchased from Dharmacon Inc (Lafayette, CO). For each 60 mm cell culture dish, 25 μl of the 20 μM stock siRNA EDG1 or C-siRNA was mixed with 500 μl of Opti-MEM medium (Invitrogen). This mixture was gently added to a solution containing 10 μl LipofectAMINETM 2000 (Invitrogen) in 500 μl Opti-MEM medium. The solution was incubated for 20 min at room temperature and gently overlaid on to the monolayer of cells in 4 ml of medium, and cells were harvested for various assays after 48 h of incubation.
Western Blot Analysis
Cell samples—dissolved in ice-cold modified radioimmunoprecipitation (RIPA) buffer (1 × PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1 μg/ml Aprotinin, 1 mM sodium orthovanadate)—were centrifuged at 12,000 rpm for 15 min at 4°C. The protein concentration of the supernatant was measured and then boiled for 5 min, and each lane was loaded with 20 μg of protein equivalent and then subjected to electrophoresis on 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels. After the transfer of protein onto nitrocellulose filters, the filters were incubated for 1 h in 5% nonfat dry milk in 1× phosphate-buffered saline (PBS)/Tween 20 [PBS-T: 15 mM NaH2PO4, 80 mM Na2HPO4, 1.5 M NaCl (pH 7.5), and 0.5% (v/v) Tween 20]. Immunological evaluation was performed for 1 h in 5% nonfat dry milk containing specific antibodies against caspase-3, p-Akt, total-Akt, and pGSK-3. The filters were subsequently washed with 1× PBS-T and incubated for 1 h with the secondary antibody conjugated to horseradish peroxidase. After extensive washing with 1× PBS-T, the immunocomplexes on the filters were reacted for 1 min with chemiluminescent reagent (NEL-100, DuPont NEN). Finally, the filters were placed in a plastic sheet protector and exposed to autoradiography film for 30 or 60 s.
Measurement of Caspase-3 Activity
The caspase-3 activity was measured by using the CaspACE Assay System Colorimetric (Promega) and performed according to the manufacturer's protocol. Briefly, cells were treated with TNF-α (20 mg/ml) and CHX (25 μg/ml) for 4 h, washed with ice-cold D-PBS, and scraped from the dishes. The collected cells were washed with D-PBS and then lysed in ice-cold cell lysis buffer (R&D Systems, Inc.). The assay for caspase-3 activity was carried out in a 96-well plate. In each well, there were 60 μg total protein, 32 μl Caspase Assay buffer, 2 μl caspase-3 colorimetric substrate, and caspase-specific peptide that is conjugated to a chromogen, p-nitroanilide (p-NA). The 96-well plate was incubated at 37°C for 4 h, during which the caspase-3 in the sample presumably cleaved the chromophore p-NA from the substrate molecule. Absorbance readings at 405 nm were made after the incubation, with the caspase-3 activity being directly proportional to the color reaction. Protein levels of each sample were determined by the method described by Bradford et al. [29].
Statistical Analysis
All data are expressed as mean ± standard error (SE) from six dishes or three separate experiments. The significance of the difference between means was determined by analysis of variance (ANOVA). Immunoblotting and Annexin V staining were repeated three times.
Results
Effect of S1P on the Apoptotic Response of IEC-Cdx2L1(Cdx-2) Cells
To examine the apoptotic response of intestinal epithelial cells we utilized TNF-α/CHX-induced apoptosis as a model. TNF-α is a pro-inflammatory cytokine which potently interacts with intestinal epithelial cells, and has been widely utilized and reported as a biological apoptotic inducer [7, 21, 30-32]. CHX is added because, in several cell types, including intestinal epithelial cells, the inhibition of de novo protein synthesis is required for the cytotoxic effects of TNF-α [21, 30, 32, 33]. The TNF-α/CHX apoptosis model is a very applicable model, especially in IECs, as various pathological and physiological conditions in the gut mucosa are associated with both release of TNF-α and downregulation of protein synthesis [1, 2, 34, 35].
