Abstract
Background: Consumption of nuts has been associated with a decreased risk of cardiovascular disease events and death. Walnuts in particular have a unique profile: they are rich in polyunsaturated fatty acids, which may improve blood lipids and other cardiovascular disease risk factors.
Objectives: We aimed to conduct a literature review and a meta-analysis to combine the results from several trials and to estimate the effect of walnuts on blood lipids.
Design: Literature databases were searched for published trials that compared a specifically walnut-enhanced diet with a control diet. We conducted a random-effects meta-analysis of weighted mean differences (WMDs) of lipid outcomes.
Results: Thirteen studies representing 365 participants were included in the analysis. Diets lasted 4–24 wk with walnuts providing 10–24% of total calories. When compared with control diets, diets supplemented with walnuts resulted in a significantly greater decrease in total cholesterol and in LDL-cholesterol concentrations (total cholesterol: WMD = −10.3 mg/dL, P < 0.001; LDL cholesterol: WMD = −9.2 mg/dL, P < 0.001). HDL cholesterol and triglycerides were not significantly affected by walnut diets more than with control diets (HDL cholesterol: WMD = −0.2, P = 0.8; triglycerides: WMD = −3.9, P = 0.3). Other results reported in the trials indicated that walnuts provided significant benefits for certain antioxidant capacity and inflammatory markers and had no adverse effects on body weight [body mass index (kg/m2): WMD = −0.4, P = 0.5; weight (kg): WMD = −0.05, P = 0.97].
Conclusions: Overall, high-walnut-enriched diets significantly decreased total and LDL cholesterol for the duration of the short-term trials. Larger and longer-term trials are needed to address the effects of walnut consumption on cardiovascular risk and body weight.
INTRODUCTION
Large prospective studies have consistently observed a reduction in cardiovascular disease (CVD) risk and mortality associated with increased nut consumption (1, 2). Clinical trials have also have shown effects on CVD risk factors such as lipid profiles, vascular inflammation, and blood pressure after various interventions that have included nuts, such as a Mediterranean diet (3–6). Nuts are a complex food composed of a number of nutrients and phytochemicals that may lower CVD risk.
Differences from other tree nuts in fatty acid composition make walnuts an interesting and intriguing target for investigation. According to the food composition database published by the US Department of Agriculture, 100 g of walnuts contain 15.2 g protein, 65.2 g fat, and 6.7 g dietary fiber. Whereas most nuts are high in monounsaturated fatty acids, walnuts are composed largely of polyunsaturated fatty acids (47.2 g), especially α -linolenic acid (18:3n−3; 9.1 g) and linoleic acid (18:2n−6; 38.1 g) (7). Several reviews have described possible explanations and mechanisms by which this unique fatty acid profile can beneficially affect cholesterol concentrations and other CVD risk factors (8). Whether by simply replacing saturated fatty acids in the diet or by explicitly acting on various pathways in CVD development, walnuts appear to have the potential to beneficially affect CVD risk.
Feeding trials have shown cholesterol-lowering trends in walnut-enriched diets compared with control diets, as reviewed by Feldman (9). Many qualitative reviews have suggested beneficial effects of walnuts on cholesterol concentrations; however, a meta-analysis of the published trials has yet to be conducted. The aim of this systematic review was to perform a comprehensive assessment of the literature and carry out a meta-analysis by examining the change in lipid concentrations induced by a walnut-enhanced diet. Additionally, we aimed to address concerns that a walnut-enriched diet might lead to weight gain, given that this outcome is reported in the published trials. Finally, we qualitatively reviewed other CVD risk factors that that have been investigated in relation to walnut consumption.
SUBJECTS AND METHODS
Search strategy and study inclusion criteria
For this review, relevant English-language articles were identified by searching the Medline database (www.pubmed.com; National Library of Medicine, Bethesda, MD) and Cochrane Reviews (www.cochrane.org/reviews; The Cochrane Collaboration) through May 2008. The search strategy was as follows: (juglans[MeSH] OR walnut[MeSH] OR walnut) AND (humans[Mesh]) AND (English[lang]). Bibliographies of accepted studies and recent reviews were screened to ensure a complete study listing.
