Abstract
Background: Although uncommon, iron deficiency (ID) occurs in breastfed infants. The regular provision of iron may prevent ID.
Objective: The objective was to test the feasibility and effectiveness of 2 modalities of providing iron (medicinal iron or iron-fortified cereal) to breastfed infants. The study tested the hypothesis that regular provision of iron improves iron status of breastfed infants without adverse effects.
Design: In this prospective, randomized, open-label trial, breastfed infants received on a regular basis either medicinal iron (n = 48) or an iron-fortified fruit-cereal combination (n = 45) from 4 to 9 mo or no intervention (control group; n = 59). The interventions provided 7.0–7.5 mg ferrous sulfate/d. Infants were enrolled at 1 mo and were followed to 2 y. Iron-status indicators were determined periodically, stool characteristics were recorded, and growth was monitored.
Results: The regular provision of iron led to improved iron status during and for some months after the intervention. Both sources of iron were about equally effective. Iron affected stool color but had no effect on feeding-related behavior. However, medicinal iron was associated with a small but significant reduction in length gain and a trend toward reduced weight gain. ID anemia was observed in 4 infants (2.3%), most of whom had a low birth iron endowment. Mild ID was common in the second year of life.
Conclusions: Regular provision of medicinal iron or iron-fortified cereal improves the iron status of breastfed infants and may prevent ID. Both modalities are equally effective, but medicinal iron leads to somewhat reduced growth. This trial was registered at clinicaltrials.gov as NCT00760890.
INTRODUCTION
The amount of iron contained in breast milk (0.2–0.4 mg/L) is so low that, despite its high bioavailability (1, 2), breast milk alone cannot meet the iron needs of infants. Because of growth, in the first year of life, the need for iron is relatively high—estimated at ≈0.7 mg absorbed Fe/d (3). To meet these needs during the first 4–6 mo of life, infants draw on their birth iron endowment, which consists of storage iron and hemoglobin iron (4, 5). When the endowment becomes exhausted, breastfed infants depend largely on iron from external sources for their iron needs. Some infants are born with diminished iron stores (6–8) and are apt to exhaust their iron endowment early. They are presumed to be at increased risk of iron deficiency (ID) and iron deficiency anemia (IDA).
Many studies have shown that breastfed infants indeed can develop ID and IDA early, ie, by 6 mo of age. Makrides et al (9) reported that 15% of 6-mo-old breastfed infants in Australia had ID and 1% had IDA. In Sweden, Domellöf et al (10) found the prevalence of IDA to be ≈1% among 5-mo-old infants and Lind et al (11) found it to be 2% among 6-mo-old infants. In Norway, Hay et al (12) reported the prevalence of low iron status to be 4% at 6 mo. Lozoff et al (13) reported that 3.6% of predominantly breastfed infants in Chile had IDA at 5–6 mo of age. In Denmark, no ID was found at 6 mo of age (14). We (15) observed early IDA in 1 of 32 breastfed infants and provided evidence that infants with IDA were born with a low iron endowment.
Because severe ID (IDA represents severe ID) in infants carries the risk of impaired behavioral and neurocognitive development (13, 16), the prevention of IDA is important. Iron can be provided to breastfed infants as medicinal iron or in the form of iron-fortified complementary foods, whereas nonfortified foods typically do not provide meaningful amounts of iron. Although medicinal iron is presumed to be effective in the prevention of ID, there is limited documentation of its efficacy in breastfed infants in industrialized countries (10). Information regarding the efficacy of dietary iron sources is similarly sparse.
One objective of the present study was to assess the feasibility and efficacy of regular consumption of medicinal iron drops or of an iron-fortified wet-pack cereal-fruit product. Both sources provided iron in the form of ferrous sulfate. Another objective was to describe the iron status and the principal sources of dietary iron of breastfed infants in whom no particular attention was being paid to the inclusion of iron-containing foods (control group). Although it would have been desirable to determine whether the provision of ferrous sulfate is effective at preventing ID, power calculations indicated that study of the requisite number of subjects was beyond the means of the study team.
Iron supplementation has in some studies been noted to affect growth negatively. Idjradinata et al (17) found that iron supplementation of preschool children slowed weight gain. Dewey et al (18) reported a significant negative effect of iron supplementation on length gain, but not on weight gain, in Swedish breastfed infants but not in Honduran infants. Majumdar et al (19) and Lind et al (20) found that iron supplementation slowed the growth of iron-replete infants and young children, whereas no such effect was seen in iron-depleted subjects.
SUBJECTS AND METHODS
Study design
The study objectives were pursued in a prospective, randomized, open-label trial with enrollment at 1 mo of age, intervention from 4 mo to 9 mo of age, and follow-up to 24 mo of age. The subjects were breastfed infants who were randomly assigned at 4 mo to 1 of 3 interventions: medicinal iron (FeMed), infant cereal (FeCer), or control. Infants in the FeMed group received 7.5 mg Fe/d in the form of ferrous sulfate. Infants in the FeCer group received daily one jar of wet-pack cereal that provided ≈7 mg Fe in the form of ferrous sulfate. Control infants were not to be given medicinal iron during the intervention period, but their feedings were entirely at the discretion of their parents.
Subjects
The subjects were term infants of both sexes with a birth weight >2500 g and who were considered healthy by their physicians and the investigators. The infants were born between August 2001 and July 2003. At recruitment at 1 mo of age, the infants were exclusively breastfed (vitamin-mineral drops were permitted) and were expected to be breastfed (partially) to 6 mo of age. The study team was not involved in providing health care to the subjects. However, when abnormal laboratory results were encountered, the subjects' parents and physicians were notified. The study protocol was reviewed and approved by the University of Iowa Institutional Review Board, and one parent provided written informed consent.