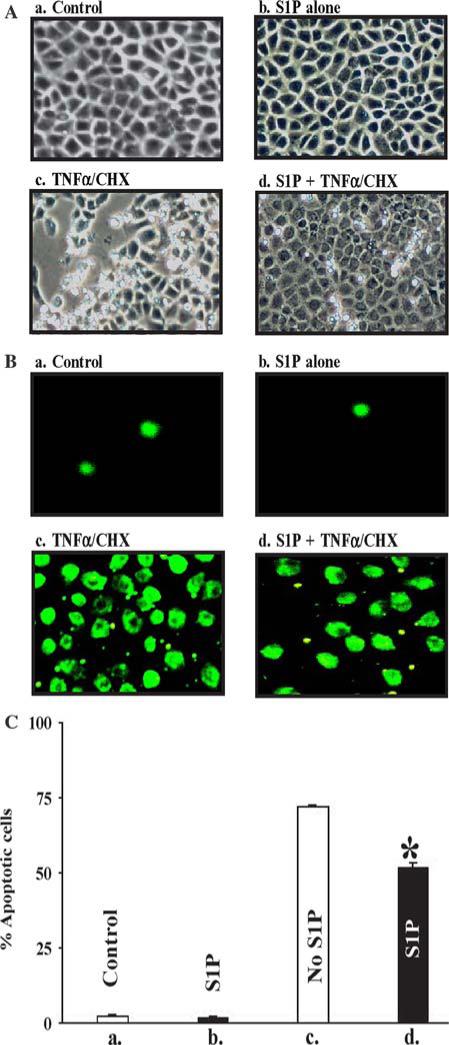
To determine the effect of S1P in protecting IECs from apoptotic stimuli, Cdx-2 cells were exposed to TNF-α/CHX as described in the “Methods” section, with or without S1P exposure (2.5 μM) for 1 h prior to the apoptosis challenge. This dose of S1P, as presented in the “Results” section, was found to be the optimal dose in dose-response experiments for a range encompassing what has been reported by several authors in other cell systems [5, 28]. TNF-α (20 mg/ml) together with CHX (25 μg/ml) induced typical apoptotic cell death in the control cells under morphologic analysis, and S1P treatment showed lesser percentage of cells undergoing apoptosis (Fig. 1). This was re-examined utilizing confocal microscopy and Annexin V staining under the same conditions, and this confirmed the protective effect of S1P for exposure to TNF-α/CHX (Fig. 1).
Fig. 1.
S1P has a protective effect from apoptotic stimuli in intestinal epithelial cells. (A) Cells were photographed before (a and b) and at 4 h after (c and d) exposure to TNF-α/CHX (doses of 20 ng/ml and 25 mcg/ml, respectively). Panels a and c are of control cells, and the right panels (b and d) are of cells that underwent a 1 h pretreatment of S1P (2.5 mcM). At 4 h after exposure to TNF-α/CHX the S1P-pretreated cells (d) were less susceptible to apoptosis compared with the control (c); the picture is representative of findings from three separate experiments. (B) The next four panels are the exact same conditions with Annexin V staining of the cells, demonstrating less apoptotic activity in the S1P-exposed cells. (C) Graphical depiction of the effect of S1P (2.5 mcM) on apoptosis by exposure to TNF-α/CHX as presented in (A); values are mean ± SE of data from three experiments. * P < 0.05 compared with control and S1P cells treated with TNF-α/CHX, respectively
Effect of S1P on Caspase-3 Protein Levels and Caspase-3 Activity in Cdx-2 Cells
Our previous studies have demonstrated that activated caspase-3 mediates apoptosis in IECs [15], so we next investigated whether S1P exposure affected protein levels of caspase-3, as well as caspase-3 activation. Cdx-2 cells were grown to confluence and exposed to TNF-α/CHX for 4 h with or without 1 h of pre-incubation with S1P at varying concentrations. As shown in Fig. 2A, S1P treatment decreased levels of caspase-3 protein compared with control cells. Dose-response and time-course experiments determined that this effect was maximal after 1 h of pretreatment of S1P at a dose of 2.5 μM (data not shown). There was no appreciable difference in the levels of procaspase-3, which is cleaved to allow formation of caspase-3.
Fig. 2.
S1P is associated with a decrease in caspase-3 levels and caspase-3 activity. (A) Cdx cells grown to confluence and exposed to control media or S1P-containing media (2.5 mcM) for 1 h. Western blotting was performed to assess for caspase-3. After 4 h exposure to TNF-α/CHX (doses of 20 ng/ml and 25 mcg/ml, respectively), the S1P-treated cells showed decreased levels of caspase-3 protein, with no appreciable difference in procaspase-3. Actin was also measured to control for loading. Results depicted below are representative of this experiment run in triplicate. In (B), caspase-3 activity was measured under identical conditions. Results shown are the average of four experiments. * P < 0.05 compared with control cells (c) also treated with TNF-α/CHX
Similar results were obtained when the caspase-3 activity was measured (Fig. 2B). Confluent cells were exposed to control conditions or 1 h S1P (2.5 μM) before apoptotic challenge by TNF-α/CHX as above. S1P-treated cells demonstrated less caspase-3 activity at 4 h, consistent with the prior results.
Effect of S1P on Akt Phosphorylation and Akt Kinase Activity in IECs
To determine the involvement of Akt activation in the cellular effects of S1P, we examined changes in the levels of Akt phosphorylation after exposure to S1P. S1P treatment for 1 h (2.5 μM) followed by exposure to TNF-α/CHX resulted in a ∼30% increase in phosphorylated Akt (p-AKT) protein versus control by densitometry, with no change in its total protein amounts (Fig. 3).
Fig. 3.