For each of the relevant abstracts, full publications were retrieved for evaluation on the basis of criteria established a priori. Studies were accepted for the meta-analysis if they were a controlled trial that evaluated a walnut-enriched diet compared with a control diet. We excluded trials that compared only the effects of other nuts. Accepted studies were required to report baseline and follow-up values, the mean change from baseline, or the mean difference between intervention groups for at least one lipid variable. Additionally, studies needed to have specifically tested walnut-based interventions and to have clearly stated the amount and frequency of walnuts included or instructed in the diet. All patient populations and age groups were included. We excluded studies evaluating only postprandial effects because this outcome was not of primary interest. Abstracts and complete articles were screened for acceptance by one author/reviewer (DKB). The decisions to exclude articles from analysis were independently evaluated by a second author/reviewer (FBH). We retained excluded publications for consideration in the review of nonlipid CVD risk factors.
Data collection
Study characteristics, which included authors, publication year, specific study design (randomized, crossover/parallel), intention-to-treat analysis, use of run-in or wash-out periods, and methods for assessment of participant dietary compliance, were extracted for quantitative and qualitative synthesis by DKB. For details of the intervention, we captured the specific amount of walnuts (percentage of energy from fat, grams per day), control intervention, number of weeks for each diet, and proportion of patients completing follow-up. Patient characteristics of interest were baseline mean age and body mass index (BMI; in kg/m2), weight, sex, percentage of smokers, and percentage of overweight or obese subjects. In addition, we also recorded the proportion of participants with relevant comorbidities, including hypercholesterolemia, dyslipidemia, diabetes, and metabolic syndrome. We also noted each study's specific inclusion and exclusion criteria for patients with these comorbidities.
We were interested in the following outcomes or endpoints, including mean serum total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride concentrations. These values were captured as the mean change from baseline to follow-up (with mean ± SD or mean ± SE, respectively). Secondary, nonlipid endpoints were also captured in this way. Nonlipid risk factors included weight change and markers of inflammation, oxidative stress, endothelial function, and antioxidant capacity (9).
Study quality was assessed by the Jadad score level-of-evidence rating for randomized controlled trials (10). Scoring is on a scale from 0 to 5 with 1 point each for randomization, blinding, and reporting of participant withdrawals. Two additional points are allotted for description of randomization and double-blinding methods. Another aspect of study quality that we evaluated was the method for monitoring participant compliance.
Statistical analysis
For the meta-analysis, we calculated the mean change from baseline to follow-up for each intervention and control group, if not reported. This was conducted by using a paired Student's t test to obtain the group mean and pooled SD. For studies providing only the mean difference between the walnut and the control groups, the control group's mean change was set as 0 and the walnut group's mean change was set as the reported mean difference. These studies were excluded from calculation of the percentage change (described below) because the control groups were artificially set at 0 and the actual mean change for each group was unknown.
Standard errors and confidence intervals were converted to SD for the analyses. All serum lipid values were converted to milligrams per deciliter, if necessary. If more than one time point for follow-up was reported, we included the value closest to the time point used in the other studies for our primary analysis. For studies with more than one comparison group, we included the control diet most like the walnut diet after the exclusion of walnuts or other nuts. Additionally, studies without SD, SE, CIs, or a P value accompanying the mean values were excluded from analysis but retained for discussion.
We used the METAN command in STATA version 9.0 (StataCorp, College Station, TX) to calculate a weighted mean difference (WMD) by conducting a random-effects meta-analysis. Cochrane's Q test was used to evaluate the significance of heterogeneity. In addition, we produced the I2 and 95% CI with the command HETEROGI to evaluate the proportion of any heterogeneity due to between-study variation, with a P < 0.1 level of significance. Sensitivity analyses were performed to evaluate the effect of outliers or “effect modifiers” (11, 12). We hypothesized that the amount of walnuts per day (% energy from fat), follow-up time, baseline comorbidity status, or type of control diet could potentially modify the observed effects between studies. A sensitivity analysis was also conducted to assess the effect of study quality on outcomes. For this, a subgroup was made up of crossover randomized trials with a Jadad score of 3 or higher. Studies reporting poor compliance (as defined by the author or authors) were excluded from this subgroup.
We derived the percentage change from the WMD for the intervention and control groups, divided by the weighted mean baseline levels, and multiplied by 100. Publication bias was assessed through Egger's and Begg's tests for each outcome at the P < 0.05 level of significance (13, 14). We also visually inspected Begg's funnel plot for publication bias, looking for any skewing to either side of the effect estimate.