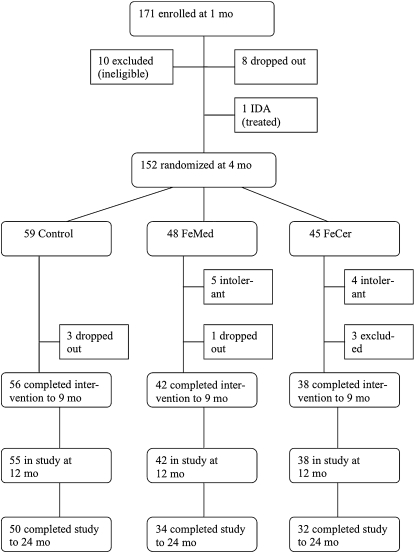
Of 171 infants enrolled at 1 mo of age, 5 were African American, 3 were Asian, 1 was Pacific Islander, 1 was Hispanic, and the remainder were white. The flow of subjects is indicated in Figure 1. Between 1 and 4 mo, 8 infants were withdrawn from the study for unknown reasons, 1 infant was excluded because he (subject 10117) developed ID at 2 mo of age and was treated with iron, and 10 infants were excluded at 4 mo because they received >200 mL formula/d, in most cases because of real or perceived insufficiency of the supply of breast milk. The remaining 152 infants were randomly assigned at 4 mo of age, and 136 completed the intervention at 9 mo; 116 were followed to 24 mo.
FIGURE 1.
Flow of participants through the trial. IDA, iron deficiency anemia; FeMed, breastfed infants who were randomly assigned at 4 mo medicinal iron; FeCer, breastfed infants who were randomly assigned at 4 mo to infant cereal.
Sample size
We hypothesized that regular consumption of iron would improve the iron status of infants. We felt that a difference of 12 μg/L in mean plasma ferritin concentration at the end of the intervention would be convincing evidence of improved iron status. Our own data (21) indicated that the mean (±SD) plasma ferritin concentration was 29.7 ± 16.5 μg/L in 9-mo old infants. Therefore, 32 subjects per group were needed for a 2-sided test. We increased the size of the control group by 50%, ie, to 48 to obtain a robust description of iron status among breastfed infants. Assuming an attrition rate of 35%, 172 infants needed to be enrolled to have 112 infants complete the intervention at 9 mo of age. Attrition was only 21%, and 136 infants completed the intervention.
Study interventions
Infants in the FeMed group were to receive each day, between 4 and 9 mo of age, 0.3 mL of the medicinal iron preparation Fer-In-Sol (Mead Johnson Nutritionals, Evansville, IN). This would provide 7.5 mg Fe/d in the form of ferrous sulfate in addition to sugar, sorbitol, and citric acid. Mothers were requested to give the medicinal iron once a day by dropper directly into the mouth of the infant and to do so just before beginning breastfeeding. The medicinal iron was to be given as long as the infant was being breastfed, regardless of how much supplemental formula was provided. Bottles of medicinal iron were weighed before dispensing and were weighed back when returned at monthly visits. The density of the solution was assumed to be 1.0 g/mL. Although parents occasionally reported spills, disappearance was assumed to represent consumption.
Infants in the FeCer group were to be fed every day wet-pack iron-fortified cereal-fruit products. Beginning at 4 mo, at each visit, the parents were provided a 1-mo supply (one jar per day) of the cereals of their choice. The choices were second Foods Rice Cereal with Applesauce, Mixed Cereal with Applesauce and Bananas, and Oatmeal with Applesauce and Bananas, all manufactured by the Gerber Products Company (Fremont, MI). Each jar provided 113 g of the cereal-fruit product with ≈7 mg Fe in the form of ferrous sulfate and 15.7 mg ascorbic acid. The predominant ingredient of these products is fruit, of which especially apples provide organic acids that may enhance iron absorption. Unused jars were returned at the next visit. Consumption of study cereal was optional between 112 and 140 d of age; however, from 140 d onward, the mothers were encouraged to feed at least half a jar each day and from 196 d on to feed one whole jar each day. The number of jars used was determined from the number dispensed and the number returned. Infants in the control group were fed entirely at the discretion of the parents, except that parents were asked to refrain from providing medicinal iron.
Study rules
With few restrictions, decisions regarding feedings were in the hands of the parents, including the duration of breastfeeding, use of supplemental formula, and use of complementary foods. However, to remain in the study and to be randomized at 4 mo, infants had to be predominantly breastfed, which was defined as receiving <200 mL supplemental formula/d, equivalent to ≈20% of estimated energy intake, and at 5.5 mo infants had to receive some breast milk at least once a day. During the intervention period, parents of infants in the control and FeCer groups were requested not to give medicinal iron, and infants in the FeMed and FeCer groups were asked not to receive any nonstudy cereal products. Infants who developed ID or IDA continued in the study, with the difference being that for infants with IDA treatment with iron in some form was recommended. Data obtained in infants with IDA after the inception of iron treatment were excluded from the data analysis.
Procedures
Infants visited the study center every 28 ± 4 d until 9 mo of age and every 3 mo ± 7 d between 12 and 24 mo. Most visits occurred within the specified limits, with only 8 visits occurring a few days outside the limits between 1 and 9 mo of age. At each visit, weight and length were measured by using established methods (22). Before each visit, parents completed a food intake record concerning the amount and type of complementary foods consumed during 2 d preceding the visit, and a stool and behavior record for the same period in which parents recorded the color, number, and consistency of stools and feeding-related behaviors such as spitting up, fussiness, and gas. At the beginning of each visit, the parents completed a questionnaire that asked about intervening illnesses (any, yes or no), feedings in general (breast, formula, complementary foods) and dietary supplements. The records and questionnaires were checked for completeness at the time of the visit by the nursing staff, and any ambiguities were clarified.
Blood collection and analysis
Blood was obtained at enrollment and at 4 mo (112 d), 5.5 mo (168 d), 7.5 mo (224 d), 9 mo (280 d), 12 mo, 15 mo, 18 mo, 21 mo, and 24 mo. Capillary blood was collected by heel stick with a disposable spring-loaded device (Tenderfoot; International Technidyne Corporation, Edison, NJ). Approximately 1 mL blood was collected into a heparin-treated tube. Hemoglobin concentrations and red blood cell indexes [mean corpuscular volume (MCV) and relative distribution width (RDW)] were measured immediately in whole blood with a Coulter AcT diff Hematology Analyzer (Coulter Corporation, Irving, TX). Blood was then centrifuged, and the plasma was used to measure ferritin by radioimmunoassay with the Quantimune kit (Bio-Rad Laboratories, Hercules, CA) and soluble transferrin receptor (TfR) by enzyme immunoassay (kit no. TF-94; Ramco, Houston, TX). C-reactive protein (CRP) was measured by using a modification of a 2-site enzyme-linked immunosorbent assay for TfR (23). The capture antibody was sheep anti-human CRP (ICN Cappel Research, Irvine, CA), the primary detection antibody was rabbit anti-human CRP (Calbiochem, San Diego, CA), and the secondary detection antibody was alkaline phosphatase–conjugated goat anti-rabbit IgG (Sigma-Aldrich, St Louis, MO).