Changes in protein levels of phosphorylated Akt (p-Akt), total Akt (T-Akt) to apoptotic stimuli in control and S1P-exposed cells. Cells were grown to confluence and exposed to control or S1P-containing media and harvested after 4 h exposure to TNF-α/CHX. Whole-cells lysates were collected and 20 μg were applied to each lane as described in the “Methods” section. The membrane was probed for p-Akt (∼60 kDa), then stripped and examined for T-Akt (∼60 kDa), p-GSK-3 (∼51 kDa), and also potential upstream proteins for Akt including P-PDK1, total PDK1, P-PTEN, and total PTEN, as pictured. Actin (∼42 kDa) immunoblotting was performed as an internal control for loading
Increased levels of p-Akt in S1P-treated Cdx-2 cells were paralleled by an increase in the phosphorylation of exogenous glycogen synthase kinase-3 (GSK-3) (Fig. 3). The levels of pGSK-3 were increased ∼30% versus control by densitometry after exposure to TNF-α/CHX following S1P pretreatment. Other potential phosphorylated downstream targets of Akt were assessed, including Bad and FKHR, however no changes in these protein levels were seen in relation to S1P exposure (data not shown).
To determine the involvement of phosphoinositide-dependent protein kinase 1 (PDK1) in the effects of S1P-induced Akt activation, we examined total PDK1 and phosphorylated PDK1 (pPDK1) after treatment with S1P. S1P-exposed cells demonstrated an increase in pPDK1 of ∼40% with no change in levels of total PDK1 (Fig. 3). There was no change observed in levels of PTEN (phosphatase and tensin homolog deleted on chromosome 10) or pPTEN, which in some cellular pathways has been shown to affect Akt activation via dephosphorylation of upstream products in Akt-activation pathways [36].
Effect of S1P on Phosphatidylinositol 3-Kinase (PI3K) Activity During Apoptotic Stimulation with TNF-α/CHX in Cdx-2 Cells
To determine the involvement of PI3K signaling in S1P regulation of apoptosis, a specific PI3K inhibitor, LY294002, was utilized in this study. We have previously shown in polyamine-deficient IEC-6 cells that LY294002 inhibits activation of Akt, however in that study only high doses (50 μM) of this compound demonstrated any appreciable change in caspase-3 protein levels in those cells.
As shown in Fig. 4A, exposure of Cdx cells to LY294002 allowed for dose-dependent decreases in Akt activation, as measured by decreased p-Akt, which could not be reversed by concomitant exposure to S1P.
Fig. 4.
Effect of treatment with LY294002 on protein levels of p-Akt and caspase-3 in S1P-exposed cells, and within the apoptotic response to TNF-α/CHX. Cells were exposed to varying levels of LY294002 in the presence or absence of S1P for 4 h. Harvesting and Western blotting were performed as described previously. (A) Membranes were probed for p-Akt and T-Akt, with actin as an internal control. (B) Membranes were probed for procaspase-3 and caspase-3. (C) Images of cells after 3 h of exposure to TNF-α/CHX. The results are depicted in (D) as total number of apoptotic cells at 3 h. * P < 0.05 compared with S1P-treated cells
Similarly, LY294002 treatment also demonstrated dose-dependent increases in caspase-3 protein levels, which was also not influenced by S1P exposure (Fig. 4B). These results indicate the S1P activation of p-Akt and repression of caspase-3 suppression occur through a pathway that is PI3K dependent.
Figure 4C depicts the effect of LY294002 on preventing the protective effect of S1P on TNF-α/CHX-induced apoptosis. At 3 h cells with PI3K inhibition undergo apoptosis at a rate greater than control cells, for which S1P is unable to offer a protective effect. Additional studies demonstrated that LY294002 alone does not itself have a toxic effect on the cells (data not shown). The results of these studies are depicted graphically in Fig. 4D.
Changes in the Effect of S1P in Stable DNMAkt-Transfected IEC Cells
To further define the role of activated Akt in the S1P-mediated suppression of apoptosis, stable dominant negative Akt mutant (DNMAkt) transfected cells were developed and characterized, as described in the “Methods” section. Figure 5a shows their characterization in standard Western blots versus control cells for the Myc-His tag, p-Akt, and t-Akt. These clones demonstrated reduced levels of p-Akt, and upon exposure to TNF-α/CHX, S1P exposure did not result in any difference in caspase-3 protein levels (data not shown).
Fig. 5.
(A) Representative Western blots in control cells and three clones (C1, C2, and C4) of DNM-Akt for the Akt proteins, the Myc tag, and actin. Transfection was performed with the expression vector containing DNM-Akt cDNA by using a LipofectAMINE kit. DNM-Akt was constructed, containing an Myc tag at the 3′ end of the Akt open reading frame and cloned to an expression pUSEamp(+) vector as previously described [15]. (B) Changes in TNF-α/CHX-induced apoptosis for clone 4 and nontransfected cells, both with and without S1P exposure (2.5 mcM). Apoptosis was measured 4 h after treatment with TNF-α/CHX and results are depicted graphically in (C) as the percentage of apoptotic cells after the various treatments. Values are mean ± SE for three experiments. * P < 0.05 versus control cells
In the DNMAkt clones, S1P was unable to offer any protective effect versus control cells upon exposure to the TNF-α/CHX apoptotic stimulation. Figure 5b depicts this experiment of control cells and DNMAkt clone 4 before and 4 h after exposure to TNF-α/CHX. The results are depicted graphically in Fig. 5c; S1P confers a protective effect in normal IEC cells, however it does not offer any protective effect to TNF-α/CHX-induced apoptosis in DNMAkt cells.