RESULTS
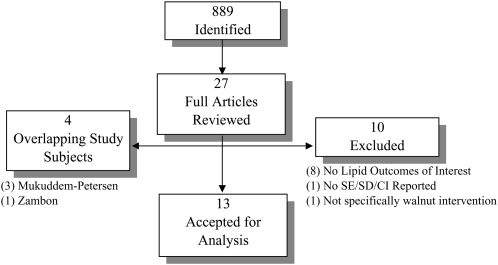
The literature search yielded 889 citations of which 27 were retrieved for complete review. Our attrition to the final 13 studies included in the meta-analysis of serum lipid concentrations, representing a total of 365 participants, is depicted in Figure 1. The study characteristics are outlined in Table 1. Of the 13 studies, 12 were randomized trials, 10 of which had a crossover design. The baseline characteristics and comorbidity status of the participants varied. Four studies recruited only those with healthy cholesterol concentrations, whereas 6 studies included participants with modest hypercholesterolemia (see Table 1 for study-specific cutoffs). The remaining studies evaluated the effect of the walnut intervention among patients with diabetes, older obese subjects, and participants with a metabolic syndrome. Ten studies were rejected from the meta-analysis for the various reasons noted in Figure 1 (see citations under “Supplemental data” in the online issue). The primary reason for exclusion of a study was including a walnut intervention trial for outcomes other than lipid concentrations. In addition, 4 studies were based on participants already represented in the database. These studies were excluded from analysis to avoid double-counting of the outcomes, but were retained for discussion of any additional nonlipid findings.
FIGURE 1.
Study attrition diagram.
TABLE 1.
Meta-analysis study characteristics1
| Study | n | Patient characteristics | Age2 | BMI (kg/m2) or weight (kg)2 | Baseline lipid values2 | Males | Study design | Diet duration | Walnut diet | Control diet | Jadad score |
| mg/dL | % | wk | |||||||||
| Spaccarotella et al, 2008 (22) | 21 | Healthy3: total PSA > 2.0 ng/mL without prostate cancer diagnosis; no lipid-altering medications | 65.9 (55–75) | 84.8 kg (13.3) | TC = 193.7 (28.4), LDL-C = 120.3 (26.6), HDL-C = 52.6 (10.6), TG = 1 16.0 (89.5) | 100 | Randomized crossover | 8 | 24% E from walnuts (75 g/d) | Average American | 2 |
| Canales et al, 2007 (16) | 22 | Overweight or obese wiith additional CHD risk factor: TC > 220 mg/dL, smoker, hypertension | 54.8 (8.3) | BMI: 29.6 (3.4) | TC = 218.1 (43.3) | 60 | Randomized crossover | 5 | 13% E from walnuts; walnut-enriched meat provided | Control: meat provided | 1 |
| Mukuddem-Petersen et al, 2007 (25) | 43 | Metabolic syndrome: lipid-altering medications allowed if consistantly used | 45 (10) | BMI: walnut—36.0 (6.3); control—35.1 (8.1) | Walnut: TC3 = 185.6 (175.6–200.3), LDL-C4 = 115.6 (101.7–127.2), HDL-C = 36.3 (32.1–40.6), TG = 168.3 (131.1–205.5); control:TC4 = 189 | 45 | Randomized parallel | 8 | 20% E from walnuts (63–108 g/d); meals provided | Control3: meals provided | 3 |
| Perez-Martinez et al, 2007 (19) | 16 | Healthy3: no lipid-altering medications | NR | NR | Normal3 | 100 | Randomized crossover | 4 | ≈ 5% E from walnuts; low fat (<30% E from fat); meals provided | Mediterranean meals provided | 1 |
| Ros et al, 2004 (24) | 20 | Hypercholesterolemia: LDL-C > 130 mg/dL; TG < 250 mg/dL; no lipid-altering medications | 55 (26–75) | 70.6 kg (10.3) | TC = 268.0 (27.1), LDL-C = 183.7 (24.0), HDL-C = 62.3 (15.9), TG = 123.1 (52.3) | 40 | Randomized crossover | 4 | 18% E from walnuts (40–65 g/d) Mediterranean | Mediterranean | 2 |
| Tapsell et al, 2004 (26) | 37 | Type 2 diabetes: no insulin therapy | Walnut: 57.2 (9.0); control: 59.3 (7.1) | BMI: walnut—30.7 (3.9); control—30.2 (4.5) | Walnut: TC = 158.9 (31.3), LDL-C = 83.9 (50.7), HDL-C = 42.5 (9.3), TG = 168.3 (65.5); control: TC = 177.1 (34.0), LDL-C = 99.8 (50.3) | Walnut: 65; control: 50 | Randomized parallel | 12, 24 | 10% E from walnuts (30 g/d); modified low-fat diet ( < 30% E from “good” fat) | Modified low-fat ( < 30% E from “good” fat) | 2 |
