Abstract
Total parenteral nutrition (TPN) results in intestinal mucosal atrophic changes due to an absence of enteral nutrition; however, the mechanisms responsible for this are not fully understood. It has been shown that bone morphogenetic protein (BMP) activation inhibits intestinal epithelial cell (EC) proliferation. Therefore, we hypothesized that the BMP pathway could be upregulated by TPN. To address this, we randomly assigned mice to receive TPN or to be enterally fed (control) for 7 d. Mucosal EC isolates were harvested from the entire length of small intestine for RNA and protein measurements. Full-thickness, mid-small bowel was processed for histological examination. TPN increased the abundance of BMP2, BMP4, and BMP type II receptor at the RNA and protein levels. Phosphorylation of Smad1, Smad5, and Smad8 also was greater in the TPN group than in the control, which helped to confirm activation of this pathway. Interestingly, the TPN and control groups did not differ in the mRNA expression of the extracellular soluble bmp antagonists, noggin, gremlin, chordin, or follistatin. Compared to the control group, the expression of c-Myc (cellular myelocytomatosis) mRNA was lower, whereas the level of p21WAF1/CIP1 was greater, in the TPN group. Because the BMP family may function through suppression of Wnt-β-catenin signaling, this pathway was also examined. mRNA expression of Wnt 3, Wnt5a, and the Wnt receptor Lrp5 were lower in the TPN group compared to controls. The results suggest that the BMP signaling pathway may be involved in the development of intestinal mucosal atrophy due to TPN administration.
Introduction
Total parenteral nutrition (TPN),6 or the absence of enteral nutrition, leads to intestinal mucosal atrophy, including a significant decline in villus height, loss of epithelial cell (EC) proliferation, and increased EC apoptosis (1,2). Numerous mechanisms may modulate such changes in intestinal homeostasis, including the bone morphogenetic protein (BMP) family and the Wnt-β-catenin signaling pathways. BMP ligands bind to a complex of the BMP receptors, the most prevalent of which are type I (Ia or Ib) and type II, leading to the phosphorylation of the BMP-specific Smad (Smad1, Smad5, and Smad8, abbreviated as Smad 1/5/8 in this paper) and subsequent DNA transcription (3). Mice with a conditional inactivation of BMP type I receptor (Bmpr1a) (4) and mice that overexpress the BMP antagonist noggin in the intestine (5) have an intestinal phenotype resembling juvenile polyposis. The Wnt/β-catenin pathway has an essential role in the maintenance of intestinal epithelial homeostasis. The Wnt/β-catenin pathway is activated in the stem cell region of intestinal crypts during developmental growth of the intestine (6) and β-Catenin is also critical for normal intestinal growth in later life (7). Increased nuclear accumulation of β-catenin promotes intestinal epithelial proliferation (8,9). Interestingly, a major mechanism by which BMP signaling inhibits EC proliferation is via suppression of the Wnt/β-catenin pathway (10). Previous studies from our laboratory have shown that β-catenin and its downstream effector genes, including cyclin D1, are markedly decreased using a mouse TPN model (11). This study also demonstrated that TPN results in a marked decline in phosphorylated-Akt expression and that this may well be responsible for the decline in β-catenin activity. This is quite intriguing in that recent studies have shown that another mechanism for the inhibitory action of BMP may be via a downregulation in the expression of phosphorylated-Akt and the subsequent loss of nuclear translocation of β-catenin (4). Taken together, this supports a potential role for BMP mediating a portion of TPN-associated intestinal atrophy. We hypothesized that the BMP pathway would be upregulated by TPN. We further hypothesized that this upregulation may be a mechanism that leads to the intestinal mucosal atrophy associated with TPN.
Materials and Methods
Parenteral nutrition model
Mice.
C57BL6J male specific-pathogen–free mice (8 wk old) were obtained from Jackson Laboratory and maintained under temperature-, humidity-, and light-controlled conditions. In some experiments, interferon-γ knockout (IFNγ-KO; C57BL/6-ifngtm1Ts, Jackson Labs) were used. Mice initially consumed nonpurified diet (Purina Chow for Rodents) (12) and water ad libitum and were allowed to acclimate. During the administration of i.v. solutions, mice were housed in metabolic cages to prevent coprophagia. The studies conformed to the Guidelines for the Care and Use of Laboratory Animals established by the University Committee on Use and Care of Animals at the University of Michigan and protocols were approved by that committee (no. 7703).