Effect of EDG-1 Receptor Interference on S1P Suppression of Apoptosis
To determine whether the S1P-mediated protective effect on apoptosis was through receptor-mediated events, we examined the effect of inhibition of EDG-1 (S1P-1) expression using siRNA specifically targeting EDG-1 mRNA (siEDG-1) on S1P's actions. We first demonstrated that IECs expressed EDG-1 (Fig. 6a) but not EDG-3 (data not shown). The siRNAs were specific mRNAs designed to cleave EDG-1 mRNA through activation of endogenous RNAse H. Exposure to the siEDG-1 for 48 h inhibited the expression of EDG-1 (Fig. 6). Transfection with control siRNA at the same concentration showed no inhibitory effect on EDG-1 expression.
Fig. 6.
Silencing of EDG-1 receptor using siRNA. (A) Representative Western blots for cells exposed to 48 h of either control conditions or containing control siRNA (Scr. siRNA, 100 mcM) or siEDG-1 using LipofectAMINE. Control cells show the presence of basal levels of the EDG-1 protein, indicative of their presence in these cells. Cells exposed to both 50 and 100 μM siEDG1 displayed diminished levels of the EDG-1 protein, whereas exposure to negative siRNA (‘scrambled’) did not affect the basal levels of this protein. (B) Changes in caspase-3 levels after knockdown of the EDG-1 receptor. Cells at 0 h showed no caspase-3 protein as expected (a and b). After 4 h exposure to TNF-α/CHX (20 ng/ml and 25 mcg/ml, respectively), control cells demonstrated caspase-3 levels (c) that were significantly reduced with S1P treatment (d). However S1P had no effect with silencing of the EDG-1 receptor (e), but maintained this effect when transfected with control siRNA (f). The results of this experiment are depicted graphically in (C). * P < 0.05 versus c. (TNF-α/CHX ‘control’ cells) + P < 0.05 versus S1P + TNF-α/CHX (d). # P < 0.05 versus (e)
We next tested whether knockdown of the EDG-1 receptor with the specific siRNA would interfere with S1P-induced decrement in caspase-3 levels induced by TNF-α/CHX. As shown in Fig. 6B, Cdx-2 cells with suppression of EDG-1 receptor levels did not respond to S1P, and caspase-3 levels were equivalent to those of control cells. S1P was still able to affect cells that were exposed to control siRNA, and these cells showed decreased caspase-3 levels. The results of these Western blots are depicted graphically in Fig. 6C.
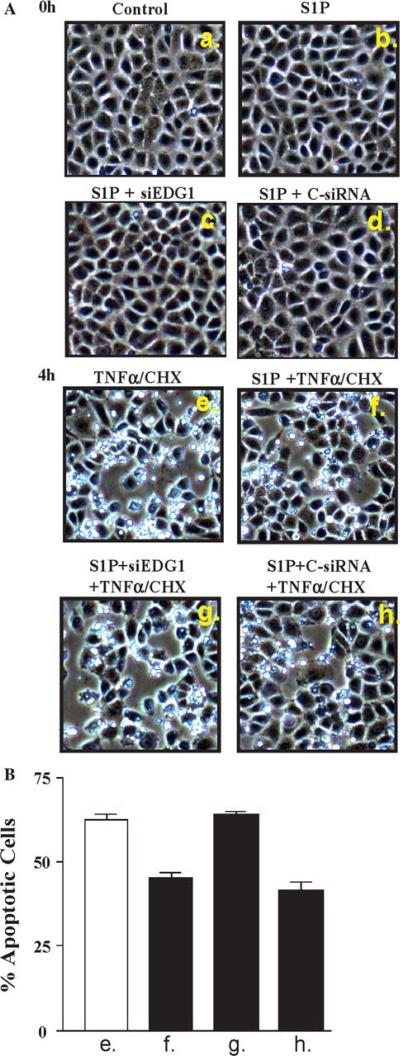
Finally, we tested whether silencing of the EDG-1 receptor would affect the S1P-mediated protective effect on apoptosis. Depicted in Fig. 7a are Cdx-2 control and siRNA transfected cells both before and after 4 h exposure to TNF-α/CHX. The 4 h results are graphically depicted in Fig. 7b, and show that S1P-exposed cells are resistant to apoptosis. This effect is lost with 48 h of transfection with siEDG-1, however this effect is intact with transfection with control siRNA (siScramble).
Fig. 7.