| Zhao et al, 2004 (15) | 23 | Hypercholesterolemia: TC: 200–240 mg/dL; LDL-C: 40th–90th percentile; no lipid-altering medications | 49.8 (7.7) | BMI: 28.1 (3.4) | TC = 226.2 (22.4), LDL-C = 153.9 (20.5), HDL-C = 44.9 (7.3), TG = 136.4 (68.2) | 87 | Randomized crossover | 6 | 18% E from walnuts and oil (62 g/d); high PUFA | Average American | 1 |
| Iwamoto et al, 2002 (17) | 40 | Healthy3 | Women: 23.6 (4.9); men: 23.8 (3.1) | BMI: women—20.7 (2.2); men—22.2 (2.2) | Women: TC = 175.2 (27.8), TG = 133.7 (166.5); men: TC = 183.7 (32.9), TG = 226.7 (123.1) | 50 | Randomized crossover | 4 | 12.5% E from walnuts (52 g/d); traditional Japanese meals provided | Traditional Japanese meals provided | 4 |
| Morgan et al, 2002 (18) | 42 | Hypercholesterolemia: TC > 200 mg/dL; no CVD | 55.7 (11.8) | BMI: 27.7 (5.8) | TC = 232.0 (30.9), LDL-C =154.7(23.2), HDL-C = 58.0 (15.5), TG = 159.4 (106.3) | 40 | Randomized crossover | 6 | 20% E from walnuts (64 g/d) low-fat/cholesterol ( < 30% E from fat, < 200 mg chol) | Low-fat/cholesterol ( < 30% E from fat, < 200 mg cholesterol) | 2 |
| Almario et al, 2001 (20) | 18 | Hypercholesterolemia: TC > 200 mg/dL; LDL-C > 130 mg/dL; no lipid-altering medications | 60 (8) | BMI: 29.0 (1.2) | TC = 230.9 (47.6), LDL-C = 138.1 (45.9), HDL-C = 49.1 (11.5), TG = 218.8 (82.7) | 28 | Nonrandomized consecutive | 6 | 16.5% E from walnuts (48 g/d); low fat ( < 20% E from fat) | Low fat ( < 20% E from fat) | NA |
| Zambón et al, 2000 (21) | 49 | Hypercholesterolemia: LDL-C > 130 mg/dL; TG < 250 mg/dL; no lipid-altering medications | 56 (11) | BMI: 27.0 (3.1) | TC = 276.9 (32.9), LDL-C = 195.3 (29.8), HDL-C = 55.7 (12.8), TG =136.4 (42.5) | 51 | Randomized crossover | 6 | 18% E from walnuts (41–56 g/d) Mediterranean | Mediterranean | 3 |
| Chisholm et al, 1998 (27) | 16 | Hypercholesterolemia: TC—212–290 mg/dL | 45 (6.8) | BMI: 28.4 (4.3) | TC = 254.4 (23.2), LDL-C = 179.0 (22.4), HDL-C = 40.6 (12.0) | 100 | Randomized crossover | 4 | 20% E from walnuts (78 g/d) low fat ( < 30% E from fat) | Low-fat ( < 30% E from fat) | 1 |
| Sabaté et al, 1993 (23) | 18 | Healthy; no lipid-altering medications | 30 (21–43) | BMI: 23.8 (18.7–30.6) | TC = 197.2 (3.54–6.47) | 100 | Randomized crossover | 4 | 20% E from walnuts (84 g/d); meals provided | Cholesterol-lowering meals provided | 4 |
n, number of participants studied; PSA, prostate-specific antigen; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; NR, not reported; E, total energy; CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; PUFA, polyunsaturated fatty acids; NA, not applicable.
Values are means; SDs or ranges in parentheses.
As stated by author.
Median.
Dietary interventions lasted from 4 to 24 wk and ≈ 6 wk on average. Walnuts represented 5–24% of the total calories in the intervention diets. One study allowed 15 g of walnut oil in addition to the 37 g of walnuts (15). Another study incorporated the walnuts into meat products to facilitate participant blinding (16). It was apparent from the remaining studies that patients were given or recommended specific daily amounts of whole walnuts. The control diets were diverse, as outlined in Table 1. Four studies used low-fat diets, with a cutoff of <30% energy from fat in 3 studies and <20% energy from fat in the fourth study. A Mediterranean-style diet was used in 3 studies. Three other studies used the average diets for the country: 2 average American and 1 traditional Japanese. One study reported that the control meals were cholesterol lowering, and another specified that control meals were provided but did not elaborate on the composition.