TPN model
Expt. 1: TPN vs. control.
Administration of TPN was performed as previously described (13). An infusion of a crystalloid solution at 4 mL/d was begun after canulation. After 24 h, mice were randomly assigned to 2 groups of 6. The control group received the same i.v. saline solution at 7 mL/d in addition to standard laboratory nonpurified diet and water ad libitum. The TPN groups received an i.v. TPN solution at 7 mL/d. The composition of the TPN solution has been described in detail previously (13) and it contained a balanced mixture of amino acids, fat, and dextrose in addition to electrolytes, trace elements, and vitamins (Supplemental Table 1). The amino acid and i.v. fats were similar as described previously (14). Energy delivery was based on estimates of energy intake by the enteral control group and from previous investigators (15), so that energy delivery was essentially the same in both groups. All mice were killed after 3 d by CO2 asphyxiation. Six additional TPN mice were killed at 7 d to better assess changes in EC proliferation.
Two additional experiments (below) were performed to determine the mechanisms potentially responsible for BMP-related changes.
Expt. 2.
Six mice received TPN with glutamine (TPN+GLN). In this experiment, 2 g glutamine (given as a 2% alanyl-glutamine solution, Sigma-Aldrich) was added to each 100 mL TPN solution. Nitrogen and energy delivery were controlled between each study group (isonitrogenous), similar to that previously described (11). Results were compared to the previous TPN and control groups.
Expt. 3.
Six IFNγ-KO mice administered a TPN solution were compared to IFNγ-KO mice that consumed a nonpurified diet. Mice were killed after 3 d.
Real-time PCR
Methods of RNA isolation, purification, and PCR were identical to those previously described (16). Primers were designed by using an optimization program (Lasergene 6; DNAStar). Real-time PCR was performed using a Rotor-Gene 6000 (Corbett Life Science) and β-actin was used as an internal control for normalization. The primer sequences for the transcripts quantified by this method are shown in Supplemental Table 2.
Western immunoblotting
Antibodies.
The following antibodies were used for Western blot analysis: anti-BMP2 (1:500; Santa Cruz Biotechnology); anti-BMP4 (1:500; Santa Cruz Biotechnology); anti-bmpr1a (1:500; Abgent); anti-phosphorylated-Smad 1/5/8 (P-Smad 1/5/8) (1:1000; Cell Signaling); anti-phosphatase and tensin homolog (PTEN) (1:1000, Cell Signaling); anti-phospho-PTEN (1:1000; Ser380/Thr382/383; Cell Signaling,); anti-phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) (1:1000; Cell Signaling); and anti-P21 WAF1 (1:1000; Santa Cruz Biotechnology). Mouse anti-β-actin antibody was from Sigma. Secondary antibodies including goat anti-mouse horseradish peroxidase, goat-anti-rabbit horseradish peroxidase, and donkey anti-goat IgG horseradish peroxidase (Santa Cruz) were used for Western blot analysis.
Western blot analysis.
Immunoblotting was identical to that previously described (17). Quantification of results was performed using Kodak 1D image quantification software (Kodak). Results of immunoblots are expressed as the expression of proteins relative to β-actin.
EC proliferation assay
An intraperitoneal injection of 5-bromo-2-deoxyuridine (BrdU) (50 mg/kg; Roche Diagnostic) was given to mice 2 h before being killed. Paraffin-embedded sections 5 μm thick were deparaffinized with xylene. Immunohistochemistry was conducted using a BrdU in situ detection kit according to the manufacturer's guidelines (BD PharMingen). Briefly, endogenous peroxidase was quenched with 3% H2O2. Slides were then incubated with biotinylated anti-BrdU Ab in a 1/10 dilution, washed, and then incubated with streptavidin-horseradish peroxidase. Slides were then exposed to diaminobenzidine substrate and counterstained with hematoxylin. An index of the crypt cell proliferation rate was calculated as the ratio of the number of crypt cells incorporating BrdU to the total number of crypt cells. The total number of proliferating cells per crypt was defined as the mean of proliferating cells in 10 crypts (counted at 45× magnification).