Data analysis
Mild ID (depleted iron stores) was defined as a plasma ferritin concentration <10 μg/L and anemia as a hemoglobin concentration <105 g/L before 9 mo of age and <100 g/L at 9 mo of age and older (24). IDA was defined as anemia in the presence of plasma ferritin <10 μg/L. Data for infants who were treated for ID (see below), although shown, were not included in the data analysis after treatment began. Although the food intake records often (but not always) indicated the amounts of complementary foods consumed, we did not attempt to quantify the intake of iron from these foods but simply recorded (yes or no) the consumption of foods.
Because ferritin is an acute phase reactant, during inflammation or infection plasma ferritin may be elevated and may not be reflective of iron stores. The presence of ID can thus be masked and the size of iron stores overestimated. Unfortunately, concurrent measurement of CRP is not a reliable means of identifying plasma ferritin values that are elevated due to acute phase reaction. In the present longitudinal study, plasma ferritin was sometimes normal in the presence of elevated CRP; conversely, plasma ferritin was at times clearly elevated when CRP was normal. Presumably the explanation is that ferritin rises sooner and remains elevated longer than CRP (25). We have classified a plasma ferritin value as “elevated” if it was >40 μg/L and was 3-fold greater than the mean of the preceding and subsequent value (or either of these values in the case of plasma ferritin at 1 and 24 mo). The classification of elevated plasma ferritin values therefore rested entirely on 2 adjacent plasma ferritin values and not on CRP. During the period 1–9 mo, a total of 4 plasma ferritin values were classified as elevated and were excluded from data analysis; between 12 and 24 mo, 13 elevated plasma ferritin values were excluded.
Plasma ferritin values showed a nonnormal distribution at most ages. Statistical analysis was therefore performed after log transformation, but only nontransformed values with arithmetic means and SD are shown. Plasma ferritin and other indicators of iron status were compared between study groups by 2-factor (group and sex) analysis of variance (ANOVA) procedures and also by analysis of covariance (ANCOVA) procedures with plasma ferritin at baseline as covariate. Tukey's test of multiple comparisons was applied. Weight and length measurements were corrected to the exact nominal ages by parabolic interpolation. Weight and length attained and gains in weight and length were compared by ANOVA procedures. Stool and behavior data were expressed as proportions of total observations for each subject. Proportions for study groups were compared by ANOVA. Total number of stools was also compared by ANOVA. All statistical analyses were performed on a per-protocol basis and included subjects who completed the intervention at 9 mo. An intention-to-treat analysis was not contemplated in this efficacy trial, and no attempt was made to obtain data after the subjects left the study, regardless of the reason. Statistical analyses were performed by using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Of 152 infants randomly assigned at 4 mo of age, 59 infants (27 girls, 32 boys) were assigned to the control, 48 (25 girls, 23 boys) to the FeMed, and 45 (22 girls, 23 boys) to the FeCer group (Figure 1). Three infants left the control group before 9 mo of age for reasons unrelated to the study.
Six infants left the FeMed group, 3 because the infants were reluctant to take the drops, 2 because the parents felt that the drops caused side effects (1 vomiting, 1 constipation), and 1 for reasons unrelated to the study. Thus, medicinal iron was tolerated by all but 5 infants (90%). Weigh-back of the medicinal iron drops indicated that in the first 28 d of the trial (112–140 d), an average (±SD) of 0.47 ± 0.23 g/d (range: 0.08–1.15 g/d) of drops were used (disappeared). Use decreased somewhat as the trial progressed, but, in the final 28 d (252–280 d), the average (±SD) consumption among 39 users was still 0.41 ± 0.18 g/d (range: 0.10–1.1 g/d). Three infants no longer used iron drops because they were no longer being breastfed. Clearly, there was a tendency to use more than the intended 0.3 g/d of the medicinal iron preparation.
Of 7 infants who left the FeCer group, 1 infant left because of “allergy” to the study cereal, 1 because the cereal was thought to cause constipation, 2 because the infant refused to take the study cereal, and 3 because of an insufficient breast milk supply. Thus, cereal was accepted and tolerated by 91% of the infants. All infants in the FeCer group consumed some study cereal in the first month of the trial, with an average (±SD) consumption of 0.79 ± 0.24 jars/d (range: 0.15–1.0 jars/d). During the final month, consumption averaged 0.92 ± 0.18 jars/d (range: 0.43–1.0 jars/d).
Feedings
At the time of randomization at 4 mo, in addition to being breastfed, a total of 8 infants were receiving some supplemental formula (<200 mL/d) and 4 infants were receiving small amounts of cereal and/or other foods (Table 1). The percentage of infants receiving any breastfeeding declined after 5.5 mo, but was still 73% at 9 mo. The use of supplemental formula increased gradually after 4 mo. Nonstudy cereals were consumed after 4 mo by most of the control infants and by the occasional infant in the FeMed group (whose parents were requested not to feed cereal). These cereals were almost exclusively dry-pack infant cereals fortified with poorly available electrolytic iron. The percentage of infants fed other complementary foods increased sharply after 4 mo and kept increasing with age. The proportion of infants in the control group who were breastfed and received neither formula nor cereal was 24% at 5.5 mo, 12.5% at 7.5 mo, and 4% at 9 mo.
TABLE 1.