Apoptosis with silencing of EDG-1. Specific siRNA to EDG-1 (siEDG1) was used in the same conditions as those of Fig. 6. Cells were exposed to 48 h of either control conditions of siRNA (Scr. siRNA 100 mcM) or siEDG-1 using LipofectAMINE. (A) At 0 h there was no evidence of apoptosis. Shown in (a) and (b) are control and S1P cells, and in (c) and (d) S1P exposure of cells that had been transfected with siEDG1 and siScramble, respectively. Panels (e) through (h) show these same four groups photographed after 4 h treatment with TNF-α/CHX (20 ng/ml and 25 mcg/ml, respectively). S1P-exposed cells (f) show significantly less apoptosis compared with control cells (e). This effect is lost with siEDG1 (g), whereas control siRNA (h) is unchanged versus (f). These results are depicted graphically in (B)
Discussion
Apoptosis plays a critical role in intestinal epithelial cell homeostasis, and an imbalance between cell proliferation and apoptosis has significant physiological consequences. The exact mechanisms that contribute to this process still remain unclear at the cellular and molecular levels. There is convincing evidence in other organ systems that the bioactive sphingolipid S1P plays a protective role for cells against apoptosis [5, 11, 12, 37-39]. In the present study, we show that S1P played a protective role in differentiated IECs. IECs that were exposed to S1P showed greater resistance to TNF-α/CHX-mediated apoptosis (Fig. 1). S1P-exposed cells also showed reduced levels of caspase-3 protein and caspase-3 activity after exposure to the apoptotic stimulator TNF-α/CHX (Fig. 2). This protective effect of S1P was also associated with activation of Akt (Fig. 3), whereas inhibition of Akt by overexpression of DNMAkt prevents the protective effect of S1P to apoptotic stimuli (Fig. 5). These findings suggest that S1P has an antiapoptotic role in differentiated intestinal epithelial cells.
It has been shown that S1P acts as an important cell survival mediator in several organ systems and in response to multiple stimuli [4, 11]. S1P is a bioactive sphingolipid that has been shown in recent literature to have strong and wide-ranging effects at the cellular level. These actions include regulation of cell adhesion and barrier function, cell proliferation, differentiation, migration, and resistance to apoptosis. S1P is rather ubiquitous, as it derives from conversion of sphingolipids, which are constituents of eukaryotic cell membranes [10, 11]. Metabolism of the sphingolipids yields a variety of biologically active metabolites, including ceramide, sphingosine, and S1P. For the most part, ceramide and sphingosine have opposing effects (proapoptotic, for example) to S1P. The level of S1P is tightly regulated, in part by the enzyme sphingosine kinase type 1 (SK1), which creates S1P via phosphorylation of sphingosine, and to a lesser degree of other enzymes such as sphingosine phosphatase and sphingosine lyase [4, 10, 40].
Although S1P is created though hydrolysis of the cell membrane and regulates a number of pathways as an intracellular second messenger, it is now believed also to have an effect extracellularly when it interacts with cell surface receptors [41]. These receptors, the endothelial differentiation gene (EDG) receptors, are G-protein coupled receptors (GPCR) that are present in a wide array of tissues including neurons, myocytes, leukocytes, and endothelial cells. The S1P pathway through the EDG receptors mandates a source of S1P release, and one common source believed to supply this is platelet release, although autocrine and paracrine release has also been shown [11]. There is strong evidence in support of S1P mediating its effects by both a direct second messenger intracellularly and via downstream effects of the EDG receptors.
The role of S1P in apoptosis has been well studied, and S1P has been found in several cellular systems to be a strong suppressor of apoptosis [11, 12]. Forced expression of S1P or of SK1 (resulting in increased S1P) has shown increased survival in several cell models under exposure to conditions that would usually result in apoptosis [5], and colon cancers have been shown in one study to possess higher SK1 levels [42]. A recent publication also indirectly demonstrated a role for S1P in GI apoptotic regulation, as they showed reduced levels of sphingosine lyase in colorectal cancers versus normal intestinal tissues [43]. S1P appears to directly stimulate MAP kinase antiapoptotic pathways, leading to Akt activation and eventual inhibition of several proteins in the caspase family, all resulting in cell survival [11]. The results shown in Fig. 1 clearly show that S1P-treated cells underwent less apoptosis upon exposure to the apoptotic stimulus TNF-α/CHX. We have previously used the TNF-α/CHX model of apoptosis in intestinal epithelial cells [15, 32, 44] and chose this model again for the present study. The advantages of it include that (a) TNF-α is widely utilized as a stimulator of apoptosis and has a potent effect on the intestinal epithelial cells [32], (b) inhibition of protein synthesis is necessary to see the cytotoxic effects of TNF-α [45], and (c) release of TNF-α in the intestinal mucosa is often seen in synchrony with inhibition of protein synthesis [46]. Because of these factors this model is the most appropriate model for the present study.
In this study we examined the Cdx-2 cells, which represent a more differentiated cell line more representative of villus cells than crypt cells. Although altered apoptosis at either crypt or villus could have drastic physiologic consequence, the latter was chosen since the majority of cellular loss at the villus tips of the small intestine and at the colonic luminal surface is via apoptosis.