The Jadad rating scale was used to score the randomized trials from 1 to 5 points (10). All studies described methods for monitoring or verifying patient compliance. All but 1 study collected either food records or food-frequency questionnaires throughout the study (15). Additionally, all but 3 studies evaluated changes in serum fatty composition or γ-tocopherol concentrations to assess achievement of dietary goals (17–19). Perez-Martinez et al (19) did not specify whether compliance was met, and the remaining authors were satisfied with adherence. Participant withdrawal from the trial was addressed in all but 3 studies (15, 16, 19). Four authors reported some withdrawals attributable to walnuts (18, 20–22). These included canker sores in 1 participant (20), gastrointestinal distress in another participant with a previous partial gastrectomy (22), and 5 participants claiming intolerance to walnuts (18, 21). Other patient-reported intolerance to walnuts that did not lead to withdrawal included softened stools in 25 participants and mild postprandial heaviness and bloating in 3 (21).
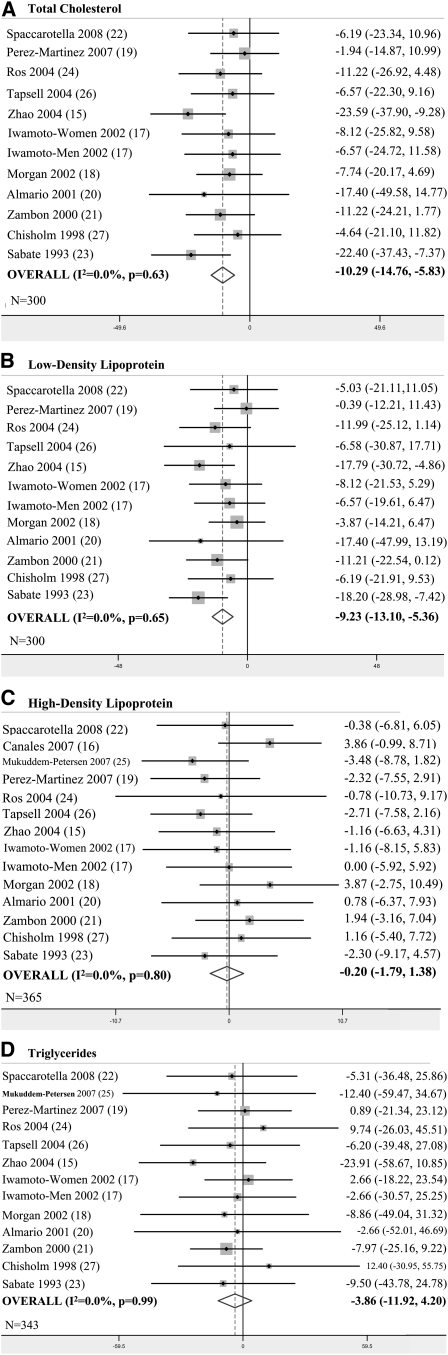
Eleven studies representing 300 participants reported results for serum TC concentrations (Figure 2A). All trials trended toward preference of the walnut-based diets. The meta-analyzed WMD shows a significantly greater reduction in TC while consuming a walnut-enriched diet than a control diet (WMD = − 10.29 mg/dL, P < 0.001). This difference represents a 4.9% greater decrease in TC concentration while consuming walnuts.
FIGURE 2.
A–D: Results of primary meta-analyses.
The same 11 studies also presented outcomes for serum LDL-cholesterol concentrations (Figure 2B). Individual study trends for LDL cholesterol were similar to those of TC. The overall result indicates a 6.7% significantly greater decrease in LDL-cholesterol concentration with the walnut intervention diets compared with the control diets (WMD = −9.23 mg/dL, P < 0.001).
All 13 trials, which represented 365 participants, reported results for HDL cholesterol. Overall, there was not a significant difference in serum HDL between walnut-enriched diets and control diets (WMD = −0.20 mg/dL, P = 0.80) (Figure 2C). Triglyceride concentrations during walnut and control diets decreased by 12.5% and 9.1% from baseline, respectively. All but one study reported this outcome, for a total of 343 participants. Although differences in triglyceride change did not reach statistical significance, we observed a trend in favor of walnut diets (WMD = −3.86 mg/dL, P = 0.35) (Figure 2D).