Number of infants in each of the 3 groups who received the specified feedings (not mutually exclusive)1
| Age |
|||||||||
| 4 mo | 5.5 mo | 7.5 mo | 9 mo | 12 mo | 15 mo | 18 mo | 21 mo | 24 mo | |
| Control | |||||||||
| All infants | 59 | 59 | 58 | 56 | 55 | 55 | 52 | 51 | 50 |
| Breast | 59 | 59 | 49 | 39 | 19 | 8 | 4 | 2 | 1 |
| Formula | 22 | 23 | 26 | 34 | 26 | 6 | 2 | 2 | 0 |
| Cow milk3 | 0 | 0 | 0 | 1 | 25 | 45 | 46 | 47 | 49 |
| Cereal4 | 2 | 39 | 47 | 48 | 41 | 35 | 36 | 26 | 25 |
| Other foods5 | 1 | 35 | 53 | 56 | 54 | 55 | 52 | 51 | 50 |
| FeMed | |||||||||
| All infants | 48 | 43 | 43 | 42 | 42 | 41 | 40 | 37 | 34 |
| Breast | 48 | 43 | 40 | 35 | 29 | 14 | 9 | 4 | 1 |
| Formula | 32 | 5 | 14 | 19 | 11 | 1 | 0 | 0 | 0 |
| Cow milk3 | 0 | 0 | 1 | 2 | 14 | 33 | 36 | 34 | 32 |
| Cereal4 | 0 | 1 | 4 | 3 | 27 | 26 | 22 | 25 | 18 |
| Other foods5 | 0 | 32 | 42 | 42 | 42 | 41 | 40 | 37 | 34 |
| FeCer | |||||||||
| All infants | 45 | 39 | 38 | 38 | 38 | 37 | 37 | 33 | 32 |
| Breast | 45 | 39 | 31 | 25 | 15 | 8 | 5 | 2 | 3 |
| Formula | 32 | 5 | 16 | 20 | 12 | 2 | 0 | 0 | 0 |
| Cow milk3 | 0 | 0 | 1 | 1 | 21 | 32 | 35 | 33 | 30 |
| Cereal4 | 0 | 39 | 38 | 38 | 28 | 28 | 27 | 23 | 21 |
| Other foods5 | 1 | 23 | 36 | 37 | 38 | 37 | 37 | 33 | 32 |
FeMed, medicinal iron; FeCer, infant cereal.
<200 mL/d.
Any liquid cow milk (skim, 2%, and full fat).
Study cereal in the FeCer group at 4–9 mo and nonstudy cereal in the control group and the FeMed group, including breakfast cereals.
Includes table foods.
After 9 mo of age, the percentage of infants who were breastfed continued to drop, but at 18 mo there were still a total of 18 infants who were breastfed and at 24 mo there were 5. After 12 mo, few subjects received formula and the great majority received some form of cow milk. Although most continued to receive cereals, these were predominantly noninfant cereals. There was a gradual switch from commercially prepared infant foods to home-prepared table foods (data not shown). Thus, whereas most of the breastfed infants received a source of iron in the form of formula or infant cereal or both beginning at ≈4 mo of age, a sizable minority of infants did not. During the second year of life, formula no longer played a role as a source of iron, whereas cereals continued to provide iron.
Iron status
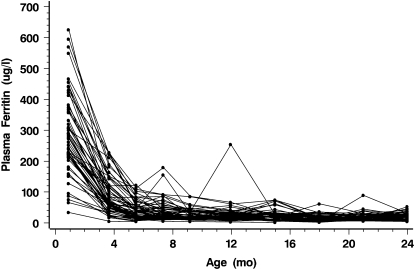
Data regarding iron status are presented in Table 2 for those 136 infants who completed the intervention at 9 mo. Data for 3 infants who became iron deficient and were treated with iron are included up to the point at which treatment began. All iron-status indicators showed the expected age-related changes, with plasma ferritin, hemoglobin, RDW, and MCV decreasing and TfR and ln[TfR/plasma ferritin] increasing between 1 and 4 mo. Individual raw plasma ferritin data for control infants presented in Figure 2 indicate the wide range of values at 1 mo and show some of the elevated values that were ignored in data analysis. The mean (±SD) plasma ferritin concentration for all groups at 1 mo was 307 ± 143 μg/L (range: 23–791 μg/L; 5th percentile: 87; 95th percentile: 575 μg/L). Plasma ferritin was significantly higher in girls than in boys at 4, 5.5, 7.5, and 9 mo but not at later ages (data not shown).
TABLE 2.
Iron status of infants in the 3 groups who completed the study to 9 mo1
| Age |
||||||||||
| 1 mo | 4 mo | 5.5 mo | 7.5 mo | 9 mo | 12 mo | 15 mo | 18 mo | 21 mo | 24 mo | |
| Ferritin (μg/L) | ||||||||||
| Control | 293 ± 146 | 89 ± 60 | 42a ± 29 | 36a ± 30 | 28a ± 17 | 25a ± 14 | 21a ± 16 | 17 ± 11 | 18 ± 9 | 21 ± 11 |
| FeMed | 318 ± 136 | 98 ± 67 | 68b ± 50 | 62b ± 55 | 50b ± 34 | 31a,b ± 19 | 23a,b ± 11 | 24 ± 14 | 19 ± 14 | 24 ± 13 |
| FeCer | 319 ± 153 | 134 ± 136 | 82b ± 67 | 61b ± 40 | 48b ± 32 | 39b ± 27 | 28b ± 16 | 22 ± 11 | 22 ± 13 | 25 ± 13 |
| TfR (mg/L) | ||||||||||
| Control | 3.19 ± 0.77 | 6.27 ± 1.06 | 6.14 ± 1.13 | 6.30 ± 1.18 | 6.14 ± 1.17 | 6.22 ± 1.12 | 6.65 ± 1.50 | 6.43 ± 1.41 | 6.41 ± 1.36 | 6.17 ± 1.20 |
| FeMed | 3.16 ± 0.78 | 6.37 ± 1.03 | 5.92 ± 1.31 | 6.00 ± 1.03 | 5.95 ± 1.10 | 6.14 ± 0.84 | 6.56 ± 0.92 | 6.34 ± 0.72 | 6.24 ± 1.03 | 6.21 ± 1.12 |