The results presented in Fig. 2 provide evidence that S1P regulates apoptosis via activation of Akt kinase activity. This activated Akt was associated with an increased resistance to TNF-α/CHX-induced apoptosis, whereas silencing of Akt by the ectopic expression of the dominant negative Akt mutant (Fig. 5) prevented this resistance to apoptosis. These findings are consistent with those of previous reports that have found that activated Akt suppresses apoptotic cell death in a number of various cell types, including IECs [15, 19, 20]. Akt is an important mediator of cell cycle progression and also suppresses apoptosis. Akt exists in three isoforms (Akt1, Akt2, and Akt3) that demonstrate ∼80% sequence homology [47]. This includes an N-terminal pleckstrin homology domain, a C-terminal regulatory region, and a catalytic domain between them. Akt activation requires phosphorylation at two sites, at Thr308 in the catalytic domain and at Ser473 in the regulatory region [48]. The exact downstream effects of Akt activation are not known. Akt has been postulated for promotion of cell survival, but also cell proliferation and cell cycle progression [49-52]. Although Akt has several potential targets, one popular pathway is via phosphorylation of GSK-3β, which inactivates it and prevents this enzyme from phosphorylating cyclin D, preventing its degradation and/or transport from nucleus to cytoplasm [49, 53, 54]. Figure 3 shows that S1P-treated cells express increases in active GSK-3, presumable via activation of Akt.
Activation of Akt has been reported in several systems as a downstream event of G protein coupled receptor activation [15, 55, 56], including S1P-mediated events through its GPCR, the EDG receptor family [56]. Although reports have mentioned several signaling pathways resulting from EDG-1 activation, including Rac1 and Src [55], the PI3K signaling pathway is most closely associated with Akt activation, which is described in some reports to be exclusively responsible for Akt activation [50, 55]. Data shown in Fig. 4 show that treatment with the specific PI3K inhibitor LY294002 blocks both activation of Akt and suppression of caspase-3 activity, indicating that PI3K activation is an upstream regulatory event for Akt activation. Others have previously reported that PI3K inhibition blocks growth-factor-induced Akt activation [18], and that upregulated PI3K activity leads to Akt activation independent of growth factor stimulation [20]. PI3K activation leads to phosphorylation of Akt at Thr308 by PDK1, and quite possibly also phosphorylation of Akt at Ser473 by PDK2, although some have also reported that this is performed by an unidentified kinase or via autophosphorylation [48, 57]. As shown in Fig. 3, S1P application to cells leads to an increase in phosphorylated PDK1 when challenged by apoptotic stimuli.
The mechanism by which S1P exerts its effects has been under debate for several years. It is traditionally considered as an intracellular second messenger [58], and inherent to this theory is that the intracellular enzyme sphingosine kinase type 1 (SK1) regulates the S1P levels tightly and serves as the critical regulator of the effects of S1P [59]. However, S1P also acts as a ligand for cell-surface GPCRs [7, 58], initiating a powerful cascade of downstream events; those who support this pathway also suggest that, if SK1 is an important regulator of S1P, the downstream effects are most likely a paracrine-autocrine effect of the S1P on the GPCRs. Similarly, in order to help maintain the critical balance in cell numbers and of apoptotic cells, S1P is released to help neighboring cells survive the same inflammatory stimulus [60]. Data from the present study demonstrates that silencing of the EDG-1 receptoxr using specific siRNA did at least partially abrogate the effect of S1P on reducing caspase-3 formation. Of interest is that we did not detect EDG-3 (S1P-3) receptor protein in the Cdx cells. The EDG-1 receptor is generally considered the predominant receptor enacting the effects of S1P, and in several publications, especially those regarding pulmonary barrier integrity [28], the EDG-3 receptor was not found to be relevant. It is worth noting that Kohno et al. [10] did detect the presence of both EDG-3 and EDG-5 (S1P-3 and S1P-2) in intestinal tissues, although they had a much lesser influence on intestinal cell physiology. EDG-1 has been thought to be the predominant receptor for apoptotic regulation by S1P, and tumors that overexpress the EDG-1 receptor are found to be more resistant to chemotherapeutic agents [41, 61]. Our data suggest that the protective effect of S1P involves, at least partially, the EDG-1 receptor. Whether additional EDG receptor subtypes are involved or if other mechanisms including alterations in SK1 activity levels are responsible remain unknown and are the goals of future studies.
In summary, these results indicate that S1P suppresses apoptosis in intestinal epithelial cells. The mechanism of this effect involves Akt activation through a PI3K-dependent pathway. The activated Akt induces phosphorylation of GSK-3 and suppression of caspase-3 activity. Inactivation of the Akt activation, either through siEDG-1 silencing of the GPCRs, inactivation of PI3K by LY294002, or silencing of Akt itself through the DNMAkt stable transfected cell, diminished responsiveness to the protective effect of S1P. This study provides new information showing that S1P has a beneficial effect on IEC survival, and these results along with the ubiquitous nature of this molecule suggest a potentially powerful role in therapeutics.
Acknowledgments
Research for this paper was supported by the Research Career Development program of the Department of Veterans’ Affairs.
Contributor Information
Jose Greenspon, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA.
Ruiyun Li, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA; Baltimore Veterans Affairs Medical Center, Baltimore, MD, USA.
Lan Xiao, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA; Baltimore Veterans Affairs Medical Center, Baltimore, MD, USA.
Jaladanki N. Rao, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA Baltimore Veterans Affairs Medical Center, Baltimore, MD, USA.
Bernard S. Marasa, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, USA.