Immunohistochemical staining
Formalin-fixed and paraffin-embedded tissue sections (4 μm) were stained using standard immunohistochemistry procedures. The following primary antibodies and dilutions were used: anti-BMP2 (1:100; Santa Cruz Biotechnology), anti-BMP4 (1:50; Santa Cruz), anti-bmpr1a (1:100; Abgent); and anti-P-Smad 1/5/8 (1:100; Cell Signaling). A biotinylated goat-anti-rabbit and donkey anti-goat IgG secondary antibody were used at a dilution of 1:200. The signal was amplified using streptavidin-horseradish peroxidase conjugate (Santa Cruz) and visualized with 3,3-diaminobenzidine substrate followed by hematoxylin.
Statistical analysis
Data are reported as means ± SD. Results were analyzed using a t test for comparison of 2 means and a 1-way ANOVA for more than 2 groups, with a Bonferroni post hoc analysis to test differences. The chi-square test was used for categorical data (Prism software; GraphPad Software). Differences were considered significant at P < 0.05. Data were inspected and approximated a uniform distribution, so nonparametric analyses were not necessary.
Results
Expression of BMP2, BMP4, and BMP7
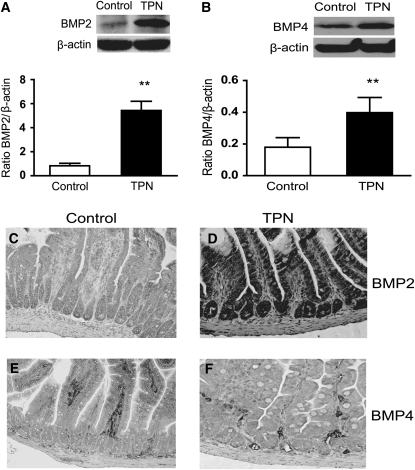
We investigated the expression of the 3 most prevalent BMP ligands in the intestinal mucosa, BMP2, BMP4, and BMP7. We found that after 3 d, the abundance of BMP2 and BMP4 mRNA was 2.4-fold and 2.6-fold greater, respectively, in the TPN group than in controls (P < 0.05; Table 1). This corresponded to the significantly greater abundance of BMP2 and BMP4 proteins in the TPN group as detected by western immunoblots (Fig. 1). Expression of BMP7 mRNA did not differ between the TPN and control groups (data not shown) and thus, immunoblotting was not performed. To further examine BMP expression, immunohistochemical staining was performed to investigate if there was a difference between the groups in the distribution and pattern of expression of these proteins along the crypt/villus axis. BMP2 expression was detected in villi and crypt EC (Fig. 1). The intensity of staining was greater than that of controls along both locations in the epithelium of TPN mice. BMP4 is normally expressed in mesenchymal cells underlying the epithelium. Staining intensity was slightly greater in the TPN group. The predominant area of increased BMP4 was in the base of the villi and crypt region in TPN mice, whereas BMP4 expression was greater in the mid- to distal villus region of control mice (Fig. 1).
TABLE 1.
Abundance of BMP2 and BMP4 mRNA from intestinal mucosa of TPN and control mice after 3 d (Expts. 1–3)1
| Groups | BMP2 | BMP4 |
|---|---|---|
| Expt. 1 | mRNA/β-actin mRNA2 | |
| Control | 4.75 ± 2.25 | 1.90 ± 1.52 |
| TPN | 16.62 ± 2.34# | 6.93 ± 3.08* |
| Expt. 2 | ||
| TPN+GLN | 16.30 ± 1.53# | 7.2 ± 1.42* |
| Expt. 3 | ||
| Control INFγ-KO | 3.93 ± 1.31 | 1.69 ± 1.22 |
| TPN given to IFNγ-KO mice | 15.44 ± 1.66## | 6.71 ± 0.83** |
Values are means ± SD, n = 5–6. Symbols indicate different from controls: *P < 0.05; #P < 0.001 or from control INFγ-KO: **P < 0.05; ##P < 0.001.
From real-time PCR.
FIGURE 1 .