| FeCer | 3.02 ± 0.85 | 6.35 ± 1.18 | 5.89 ± 1.14 | 6.14 ± 0.86 | 5.91 ± 1.05 | 6.24 ± 0.89 | 6.34 ± 1.06 | 6.39 ± 1.02 | 6.32 ± 1.04 | 5.75 ± 1.00 |
| ln[TfR × 103/plasma ferritin] | ||||||||||
| Control | 2.50 ± 0.66 | 4.48 ± 0.81 | 5.24a ± 0.85 | 5.39a ± 0.76 | 5.56a ± 0.66 | 5.69a ± 0.70 | 6.01a ± 0.80 | 6.11 ± 0.72 | 6.00 ± 0.61 | 5.84 ± 0.73 |
| FeMed | 2.38 ± 0.62 | 4.41 ± 0.78 | 4.69b ± 0.82 | 4.83b ± 0.78 | 4.96b ± 0.69 | 5.46b ± 0.71 | 5.79b ± 0.60 | 5.78 ± 0.77 | 5.95 ± 0.66 | 5.75 ± 0.80 |
| FeCer | 2.33 ± 0.57 | 4.25 ± 1.00 | 4.55b ± 0.90 | 4.80b ± 0.69 | 4.99b ± 0.71 | 5.27b ± 0.72 | 5.55b ± 0.60 | 5.78 ± 0.68 | 5.80 ± 0.64 | 5.55 ± 0.64 |
| Hemoglobin (g/L) | ||||||||||
| Control | 127 ± 15 | 115 ± 7 | 115 ± 8 | 114 ± 8 | 116 ± 7 | 118 ± 7 | 116 ± 6 | 119 ± 9 | 123 ± 9 | 124 ± 7 |
| FeMed | 123 ± 14 | 114 ± 8 | 117 ± 9 | 114 ± 10 | 113 ± 8 | 118 ± 14 | 118 ± 9 | 118 ± 10 | 122 ± 10 | 124 ± 11 |
| FeCer | 124 ± 18 | 115 ± 8 | 115 ± 8 | 112 ± 8 | 115 ± 8 | 115 ± 8 | 118 ± 9 | 119 ± 9 | 122 ± 10 | 124 ± 14 |
| RDW (%) | ||||||||||
| Control | 15.0 ± 1.0 | 12.4 ± 0.9 | 12.8 ± 1.0 | 14.1 ± 1.1 | 13.9 ± 1.3 | 13.9 ± 1.1 | 14.0 ± 1.3 | 14.0 ± 1.3 | 13.9 ± 1.3 | 13.5 ± 1.3 |
| FeMed | 15.2 ± 0.9 | 12.4 ± 1.0 | 13.2 ± 1.2 | 14.1 ± 1.1 | 13.6 ± 1.0 | 13.6 ± 1.2 | 13.8 ± 1.0 | 14.1 ± 1.2 | 13.8 ± 1.4 | 13.5 ± 1.0 |
| FeCer | 15.0 ± 1.1 | 12.3 ± 0.9 | 12.8 ± 1.2 | 13.8 ± 1.2 | 13.5 ± 1.2 | 13.4 ± 1.4 | 14.1 ± 1.5 | 13.8 ± 1.3 | 13.5 ± 1.3 | 13.3 ± 1.0 |
| MCV (fL) | ||||||||||
| Control | 98 ± 3 | 82 ± 3 | 78 ± 4 | 78 ± 4 | 79 ± 4 | 80 ± 4 | 79 ± 4 | 79 ± 4 | 80 ± 4 | 81 ± 4 |
| FeMed | 98 ± 3 | 81 ± 3 | 78 ± 4 | 79 ± 3 | 79 ± 4 | 79 ± 4 | 79 ± 3 | 79 ± 3 | 80 ± 4 | 81 ± 4 |
| FeCer | 98 ± 3 | 82 ± 3 | 78 ± 3 | 79 ± 4 | 79 ± 3 | 80 ± 3 | 79 ± 4 | 79 ± 4 | 81 ± 4 | 80 ± 4 |
All values are means ± SDs. FeMed, medicinal iron; FeCer, infant cereal; TfR, transferrin receptor; RDW, relative distribution width; MCV, mean corpuscular volume. Values in same column with different superscript letters are significantly different, P < 0.05 (ANOVA); data for 3 infants who were treated with iron are included only up to the point at which treatment began (Table 3), and data for subject 10117 are not included.
FIGURE 2.
Raw plasma ferritin concentrations in individual infants in the control group, including elevated values that were not included in the data analysis.
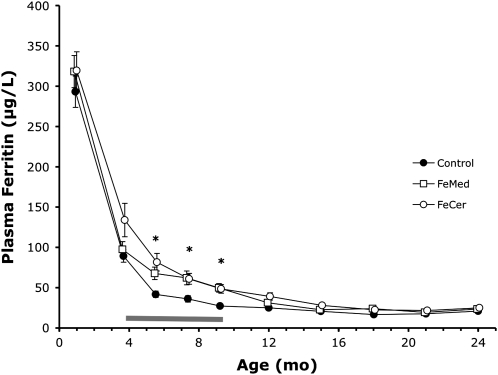
At 1 mo and at 4 mo, there were no significant differences between study groups for any of the iron-status indicators. During the intervention period, plasma ferritin was significantly lower at 5.5, 7.5, and 9 mo (Table 2, Figure 3), and ln[TfR/plasma ferritin] was significantly higher in control infants than in the FeMed and FeCer infants. The differences in plasma ferritin remained significant after correction for differences in plasma ferritin at 1 mo and at 4 mo by ANCOVA with plasma ferritin concentrations at 1 mo and at 4 mo, respectively, as covariates. There were no significant differences in TfR or any of the hematological indexes between study groups at any age. At no time was there a significant difference in iron status between the FeMed and FeCer groups. Within the control group, the iron status of those (few) infants who received neither formula nor cereal was not significantly lower than that of infants who did receive formula and/or cereal (data not shown). After the intervention, plasma ferritin remained significantly higher in the FeCer than in the control infants and ln[TfR/plasma ferritin] remained lower in the FeCer and FeMed infants than in the control infants at 12 and 15 mo. Thereafter, there were no significant differences in iron status. However, fewer infants in the FeCer and FeMed groups than in the control group became iron deficient during the second year of life (see below).
FIGURE 3.
Mean (±SE) plasma ferritin concentrations between 1 and 24 mo. Number of subjects at 4 mo was n = 59 in the control group, n = 48 in the medicinal iron (FeMed) group, and n = 45 in the infant cereal (FeCer) group; there were fewer subjects at later ages. The horizontal bar indicates the intervention period. *Significant differences between the control and FeMed plus FeCer groups, P < 0.05 (ANOVA and ANCOVA).