Tests for heterogeneity indicated that the treatment effect was not significantly different between studies (TC: P = 0.63; LDL cholesterol: P = 0.66; HDL cholesterol: P = 0.79; triglycerides: P = 0.99). I2 values were 0% for all lipid variables, with the widest 95% CI ranging from 0% to 58%. Because of the lack of significant between-study variation, we did not expect the presence of significant effect modification. However, we proceeded with sensitivity analyses to see if there were any noteworthy trends. The 6 studies among participants with hypercholesterolemia produced results similar to the overall meta-analyses, which slightly favored walnut diets (TC: WMD = −12.0, P < 0.001; LDL cholesterol: WMD = − 10.1, P < 0.001; HDL cholesterol: WMD = 1.1, P = 0.4; triglycerides: WMD = −6.0, P = 0.3). The subgroup of studies meeting our quality criteria consisted of 3 trials (17, 21, 23). Results of this analysis also slightly favored walnut diets over the overall results (TC: WMD = −12.8, P =0.001; LDL cholesterol: WMD = −11.7, P < 0.001; HDL cholesterol: WMD = −0.8, P = 0.5; triglycerides: WMD = −4.6, P = 0.4). Although it was not a specific criterion for this subgroup, lipid-altering medications were not allowed in these 3 trials, which reduced the concern of this potential confounder biasing the results. We evaluated the dose-response effect of walnut amount (% total energy) and weeks of intervention with meta-regression; however, results were null (data not shown). Finally, we meta-analyzed only randomized trials, which produced results no different from the overall analyses. There were no signs of publication bias when examining the funnel plots. Results from Begg's and Egger's tests also did not indicate evidence of publication bias (TC: Begg P = 1.0, Egger P = 0.79; LDL cholesterol: Begg P = 1.0, Egger P = 0.95; HDL cholesterol: Begg P = 0.70, Egger P = 0.95; triglycerides: Begg P = 0.67, Egger P = 0.87).
BMI and weight change
There has been some concern about recommending increased nut consumption for patients with high cholesterol concentrations because of the high fat content and ostensible risk of weight gain. Multiple authors have reviewed this topic and conclude that short-term intervention data do not suggest a significant weight gain and, to the contrary, sometimes indicate weight loss with increased nut consumption (28, 29). Of the studies identified in our literature review, none reported significant weight change while on a walnut-based diet intervention. The 4 studies reporting BMI change (16, 20, 25, 26) and the 8 studies reporting weight change (20–22, 24–26) outcomes actually observed slight nonsignificant decreases from baseline. Results are mixed when compared with the control diets in a meta-analysis, producing an overall null difference between them (BMI: WMD = −0.40, P = 0.5; weight (kg): WMD = −0.05, P = 0.97). Importantly, weight gain did not occur during short-term dietary interventions with walnuts.
Other markers of cardiovascular disease
Other identified risk factors for CVD were mentioned in our systematic literature search of clinical trials of walnut-based diets. Of these risk factors, few reached statistical significance and were consistent across studies. Apolipoproteins A-I and B (apo A-1, apo B) outcomes were reported in 7 studies, 3 of which found significantly greater decreases in apo B for the walnut group than for the control group (15, 17, 29). Oxidative stress is a feature of atherosclerosis and was measured via LDL-cholesterol-conjugated diene formation in 3 walnut intervention trials (17, 21, 30). There was no effect of walnuts on in vitro lag time in these studies. Other markers of oxidative stress, which included oxidized LDL cholesterol, malondialdehyde, lipid peroxidation, and uric acid, all maintained baseline concentrations across all interventions, indicating no increased oxidative stress over the duration of the trials (16, 24, 25). Importantly, resistance to oxidation was maintained despite reported increases in lipid particle enrichment with polyunsaturated fatty acids (30). General antioxidant capacity was evaluated in 2 studies, both of which found significant decreases in oxidized glutathione during the walnut diet phase compared with the control phase (16, 30). The other common marker between the 2 studies was glutathione, but only 1 study found significantly decreased concentrations (16). Other markers of antioxidant capacity were improved with a walnut diet in individual studies, but have yet to be evaluated in other trials.
Several walnut intervention trials explored the effects on markers of inflammation and endothelial function (15, 19, 24, 25). Results for C-reactive protein were inconsistent across 3 trials, showing a significant reduction, increase, and no change for walnut groups from baseline (15, 24, 25). The study showing a significant increase was not of a crossover design, and although the walnut diet group experienced a significant increase from baseline, their follow-up concentration of C-reactive protein was actually still less than that of the control groups (25). Another inflammatory marker, nuclear factor κB, showed no difference between a walnut and a Mediterranean control diet for 16 healthy men (19). Markers of endothelial function included monocyte chemoattractant protein 1, interleukin-6, tumor necrosis factor-α, intracellular adhesion molecule 1, vascular cell adhesion molecule 1 (VCAM-1), hyperemic flow, and vasodilation of the brachial artery (15, 19, 24). VCAM-1 concentrations were consistently significantly lowered for participants during the walnut diets compared with the control diets across the 3 studies (15, 19, 24). Endothelium-dependent vasodilation of the brachial artery significantly improved under the walnut diet compared with the control diet during the one study that evaluated this intermediate endpoint (24). Overall, evidence of decreasing VCAM-1 and increasing endothelium-dependent vasodilation suggests that walnut-rich diets may have benefits for vascular endothelial function, a mediator of CVD risk.