Expression of intestinal mucosal BMP2 (A,C,D) and BMP4 (B,E,F) detected by Western blotting and immunohistochemical staining in control and TPN mice after 3 d (Expt. 1). Values are means ± SD, n = 6–8. **Different from TPN, P < 0.001. Immunohistochemical staining was moderately weak in controls and greater in the TPN group. Staining was predominately in the lower one-third of villi and the crypt region. A color version of this figure is available (Supplemental Fig. 1).
BMP signaling pathway is upregulated with TPN
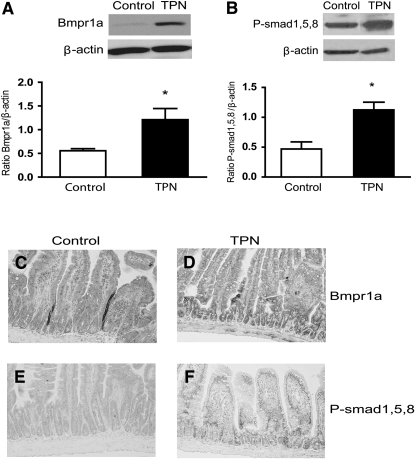
To investigate if the greater abundance of BMP led to an increase in the intracellular BMP signaling pathway, we examined the expression of the BMP receptor and phosphorylation of Smad 1/5/8 in the intestinal mucosa. The abundance of bmpr1a mRNA (Table 2) and protein (Fig. 2A) was greater in TPN mice than in the control group (P < 0.05). However, the mRNA expression of other receptors did not differ between the TPN (bmpr1b, 5.4 ± 1.54; bmpr2, 3.23 ± 0.38) and control group (bmpr1b, 3.73 ± 2.10; bmpr2, 3.45 ± 0.78). Bmpr1a immunoreactivity in TPN mice (Fig. 2D) had a stronger expression in the crypt and villus epithelium in TPN mice compared to controls. BMP signaling in the intestinal epithelium was also assessed by measuring the abundance of P-Smad 1/5/8. P-Smad 1/5/8 immunostaining in TPN mice was predominately located in the proximal one-third of villus EC as well as within the crypt/villus junction and the base of the crypts. Immunoreactivity to P-Smad 1/5/8 was fairly weak in control mice and was much stronger in the TPN group (Fig. 2F). Western blot results showed that the level of the P-Smad 1/5/8 protein was significantly greater in the intestinal mucosa in the TPN group (Fig. 2B).
TABLE 2.
Abundance of mRNA from BMP and Wnt signaling pathways in the small intestinal mucosa of control and TPN mice after 3 d (Expt. 1)1
| Gene symbol | Controls | TPN |
|---|---|---|
| BMP receptors | mRNA/β-actin mRNA2 | |
| BMPr1a | 2.84 ± 2.21 | 17.50 ± 4.83# |
| BMPr1b | 5.40 ± 1.54 | 3.73 ± 2.10 |
| BMPr2 | 3.45 ± 0.78 | 3.23 ± 0.38 |
| BMP antagonists | ||
| Noggin | 3.04 ± 0.78 | 3.20 ± 0.35 |
| Gremlin | 1.39 ± 0.44 | 2.13 ± 0.36 |
| Chordin | 3.91 ± 0.83 | 3.58 ± 0.28 |
| Follistatin | 3.91 ± 1.69 | 5.02 ± 0.94 |
| Wnt signaling pathway | ||
| Wnt3 | 2.62 ± 0.94 | 0.60 ± 0.27* |
| Wnt4 | 7.30 ± 1.57 | 5.75 ± 2.64 |
| Wnt5a | 60.13 ± 6.48 | 17.08 ± 3.58* |
| Wnt6 | 4.08 ± 1.43 | 8.11 ± 1.05 |
| Wnt9b | 6.97 ± 2.42 | 7.90 ± 1.44 |
| Lrp5 | 135.25 ± 30.72 | 33.07 ± 8.21* |
| Related signaling pathways | ||
| PTEN | 0.11 ± 0.02 | 0.12 ± 0.01 |
| c-Myc | 9.40 ± 1.98 | 4.55 ± 1.65* |
| p21waf1/cip1 | 6.67 ± 0.41 | 11.48 ± 1.86* |
| P21 protein | 0.39 ± 0.09 | 0.80 ± 0.05* |
Values are means ± SD, n = 5–6. Symbols indicate different from controls: *P < 0.05; #P < 0.001.