Iron deficiency
Data for infants who developed ID before and during the intervention are summarized in Table 3. A more detailed description of some of these infants is presented in Appendix A. If treatment with iron was recommended, infants were still followed. Values obtained after initiation of treatment are provided in Table 3, but were excluded from the data analysis.
TABLE 3.
Plasma ferritin and hemoglobin concentrations in infants in each of the 3 groups who developed iron deficiency before and during the intervention period1
| Age |
||||||
| Subject no. and group | 1 mo | 4 mo | 5.5 mo | 7.5 mo | 9 mo | 12 mo |
| 10117, Not randomized | ||||||
| Plasma ferritin (μg/L) | 23 | 132 | 262 | 252 | 102 | 102 |
| Hemoglobin (g/L) | 94 | 1032 | — | — | 1082 | 1242 |
| 09905, Control | ||||||
| Plasma ferritin (μg/L) | 2223 | 17 | 5 | 242 | 82 | 272 |
| Hemoglobin (g/L) | 103 | 112 | 98 | — | 1132 | 1062 |
| 09914, Control | ||||||
| Plasma ferritin (μg/L) | 87 | 39 | 13 | 14 | 9 | 6 |
| Hemoglobin (g/L) | 157 | 112 | 122 | 114 | 118 | 120 |
| 09931, Control | ||||||
| Plasma ferritin (μg/L) | 229 | 25 | 9 | 12 | 12 | 26 |
| Hemoglobin (g/L) | 95 | 108 | 112 | — | — | 121 |
| 09938, Control | ||||||
| Plasma ferritin (μg/L) | 159 | 20 | 7 | 12 | 23 | 15 |
| Hemoglobin (g/L) | 118 | 114 | 109 | 120 | 106 | 106 |
| 09943, Control | ||||||
| Plasma ferritin (μg/L) | 65 | 32 | 12 | 5 | 4 | 3 |
| Hemoglobin (g/L) | 142 | 116 | 128 | 121 | 123 | 119 |
| 09950, Control | ||||||
| Plasma ferritin (μg/L) | 34 | 5 | 4 | 152 | 222 | 132 |
| Hemoglobin (g/L) | 109 | 115 | 104 | 1062 | 1192 | 1232 |
| 10031, Control | ||||||
| Plasma ferritin (μg/L) | 422 | 71 | 23 | 23 | 9 | 13 |
| Hemoglobin (g/L) | 121 | 114 | 117 | 121 | 117 | 132 |
| 10043, Control | ||||||
| Plasma ferritin (μg/L) | 75 | 31 | 15 | 9 | 16 | 15 |
| Hemoglobin (g/L) | 143 | 116 | 112 | 109 | 120 | 125 |
| 10068, FeMed | ||||||
| Plasma ferritin (μg/L) | 51 | 9 | 7 | 162 | 102 | 132 |
| Hemoglobin (g/L) | 138 | 109 | 99 | 1102 | 1202 | 1162 |
| 09906, FeCer | ||||||
| Plasma ferritin (μg/L) | 109 | 23 | — | 19 | 9 | 52 |
| Hemoglobin (g/L) | — | 124 | 117 | 103 | 106 | 1062 |
FeMed, medicinal iron; FeCer, infant cereal.
Values were obtained during and after treatment with medicinal iron.
Probably elevated because the concurrent C-reactive protein concentration was 37.1 μg/L.
Before the intervention, one infant (subject 10117) developed IDA at 2 mo of age (plasma ferritin: 6 μg/L; hemoglobin: 93 g/L) (Table 3; also see Appendix A). This infant at 1 mo of age had the lowest plasma ferritin concentration (23 μg/L) of all infants in the study. The infant was treated with iron and was followed but was not randomly assigned for treatment, and the data are not included in Table 2. Two infants (subjects 09950 and 10068) had ID at the time of randomization at 4 mo (subject 09950 to the control group and subject 10068 to the FeMed group) and each went on to develop IDA within the next 2 mo (see below).
During the intervention, 2 infants in the control group developed IDA. Subject 09905 at 5.5 mo had a plasma ferritin concentration of 5 μg/L and a hemoglobin concentration of 98 g/L, was treated with iron, and remained free of anemia thereafter. Subject 09950, as mentioned previously, already had ID at the time of randomization and developed IDA by 5.5 mo with a plasma ferritin concentration of 4 μg/L and a hemoglobin concentration of 104 g/L. The infant was withdrawn from the study, and treatment with iron was recommended. Subsequently, the infant remained in good iron status. Also in the control group, ID without anemia developed in 2 infants at 5.5 mo, in 2 at 7.5 mo, and in 2 at 9 mo (Table 3). In all of these infants during follow-up, plasma ferritin returned to >10 μg/L without treatment, except for subject 09943, whose plasma ferritin concentration remained low but whose hemoglobin concentration remained >119 g/L (Appendix A). In the FeMed group, one infant (subject 10068) developed IDA at 5.5 mo with plasma ferritin concentration of 7 μg/L and hemoglobin concentration of 99 g/L. As mentioned, this infant already had ID at the time of randomization. This infant was frequently spitting up the medicinal iron drops and thus may not have received the full intended amount of iron. Treatment with a greater dose of iron was initiated, and the infant remained in good iron status. In the FeCer group, one infant (subject 09906) had ID without anemia at 9 mo (plasma ferritin: 9 μg/L; hemoglobin concentration: 106 gL). The proportion of infants with IDA did not differ significantly between the control (n = 2) and FeMed (n = 1) groups (P = 0.568, Fisher's exact test). However, the proportion of infants with ID + IDA in the control group (n = 8, prevalence of 14.3%) was significantly higher than the proportion (n = 2, prevalence 2.5%) in the combined FeCer and FeMed groups (P = 0.016, Fisher's exact test).