Eric D. Strauch, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA
Jian-Ying Wang, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA; Baltimore Veterans Affairs Medical Center, Baltimore, MD, USA; Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, USA.
Douglas J. Turner, Department of Surgery, Cell Biology Group, Baltimore Veterans Affairs Medical Center, University of Maryland School of Medicine, 10 N. Greene Street, Baltimore, MD 21201, USA Baltimore Veterans Affairs Medical Center, Baltimore, MD, USA.
References
- 1.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15:82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- 3.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel S, Milstien S. Sphingosine–1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. doi:10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 5.Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta. 2002;1585:153–162. doi: 10.1016/s1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 6.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, et al. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. doi:10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 7.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–1085. doi: 10.1002/jcb.20088. doi:10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 8.Thamilselvan V, Li W, Sumpio BE, Basson MD. Sphingosine–1-phosphate stimulates human caco-2 intestinal epithelial proliferation via p38 activation and activates erk by an independent mechanism. In Vitro Cell Dev Biol Anim. 2002;38:246–253. doi: 10.1290/1071-2690(2002)038<0246:SPSHCI>2.0.CO;2. doi:10.1290/1071−2690(2002)038≤0246:SPSHCI≥2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Yatomi Y, Welch RJ, Igarashi Y. Distribution of sphingosine 1-phosphate, a bioactive sphingolipid, in rat tissues. FEBS Lett. 1997;404:173–174. doi: 10.1016/s0014-5793(97)00121-x. doi:10.1016/S0014−5793(97)00121-X. [DOI] [PubMed] [Google Scholar]
- 10.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23. doi: 10.1128/MCB.02341-05. doi:10.1128/MCB.02341−05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 12.Suomalainen L, Hakala JK, Pentikainen V, Otala M, Erkkila K, Pentikainen MO, et al. Sphingosine-1-phosphate in inhibition of male germ cell apoptosis in the human testis. J Clin Endocrinol Metab. 2003;88:5572–5579. doi: 10.1210/jc.2003-030776. doi:10.1210/jc.2003−030776. [DOI] [PubMed] [Google Scholar]
- 13.Johnson LR. Regulation of gastrointestinal mucosal growth. Physiol Rev. 1988;68:456–502. doi: 10.1152/physrev.1988.68.2.456. [DOI] [PubMed] [Google Scholar]
- 14.Brazil DP, Hemmings BA. Ten years of protein kinase b signalling: a hard akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. doi:10.1016/S0968−0004(01)01958−2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, et al. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem. 2004;279:22539–22547. doi: 10.1074/jbc.M314337200. doi:10.1074/jbc.M314337200. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Wang X, Hernandez A, Hellmich MR, Gatalica Z, Evers BM. Regulation of trail expression by the phosphatidylinositol 3-kinase/akt/gsk-3 pathway in human colon cancer cells. J Biol Chem. 2002;277:36602–36610. doi: 10.1074/jbc.M206306200. doi:10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- 17.Suhara T, Magrane J, Rosen K, Christensen R, Kim HS, Zheng B, et al. Abeta42 generation is toxic to endothelial cells and inhibits enos function through an akt/gsk-3beta signaling-dependent mechanism. Neurobiol Aging. 2003;24:437–451. doi: 10.1016/s0197-4580(02)00135-5. doi:10.1016/S0197−4580(02)00135−5. [DOI] [PubMed] [Google Scholar]
- 18.Edwards E, Geng L, Tan J, Onishko H, Donnelly E, Hallahan DE. Phosphatidylinositol 3-kinase/akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002;62:4671–46777. [PubMed] [Google Scholar]
- 19.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serinethreonine protein kinase akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. doi:10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 20.Franke TF, Kaplan DR, Cantley LC. Pi3k: downstream aktion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. doi:10.1016/S0092−8674(00)81883−8. [DOI] [PubMed] [Google Scholar]
- 21.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. doi:10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 22.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. doi:10.1016/S0092−8674(00)80595−4. [DOI] [PubMed] [Google Scholar]
- 23.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JX, et al. Activation of k(+) channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol. 2002;282:C885–C898. doi: 10.1152/ajpcell.00361.2001. [DOI] [PubMed] [Google Scholar]
- 25.Rao JN, Li J, Li L, Bass BL, Wang JY. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin ii. Am J Physiol. 1999;277:G1149–G1158. doi: 10.1152/ajpgi.1999.277.6.G1149. [DOI] [PubMed] [Google Scholar]
- 26.Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, Yuan JX, et al. Ca2+-rhoa signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol. 2001;280:C993–C1007. doi: 10.1152/ajpcell.2001.280.4.C993. [DOI] [PubMed] [Google Scholar]
- 27.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. doi:10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by s1p1 receptor, pi3 kinase, tiam1/rac1, and alpha-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. doi:10.1096/fj.05−3928com. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. doi:10.1016/0003−2697(76)90527−3. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya S, Ray RM, Viar MJ, Johnson LR. Polyamines are required for activation of c-jun nh2-terminal kinase and apoptosis in response to tnf-alpha in iec-6 cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G980–G991. doi: 10.1152/ajpgi.00206.2003. [DOI] [PubMed] [Google Scholar]