From real-time PCR.
FIGURE 2 .
Expression of intestinal mucosal Bmpr1a (A,C,D) and p-Smad 1/5/8 (B,E,F) as detected by Western blotting and immunohistochemical staining (Expt. 1). Values are means ± SD, n = 6–8. *Different from TPN, P < 0.05. Immunohistochemical staining of Bmpr1a and p-Smad 1/5/8 increased in the TPN group. Immunoreactivity to p-Smad 1/5/8 proteins showed a predominant expression in the lower one-third of villi and in the crypt region. A color version of this figure is available (Supplemental Fig. 2).
Expression of BMP antagonists
BMP activities are controlled in part by specific antagonists (18), which prevent BMP interactions with their specific receptors and counteract the effect of BMP, so that Wnt activity is maintained in the crypt epithelium. The expression of a number of BMP antagonists (noggin, chordin, gremlin, and follistatin) was studied using real-time-PCR (Table 2). Interestingly, the expression of these BMP antagonists did not differ between the TPN and control groups.
BMP signaling pathways
Phosphatase and tensin homolog.
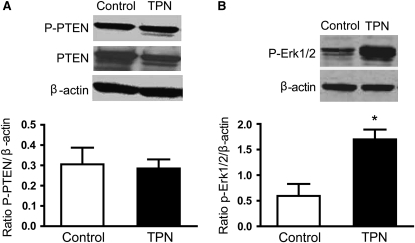
Aside from BMP mediating its action through Smad4, signaling via PTEN and the Ras/Erk signaling pathway are other control mechanisms by which BMP inhibits EC proliferation (19). To further address the connection of the BMP signaling pathway and the PTEN/AKT pathway, we examined PTEN expression. Interestingly, the abundance of PTEN and phosphorylated-PTEN protein expression did not differ between the TPN and control groups (Table 2; Fig. 3A).
FIGURE 3 .
Protein expression of the total PTEN and p-PTEN (A) and p- Erk1/2 (B) from intestinal mucosal specimens as measured by immunoblot analysis (Expt. 1). Values are means ± SD, n = 6–8. *Different from TPN, P < 0.05.
RAS/ERK expression in TPN mice
Although we did not detect a difference in PTEN expression, PTEN requires Erk signaling for adequate suppression of EC proliferation. Protein levels of p-Erk1/2 in intestinal mucosa were therefore examined using Western blot analysis. The level of p-Erk1/2 was significantly greater in the TPN group compared with controls (Fig. 3B) (P < 0.02). Thus, our data suggest that although the BMP signaling pathway does not affect PTEN expression, the increased p-Erk1/2 suggests that BMP may still signal through the PTEN pathway to affect some of its suppressive effects.
Expression of cellular myelocytomatosis and P21WAF1
Previous studies have shown that the BMP signaling pathway inhibition of cell growth is associated with an upregulation of the cyclin-dependent kinase inhibitor p21WAF1/CIP1 (20), which functions to inhibit cell proliferation. Transcription factor cellular myelocytomatosis (c-Myc), a Wnt/β-catenin target gene, is critical for regulating (typically decreasing) p21WAF1/CIP1 gene induction. We therefore examined the expression of c-Myc and p21WAF1/CIP1. Expression of c-Myc mRNA was lower than controls in the TPN mice (P < 0.01) (Table 2). Conversely, the abundance of p21WAF1/CIP1 mRNA and protein in the intestinal mucosa of TPN mice was greater than in controls (P = 0.03) (Table 2).
EC proliferation
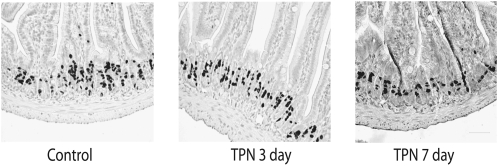
BrdU uptake was assessed after 3 and 7 d of TPN to more fully examine how these regulatory factors might affect EC proliferation. BrdU-positive EC (Fig. 4) were slightly lower than in controls (24.4 ± 4.2% BrdU-positive cells per crypt) (not significant) after 3 d TPN (22.3 ± 3.3%) and decreased (P < 0.001) after 7 d of TPN (13.5 ± 2.4%) compared with controls.