During the second year of life (15–24 mo), a total of 42 infants (37%) had ID on 1–3 occasions, but no infant had anemia. Among former control infants, 27 (56%) were iron deficient on one or more occasions (total of 49 values <10 μg/L) compared with 15 infants (23%) (total 27 values <10 μg/L) in the combined FeMed and FeCer groups; the difference was statistically significant (P < 0.001, chi-square test). Comparison of iron-status indicators of the 42 subjects who had ID with the 69 subjects who did not have ID indicated no differences in hemoglobin or MCV, but a significantly higher TfR concentration at all ages and a higher RDW at 21 and 24 mo in those with ID.
Tracking of plasma ferritin and hemoglobin
As shown in Table 4, correlations of plasma ferritin values were very high for short age intervals in the first year of life and became less strong with increasing length of the age interval but remained statistically significant, with a single exception, during the entire first 2 y of life. Correlations among hemoglobin values, in contrast, were low and mostly nonsignificant if they included the first mo of life but were strong and significant for later age intervals.
TABLE 4.
Linear correlation coefficients (r) between plasma ferritin and hemoglobin concentrations at different ages
| Age |
|||||||||
| 4 mo | 5.5 mo | 7.5 mo | 9 mo | 12 mo | 15 mo | 18 mo | 21 mo | 24 mo | |
| Plasma ferritin | |||||||||
| 1 mo | 0.7791 | 0.5971 | 0.5721 | 0.4621 | 0.4601 | 0.4221 | 0.2151 | 0.2031 | 0.180 |
| 4 mo | 0.7951 | 0.6861 | 0.5611 | 0.4571 | 0.3821 | 0.2091 | 0.2241 | 0.2271 | |
| 5.5 mo | 0.8321 | 0.7271 | 0.6531 | 0.4671 | 0.2901 | 0.2631 | 0.3091 | ||
| 7.5 mo | 0.8011 | 0.6891 | 0.5541 | 0.2961 | 0.3391 | 0.2701 | |||
| 9 mo | 0.6451 | 0.5801 | 0.3881 | 0.3701 | 0.4221 | ||||
| 12 mo | 0.5511 | 0.4131 | 0.3141 | 0.3361 | |||||
| 15 mo | 0.4891 | 0.2251 | 0.3281 | ||||||
| Blood hemoglobin | |||||||||
| 1 mo | 0.100 | 0.1901 | 0.060 | 0.2341 | 0.177 | 0.051 | −0.082 | 0.103 | −0.062 |
| 4 mo | 0.5841 | 0.5711 | 0.4981 | 0.3731 | 0.3811 | 0.3941 | 0.4241 | 0.2941 | |
| 5.5 mo | 0.7371 | 0.5281 | 0.4261 | 0.4661 | 0.4311 | 0.4291 | 0.3211 | ||
| 7.5 mo | 0.6011 | 0.4781 | 0.5191 | 0.5021 | 0.4681 | 0.3341 | |||
| 9 mo | 0.4081 | 0.4081 | 0.4361 | 0.3061 | 0.2691 | ||||
| 12 mo | 0.4151 | 0.3061 | 0.2581 | 0.2401 | |||||
| 15 mo | 0.6111 | 0.5051 | 0.4141 | ||||||
Statistically significant (P < 0.05).
In the control group, the higher the plasma ferritin concentration at 1 mo, the lower the risk of having ID during the first 9 mo of life (P for trend = 0.01). The odds ratio for ID decreased by 12% with every 10- μg/L increase in plasma ferritin at 1 mo. For subjects with a plasma ferritin concentration at 1 mo in the lowest quartile (plasma ferritin <211 μg/L), the odds ratio of developing ID in the first year of life was 7.7 (95% CI: 1.6, 35.7).
Stool characteristics, side effects, and illness
For each infant, 12 d worth of stool records were obtained (2 d preceding each visit) during the intervention period. There were no differences in stool number and consistency between study groups at any time, but there were differences in stool color. Infants receiving supplemental iron (FeMed + FeCer groups) had significantly (P = 0.004) more green and black stools than did the control infants. Differences in stool color between the FeMed and FeCer groups were not significant. There were no differences in the frequency of regurgitation, fussiness, colic, or gassiness between groups. There were no differences in the frequency of illnesses during the intervention period.
Growth
There were no differences in weight (Table 5) or length (Table 6) at trial entry (data not shown). At 4 mo and through 21 mo, boys were significantly heavier and longer than girls. On a sex-specific basis, mean weight and length of the 3 study groups were similar. However, weight gain between 4 and 9 mo of both boys and girls was less in the FeMed group than in either the control or FeCer groups. The treatment effect was not significant (P = 0.083) for weight gain but was significant (P = 0.039) for length gain. In post hoc tests, the difference between the FeMed and control groups was significant for weight gain (P < 0.027) and length gain (P < 0.011). On the other hand, differences in gains between the FeMed and FeCer groups were not significant, nor were differences between the FeCer and control groups or between the FeMed and FeCer groups combined and the control group. There were no differences in weight or length gain during the second year of life, nor were there differences in weight or length at the end of the study at 2 y (data not shown).
TABLE 5.
Growth (weight) data for the 3 groups of infants1
| Weight (g) |
|||||||||
| 4 mo |
9 mo |
Weight gain (g/d) 4–9 mo |
|||||||
| Control | FeMed | FeCer | Control | FeMed | FeCer | Control | FeMed | FeCer | |
| Boys | 6838 ± 726 (32) | 6993 ± 692 (23) | 6736 ± 752 (23) | 9179 ± 877 (28) | 9114 ± 970 (18) | 8883 ± 755 (19) | 13.9 ± 3.30 (28) | 12.7 ± 2.39 (18) | 13.0 ± 2.57 (19) |
| Girls | 6291 ± 558 (27) | 6299 ± 455 (25) | 6200 ± 664 (22) | 8555 ± 772 (26) | 8197 ± 732 (23) | 8496 ± 871 (19) | 13.3 ± 3.39 (26) | 11.6 ± 3.23 (23) | 13.2 ± 2.73 (19) |
| Both sexes combined | — | — | — | — | — | — | 13.58 | 12.182 | 13.13 |
| P (ANOVA) | |||||||||
| Sex | 0.001 | 0.001 | 0.351 | ||||||
| Treatment | 0.420 | 0.410 | 0.083 | ||||||
| Sex × treatment | 0.800 | 0.369 | 0.603 | ||||||
All values are means ± SDs; n in parentheses. FeMed, medicinal iron; FeCer, infant cereal.