DISCUSSION
Results of our meta-analysis support the consumption of walnuts for the lowering of serum cholesterol concentrations. Compared with various control diets, substitution with walnuts was consistently better at lowering total and LDL cholesterol concentrations. Trends toward decreases in triglyceride concentrations also favored a walnut diet. HDL cholesterol was not affected significantly.
The macronutrient and micronutrient composition of walnuts has led researchers to investigate the effect of walnuts on factors associated with CVD risk. Suspected increases in BMI were not confirmed with these studies, and some studies suggested a trend toward lower BMI with walnut-rich diets. The high levels of antioxidants found in walnuts conferred an improvement in antioxidant status as noted by increased enzyme activity and stable oxidation of LDL cholesterol. Some inflammatory markers also improved with walnut consumption compared with other diets. Evidence for C-reactive protein reduction was inconsistent, although decreases in VCAM-1 were apparent among subjects during walnut-rich diets. Overall, walnuts significantly improved lipid profiles and favorably affected a number of other factors associated with CVD risk. There was no evidence, however, which suggests that walnut-enriched diets beneficially affected insulin resistance or blood pressure.
Although certain positive results were seen with these trials, there are some limitations. Primarily, the studies had relatively small sample sizes and short durations of follow-up. For the 3 parallel design studies, the small sample sizes could have led to ineffective randomization and potential confounding (20, 25, 26). Although authors adjusted for some covariates, it might not have been enough to eliminate bias. However, when we excluded these studies from the subgroup analysis of higher quality trials, it was apparent that they did not appreciably affect the results. The small sample sizes also decrease statistical power to detect minor, but clinically significant, changes. Long-term effects are clinically important for lipid profiles and other CVD risk factors. The longest follow-up time was 6 mo so presumed health benefits cannot be extrapolated beyond the duration of these studies. Furthermore, because lipid profiles change soon after switching diets, walnut consumption would need to be maintained indefinitely to maintain lower lipid concentrations. Compliance with the diets was acceptable according to authors, but long-term adherence is often a concern with dietary interventions. Finally, the amount of walnuts consumed in these trials was relatively large, representing 5–25% of total calories (30–108 g/d). This level of consumption might be difficult to maintain in a nonresearch setting. Nevertheless, modest lipid improvements were seen in trials incorporating smaller amounts of walnuts.
There are limitations with meta-analysis as well. Primarily, a meta-analysis is limited by the methods, reported outcomes, and quality of the individual studies. When individual studies clearly describe their methods, fewer assumptions are made in data extraction and analysis when pooling multiple studies together. Errors made by authors might go unnoticed and could potentially change the results. However, larger numbers of studies in an analysis make it less likely that such errors could materially alter a final result. Publication bias is another concern with meta-analyses; ie, negative or null findings fail to be submitted and/or accepted for publication. Despite extensive literature searches, meta-analysis can include only those studies that are actually published. With tests and visual inspections, such as the Begg's and Egger's methods used in this review, we were able to exclude publication bias with some confidence. However, these tests rely on the inclusion of larger trials for proper interpretation, and our largest trial was <50 participants. When research on a particular topic is limited to small trials with less-than-definitive conclusions, a meta-analysis is able to compile results into a comprehensive analysis, which allows greater statistical power. No single walnut trial enrolled >50 participants, but the meta-analysis was able to incorporate results from ≥300 for each outcome. The various patient backgrounds included in our review should enhance the generalizability of the results. Baseline comorbidity status, ethnicity, and diet composition varied across studies, indicating that our findings may apply to a broader population. However, given the limitations of the individual studies, such as a short follow-up time, there is still a need for further research.
In conclusion, this meta-analysis found significant improvements in lipid profiles with high walnut consumption compared with various control diets. Walnuts may also have potential benefits on oxidative stress and inflammatory markers. Despite their high fat content, walnuts do not appear to adversely affect body weight. Although larger and longer-term trials are needed to address the effects of walnuts on cardiovascular risk and body weight, the evidence summarized in this review indicates that walnuts can be incorporated into one's diet for the improvement of CVD risk factors, namely lipid profiles.