FIGURE 4 .
Representative histologic images of EC proliferation as detected by BrdU staining in mouse jejunum. Note a marked decrease in BrdU staining at 7 d of TPN. See text for quantitative results. A color version of this figure is available (Supplemental Fig. 3).
Wnt ligands and the Wnt receptor Lrp 5 expression are decreased with TPN
After 3 d of TPN administration, Wnt 3 and Wnt5a abundances were 68% less than in controls (Table 2). The expression of the Wnt receptor Lrp 5 was also less (P < 0.01) than in controls. The expressions of Wnt 4, Wnt 6, and Wnt 9b did not differ between the TPN and control groups. The results suggest that the Wnt/β-catenin pathway has a role in the development of intestinal mucosa atrophy due to TPN.
Potential mechanisms influencing TPN-associated changes in BMP2 and BMP4
In Expts. 2 and 3, BMP expression did not differ between the TPN and TPN+GLN groups (Table 1). Additionally, the use of IFNγ-KO mice has been shown to lead to a preservation of epithelial growth and epithelial barrier function with TPN administration (17). To test whether IFNγ expression may influence BMP expression, IFNγ-KO mice were used. The control IFNγ- group did not differ in either BMP2 or BMP4 expression compared to controls (Table 1). In addition, there was no change in BMP expression between the TPN group compared to the TPN+ IFNγ- group (Expt. 1 compared to Expt. 3).
Discussion
BMP belongs to the transforming growth factor-β superfamily and plays an important role during embryonic development and in postnatal homeostasis of the intestine. Conditional inactivation of the BMP type II receptor in the stroma leads to epithelial hyperplasia throughout the colon, resulting in increased EC proliferation, which in turn leads to multiple polyposis (21). Because of the critical role that BMP have on EC growth, we hypothesized that the BMP signaling pathway would contribute to the mechanisms promoting TPN-associated mucosal atrophy. Our data showed a significantly increased abundance of BMP2, BMP4, and Bmpr1a in the intestinal mucosa of mice administered TPN solutions. Also, P-Smad 1/5/8, the hallmark of active BMP signaling, was significantly greater in the intestinal mucosa of TPN mice. This strongly suggests that the BMP signaling pathway was upregulated by TPN and may be a contributory mechanism in the loss of intestinal EC proliferation with TPN.
BMP actions are controlled in part by specific antagonists that prevent BMP interactions with their specific receptors. Interestingly, none of the BMP antagonists were affected by TPN administration. It has been shown that BMP signaling is also regulated at other intracellular molecular levels, including BMP-induced expression of Smad6 and Smad7, each of which can act as feedback inhibitor to BMP signaling. Additionally, other regulators may also be involved in the repression of BMP responses. These include ubiquitin-proteasome–mediated degradation at the level of Smad as well as Smad-ubiquitination-regulatory factor 1, which interacts with Smad1 and Smad5, targeting them for degradation (22). It is important for future investigations to examine these other pathways.
We next investigated what intracellular pathways would be used to mediate the actions of BMP2 and BMP4. Three major pathways were investigated: PTEN, Erk1/2, and Wnt/β-catenin. BMP2 has been shown to increase the tumor suppressor PTEN in MCF7 (Michigan Cancer Foundation 7) breast cancer cells (23). Phosphorylation of PTEN leads to PTEN inactivation, with the subsequent activation of Akt and cellular proliferation (23). We found no change in either PTEN or p-PTEN expression, suggesting that this pathway was not involved in mediating BMP activity. The Erk family belongs to the mitogen-activated protein kinase pathway. We examined this pathway as a major BMP signaling pathway, because inhibition of intestinal EC proliferation is through repression of β-catenin activity. However, recent studies have shown that mice lacking the Bmpr1a exclusively in the intestinal epithelium had increased proliferation within the crypts, but this was not mediated via an increase in Wnt/β-signaling (24). Therefore, it has been suggested that BMP also inhibits intestinal EC proliferation through other pathways, including Ras/Erk (19). This may well be supported by our data, which showed an increased abundance of p-Erk1/2 with TPN. Such findings are quite similar to those of Sheng et al. (25), who found that mice given an elemental liquid diet had increased EC levels of p-ERK1/2. This study showed that this restricted diet led to a significantly reduced number of proliferating EC. These findings were consistent with our finding of decreased c-Myc expression and EC proliferation.