- 31.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)–8-phenyl-4 h–1-benzopyran-4-one (ly294002). J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 32.Li L, Rao JN, Bass BL, Wang JY. Nf-kappab activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G992–G1004. doi: 10.1152/ajpgi.2001.280.5.G992. [DOI] [PubMed] [Google Scholar]
- 33.Zou T, Rao JN, Guo X, Liu L, Zhang HM, Strauch ED, et al. Nf-kappab-mediated iap expression induces resistance of intestinal epithelial cells to apoptosis after polyamine depletion. Am J Physiol Cell Physiol. 2004;286:C1009–C1018. doi: 10.1152/ajpcell.00480.2003. doi:10.1152/ajpcell.00480.2003. [DOI] [PubMed] [Google Scholar]
- 34.Potten CS. Epithelial cell growth and differentiation. Ii. Intestinal apoptosis. Am J Physiol. 1997;273:G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- 35.Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- 36.Besson A, Robbins SM, Yong VW. Pten/mmac1/tep1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605–611. doi: 10.1046/j.1432-1327.1999.00542.x. doi:10.1046/j.1432−1327.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 37.Castillo SS, Teegarden D. Sphingosine-1-phosphate inhibition of apoptosis requires mitogen-activated protein kinase phosphatase-1 in mouse fibroblast c3h10t 1/2 cells. J Nutr. 2003;133:3343–3349. doi: 10.1093/jn/133.11.3343. [DOI] [PubMed] [Google Scholar]
- 38.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD. Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet beta-cells. Endocrinology. 2006;147:4705–4712. doi: 10.1210/en.2006-0456. doi:10.1210/en.2006−0456. [DOI] [PubMed] [Google Scholar]
- 40.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through s1p lyase inhibition and disruption of s1p gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. doi:10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 41.Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer. 2006;95:1131–1135. doi: 10.1038/sj.bjc.6603400. doi:10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 43.Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci USA. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. doi:10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang HM, Keledjian KM, Rao JN, Zou T, Liu L, Marasa BS, et al. Induced focal adhesion kinase expression suppresses apoptosis by activating nf-kappab signaling in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1310–C1320. doi: 10.1152/ajpcell.00450.2005. doi:10.1152/ajpcell.00450.2005. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. doi:10.1016/S1097−2765(02)00482−3. [DOI] [PubMed] [Google Scholar]
- 46.Loeffler M, Birke A, Winton D, Potten C. Somatic mutation, monoclonality and stochastic models of stem cell organization in the intestinal crypt. J Theor Biol. 1993;160:471–491. doi: 10.1006/jtbi.1993.1031. doi:10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- 47.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. doi:10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, et al. Dual role of phosphatidylinositol-3, 4, 5-trisphosphate in the activation of protein kinase b. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. doi:10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 49.Radeff-Huang J, Seasholtz TM, Chang JW, Smith JM, Walsh CT, Brown JH. Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent akt activation and cyclin d expression. J Biol Chem. 2007;282:863–870. doi: 10.1074/jbc.M601698200. doi:10.1074/jbc.M601698200. [DOI] [PubMed] [Google Scholar]
- 50.Liang J, Slingerland JM. Multiple roles of the pi3k/pkb (akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 51.Testa JR, Bellacosa A. Akt plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. doi:10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim D, Chung J. Akt: Versatile mediator of cell survival and beyond. J Biochem Mol Biol. 2002;35:106–115. doi: 10.5483/bmbrep.2002.35.1.106. [DOI] [PubMed] [Google Scholar]
- 53.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin d1 proteolysis and sub-cellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. doi:10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin d1 nuclear export and cyclin d1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. doi:10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. doi:10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 56.Brizuela L, Rabano M, Gangoiti P, Narbona N, Macarulla JM, Trueba M, et al. Sphingosine–1-phosphate stimulates aldosterone secretion through a mechanism involving the pi3k/pkb and mek/erk 1/2 pathways. J Lipid Res. 2007;48:2264–2274. doi: 10.1194/jlr.M700291-JLR200. doi:10.1194/jlr.M700291-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Toker A, Newton AC. Cellular signaling: Pivoting around pdk-1. Cell. 2000;103:185–188. doi: 10.1016/s0092-8674(00)00110-0. doi:10.1016/S0092−8674(00)00110−0. [DOI] [PubMed] [Google Scholar]
- 58.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. doi:10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 59.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer mcf-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. doi:10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 60.Weigert A, Johann AM, von Knethen A, Schmidt H, Geisslinger G, Brune B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. doi:10.1182/blood-2006−04−014852. [DOI] [PubMed] [Google Scholar]
- 61.Annabi B, Thibeault S, Lee YT, Bousquet-Gagnon N, Eliopoulos N, Barrette S, et al. Matrix metalloproteinase regulation of sphingosine-1-phosphate-induced angiogenic properties of bone marrow stromal cells. Exp Hematol. 2003;31:640–649. doi: 10.1016/s0301-472x(03)00090-0. doi:10.1016/S0301−472X(03)00090−0. [DOI] [PubMed] [Google Scholar]