Acknowledgments
Both authors contributed to the analysis and text of this manuscript. Neither of the authors had a conflict of interest.
REFERENCES
- 1.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev 2001;59:103–11 [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ. Nut consumption and risk of coronary heart disease: a review of epidemiologic evidence. Curr Atheroscler Rep 1999;1:204–9 [DOI] [PubMed] [Google Scholar]
- 3.Spiller GA, Miller A, Olivera K, et al. Effects of plant-based diets high in raw or roasted almonds, or roasted almond butter on serum lipoproteins in humans. J Am Coll Nutr 2003;22:195–200 [DOI] [PubMed] [Google Scholar]
- 4.Griel AE, Cao Y, Bagshaw DD, Cifelli AM, Holub B, Kris-Etherton PM. A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J Nutr 2008;138:761–7 [DOI] [PubMed] [Google Scholar]
- 5.Mercanligil SM, Arslan P, Alasalvar C, et al. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur J Clin Nutr 2007;61:212–20 [DOI] [PubMed] [Google Scholar]
- 6.Fitó M, Guxens M, Corella D, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med 2007;167:1195–203 [DOI] [PubMed] [Google Scholar]
- 7.Li L, Tsao R, Yang R, Kramer JK, Hernandez M. Fatty acid profiles, tocopherol contents, and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.). J Agric Food Chem 2007;55:1164–9 [DOI] [PubMed] [Google Scholar]
- 8.Ros E, Mataix J. Fatty acid composition of nuts: implications for cardiovascular health. Br J Nutr 2006;96(suppl 2):S29–35 [DOI] [PubMed] [Google Scholar]
- 9.Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr 2002;132:1062S–101S [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88 [DOI] [PubMed] [Google Scholar]
- 12.Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med 2001;20:3625–33 [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101 [PubMed] [Google Scholar]
- 15.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr 2007;85:385–91 [DOI] [PubMed] [Google Scholar]
- 16.Canales A, Benedí J, Nus M, Librelotto J, Sánchez-Montero JM, Sánchez-Muniz FJ. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J Am Coll Nutr 2007;26:225–32 [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto M, Imaizumi K, Sato M, et al. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr 2002;56:629–37 [DOI] [PubMed] [Google Scholar]
- 18.Morgan JM, Horton K, Reese D, Carey C, Walker K, Capuzzi DM. Effects of walnut consumption as part of a low-fat, low-cholesterol diet on serum cardiovascular risk factors. Int J Vitam Nutr Res 2002;72:341–7 [DOI] [PubMed] [Google Scholar]
- 19.Perez-Martinez P, Lopez-Miranda J, Blanco-Colio L, et al. The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor kappaB activation in peripheral blood mononuclear cells from healthy men. Atherosclerosis 2007;194:e141–6 [DOI] [PubMed] [Google Scholar]
- 20.Almario RU, Vonghavaravat V, Wong R, Kasim-Karakas SE. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr 2001;74:72–9 [DOI] [PubMed] [Google Scholar]
- 21.Zambón D, Sabaté J, Muñoz S, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med 2000;132:538–46 [DOI] [PubMed] [Google Scholar]
- 22.Spaccarotella KJ, Kris-Etherton PM, Stone WL, et al. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr J 2008;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med 1993;328:603–7 [DOI] [PubMed] [Google Scholar]
- 24.Ros E, Núñez I, Pérez-Heras A, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004;109:1609–14 [DOI] [PubMed] [Google Scholar]
- 25.Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr 2007;97:1144–53 [DOI] [PubMed] [Google Scholar]
- 26.Tapsell LC, Gillen LJ, Patch CS, et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27:2777–83 [DOI] [PubMed] [Google Scholar]
- 27.Chisholm A, Mann J, Skeaff M, et al. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr 1998;52:12–6 [DOI] [PubMed] [Google Scholar]
- 28.Sabaté J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr 2005;94:859–64 [DOI] [PubMed] [Google Scholar]
- 29.García-Lorda P, Megias Rangil I, Salas-Salvadó J. Nut consumption, body weight and insulin resistance. Eur J Clin Nutr 2003;57(suppl 1):S8–11 [DOI] [PubMed] [Google Scholar]
- 30.Davis L, Stonehouse W, Loots du T, et al. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr 2007;46:155–64 [DOI] [PubMed] [Google Scholar]