c-Myc was significantly decreased and p 21WAF1/CIP1 was significantly increased in our TPN model. c-Myc is a major biological regulator of intestinal mucosal development and homoeostasis (26). Increased c-Myc expression is not only essential for the induction of crypt formation during early intestinal development (26), but c-Myc also stimulates healing of damaged mucosa in adult mice (27). c-Myc abundance increases with T-cell factor 4 (tcf4) transcriptional activation via the canonical Wnt pathway. Overexpression of p21WAF1/CIP1 has been shown to have a negative effect on the cell cycle. p21WAF1/CIP1 is expressed in intestinal EC during the nonproliferative state and p21WAF1/CIP1 expression is inversely related to proliferation and directly related to terminal differentiation in normal colonic mucosa (28). Conversely, treatment with the BMP antagonist gremlin 1 resulted in decreased P21WAF1/CIP1 gene expression in Caco-2 cells (29). The increased BMP signaling in TPN mice may well act to increase p21WAF1/CIP1 transcription by repressing the Wnt/β-catenin pathway. This latter point is consistent with our finding of a marked decline in Wnt 3, Wnt 5a, and Lrp5 and suggests that BMP signaling inhibited Wnt/β-catenin signaling activity in our TPN-treated mice. This decline in Wnt expression corresponded well with the loss of tcf4 transcription, as supported by the decline in c-Myc in our results and the decline in cyclin D1 recently published by our group (11).
Glutamine is the primary fuel source for enterocytes and is essential for gut homeostasis (30). Further, the addition of glutamine to TPN, or to a liquid, elemental diet, reduces gut mucosal atrophy (31,32). To assess whether this glutamine-associated, sustained EC growth was due to a modulation of BMP expression, BMP abundance was examined in mice administered a TPN solution supplemented with glutamine. The fact that BMP2 and BMP4 did not significantly change suggests that this pathway is not used by glutamine. As stated above, cytokine modulation within the intestinal mucosa may have a significant role in modulating EC growth (16). Additionally, BMP expression has been shown to be modulated by the expression of a variety of cytokines (33). Interestingly, IFNγ-KO mice given TPN did not influence BMP expression compared to wild-type TPN mice. Thus, future work will need to be done to better understand the mechanisms guiding the increased abundance of BMP in our TPN model.
In conclusion, TPN administration upregulated the BMP signaling pathway. Because BMP plays an important role in inhibiting intestinal epithelium cell proliferation, the increased signaling through this pathway may well have an important role in the loss of EC proliferation in our mouse model of TPN administration.
Supported by the NIH grant 5R01 AI044076-10 (to D. H. Teitelbaum) and the National Cancer Institute through the University of Michigan's Cancer Center Support grant (5 P03 CA46592).
Author disclosures: C. Zhang, Y. Feng, H. Yang, H. Koga, and D. H. Teitelbaum, no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figures 1–3 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BMP, bone morphogenetic protein; Bmpr1a, BMP type I receptor; BrdU, 5-bromo-2-deoxyuridine; c-Myc, cellular myelocytomatosis; EC, epithelial cell; ERK, extracellular signal-regulated kinase; IFNγ-KO, interferon-γ knockout; LRP, low density lipoprotein receptor related protein; MCF7, Michigan Cancer Foundation 7; p-ERK, Erk1/2 phosphorylation; P-Smad 1/5/8, phosphorylated-Smad 1/5/8; PTEN, phosphatase and tensin homolog; tcf4, T-cell factor 4; TPN, total parenteral nutrition; TPN+GLN, total parenteral nutrition with glutamine.
References
- 1.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–8. [DOI] [PubMed] [Google Scholar]
- 2.Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH. Lack of enteral nutrition–effects on the intestinal immune system. J Surg Res. 2005;123:8–16. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. [DOI] [PubMed] [Google Scholar]
- 4.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–21. [DOI] [PubMed] [Google Scholar]
- 5.Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus G, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–6. [DOI] [PubMed] [Google Scholar]
- 6.Camac KS, Thompson FM, Cummins AG. Activation of beta-catenin in the stem cell region of crypts during growth of the small intestine in infant rats. Dig Dis Sci. 2007;52:1242–6. [DOI] [PubMed] [Google Scholar]
- 7.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gastrointestinal surgery for severe obesity. Proceedings of a National Institutes of Health Consensus Development Conference. Am J Clin Nutr. 1992;55:S487–619. [DOI] [PubMed] [Google Scholar]
- 9.Bernal NP, Stehr W, Zhang Y, Profitt SA, Erwin CR, Warner BW. Evidence for active Wnt signaling during postresection intestinal adaptation. J Pediatr Surg. 2005;40:1025–9. [DOI] [PubMed] [Google Scholar]
- 10.Tian Q, He X, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14–3-3zeta. Cell Cycle. 2005;4:215–6. [PubMed] [Google Scholar]
- 11.Feng Y, Sun X, Yang H, Teitelbaum D. Dissociation of E-cadherin and beta-catenin in a mouse model of total parenteral nutrition: a mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol. 2009;587:641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purina Mills. Product information for Rodent Chow 5001 [cited 2009 21 May]. Available from: http://wwwlabdietcom/indexlabdiethomehtm.
- 13.Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res. 1998;79:91–6. [DOI] [PubMed] [Google Scholar]
- 14.Kritsch KR, Murali S, Adamo M, Clayton M, Ney D. Hypoenergetic high-carbohydrate or high-fat parenteral nutrition induces a similar metabolic response with differential effects on hepatic IGF-I mRNA in dexamethasone-treated rats. J Nutr. 2005;135:479–85. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Gocinski B, Henken B, Kudsk K. Effects of parenteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–52. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G629–37. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, Antony PA, Zhou H, Teitelbaum DH. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002;236:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005;16:309–17. [DOI] [PubMed] [Google Scholar]
- 19.Beck SE, Carethers J. BMP suppresses PTEN expression via RAS/ERK signaling. Cancer Biol Ther. 2007;6:1313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosselet FP, Magnaldo T, Culerrier RM, Sarasin A, Ehrhart JC. BMP2 and BMP6 control p57 (Kip2) expression and cell growth arrest/terminal differentiation in normal primary human epidermal keratinocytes. Cell Signal. 2007;19:731–9. [DOI] [PubMed] [Google Scholar]
- 21.Beppu H, Mwizerwa ON, Beppu Y, Dattwyler MP, Lauwers GY, Bloch KD, Goldstein AM. Stromal inactivation of BMPRII leads to colorectal epithelial overgrowth and polyp formation. Oncogene. 2008;27:1063–70. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Wang X. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887–96. [DOI] [PubMed] [Google Scholar]
- 25.Sheng H, Shao J, Townsend CJ, Evers B. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettess MD, Dubois N, Murphy M, Dubey C, Roger C, Robine S, Trumpp A. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol. 2005;25:7868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JY, Johnson LR. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am J Physiol. 1994;266:G878–86. [DOI] [PubMed] [Google Scholar]
- 28.Doglioni C, Pelosio P, Laurino L, Macri E, Meggiolaro E, Favretti F, Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996;179:248–53. [DOI] [PubMed] [Google Scholar]
- 29.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA. 2007;104:15418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avissar NE, Sax H, Toia L. In human entrocytes, GLN transport and ASCT2 surface expression induced by short-term EGF are MAPK, PI3K, and Rho-dependent. Dig Dis Sci. 2008;53:2113–25. [DOI] [PubMed] [Google Scholar]
- 31.Buchman AL, Moukarzel A, Bhuta S, Belle M, Ament M, Eckhert C, Hollander D, Gornbein J, Kopple J, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN Parenter Enteral Nutr. 1995;19:453–60. [DOI] [PubMed] [Google Scholar]
- 32.Estívariz C, Griffith D, Luo M, Szeszycki E, Bazargan N, Dave N, Daignault N, Bergman G, McNally T, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. JPEN. 2008;32:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lories R, Derese I, Ceuppens J. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48:2807–18. [DOI] [PubMed] [Google Scholar]