Significantly different from control, P = 0.027.
TABLE 6.
Growth (length and length gain) data for the 3 groups of infants1
| Length (cm) |
|||||||||
| 4 mo |
9 mo |
Length gain (mm/d) 4–9 mo |
|||||||
| Control | FeMed | FeCer | Control | FeMed | FeCer | Control | FeMed | FeCer | |
| Boys | 62.2 ± 4.11 (32) | 63.5 ± 1.57 (23) | 63.2 ± 1.60 (23) | 71.5 ± 1.84 (28) | 72.0 ± 1.92 (18) | 71.7 ± 1.50 (19) | 0.51 ± 0.060 (28) | 0.50 ± 0.071 (18) | 0.50 ± 0.073 (19) |
| Girls | 61.3 ± 1.46 (27) | 61.5 ± 1.56 (25) | 61.1 ± 2.04 (22) | 70.1 ± 1.96 (26) | 69.2 ± 1.79 (23) | 69.7 ± 2.32 (19) | 0.52 ± 0.072 (26) | 0.47 ± 0.062 (23) | 0.50 ± 0.055 (19) |
| Both sexes combined | — | — | — | — | — | — | 0.519 | 0.4842 | 0.503 |
| P (ANOVA) | |||||||||
| Sex | 0.001 | 0.001 | 0.646 | ||||||
| Treatment | 0.303 | 0.839 | 0.039 | ||||||
| Sex × treatment | 0.323 | 0.220 | 0.321 | ||||||
All values are means ± SDs; n in parentheses. FeMed, medicinal iron; FeCer, infant cereal.
Significantly different from control, P = 0.011.
DISCUSSION
The present study showed that providing iron to breastfed infants is feasible and effective at enhancing iron status. Medicinal iron and an iron-fortified cereal-fruit combination were about equally effective at improving iron status, and both were well tolerated. Our study showed that without attention to iron (control infants), intake of iron-containing complementary foods tends to be insufficient to prevent ID. The study showed that breastfed infants are susceptible to early severe ID and that a low birth endowment is primarily responsible for early deficiency. Medicinal iron was associated with a significant but small decrease in growth.
The infants we studied were exclusively breastfed until 4 mo of age and during that period depended entirely on their birth iron endowment to meet iron needs for growth. Complementary foods and formula were gradually introduced after 4 mo. A rising proportion of study infants ceased to be breastfed after age 5.5 mo. Our infants tended to receive cereals and formula somewhat later in life than was the national average for breastfed infants in the United States in 2005–2007 (26, 27). It appears that breastfeeding was more exclusive in other studies of breastfed infants, although actual consumption of supplemental foods was not always reported (10, 11).
The present study showed that it is feasible to provide ferrous sulfate, a well-absorbed form of iron, to breastfed infants on a regular basis. Ferrous sulfate was about equally effective whether it was provided as medicinal iron or as fortification iron as a wet-pack cereal-fruit combination. Both forms were equally well tolerated. In only 10% of infants was medicinal iron perceived by the parents to have side effects or to be poorly tolerated and, similarly, in only 9% of infants was cereal unacceptable. Thus, ≥90% of infants accepted and tolerated either form of iron. Both sources of iron led to stools being green or dark more often than when iron was not provided regularly, but there was no effect on stool consistency or feeding-related behaviors.
The study showed that the daily consumption of ≈7 mg Fe as ferrous sulfate was effective at increasing iron stores of breastfed infants as indicated by plasma ferritin concentration. The effect was comparable in size with the effect observed when a daily supplement of 7 mg was provided to breastfed infants from 1 to 5.5 mo of age (15). The efficacy was the same whether iron was provided as medicinal iron or as an iron-fortified cereal-fruit combination, except that iron provided from cereal seemed to enhance iron stores beyond the period of supplementation, whereas medicinal iron did not. The apparent extended effect of cereal on iron stores could have been the result of parents continuing the practice of regularly feeding cereal after having done so during the study. Our food intake records did not allow us to confirm or refute this possible explanation. That a similar extended effect was not observed with medicinal iron may suggest that the latter was less well accepted than was cereal. No extended effect of medicinal iron supplementation was evident in our earlier study (15).
Besides plasma ferritin, none of the other indicators of iron status were influenced by iron supplementation, except for ln[TfR/plasma ferritin], which was decreased. However, ln[TfR/plasma ferritin] was highly inversely correlated with plasma ferritin at all ages (data not shown), which suggests that its variation is largely reflective of variation in plasma ferritin. Others (28) have also concluded that plasma ferritin provides as good an estimate of iron stores as any of the TfR/plasma ferritin ratios. The absence of effects on indicators that reflect tissue iron availability suggests that, on average, the availability of iron to tissues was not compromised.
Although the study was not designed and powered to determine whether the provision of iron prevents ID, a difference in the prevalence of ID between the control and the intervention groups was evident (Table 3). Of the 56 control infants, 6 had ID and 2 had IDA (prevalence: 14.3%). Of the 80 infants who received supplemental iron (FeMed and FeCer groups), 1 had ID and 1 had IDA (prevalence: 2.5%; P = 0.016). Thus, we conclude that supplemental iron protects significantly, albeit not completely, against ID. Why medicinal iron failed to prevent the progression from ID to IDA in the case (subject 09905) who had ID at randomization is unclear. It is possible that this infant did not receive or retain all of the iron he was intended to receive. However, it is also possible that the intended amount of iron (≈7 mg/d) was insufficient to correct the existing ID.
Medicinal iron was associated with decreased growth. The effect was small and seemed to be more pronounced with regard to length gain than weight gain. Dewey et al (18) similarly reported a significant negative effect of iron supplementation on length gain among Swedish infants, but not among Honduran infants, between 4 and 9 mo of age. In young Indonesian children (12–18 mo), Idjradinata et al (17) showed that iron supplementation slowed growth significantly. Majumdar et al (19) and Lind et al (20) found that iron slowed the growth of iron-replete infants and children but not that of iron-depleted infants and children. In our previous study (15) of infants with replete iron stores, iron supplementation led to decreased weight gain but not length gain in female infants. In male infants, interpretation of growth data was compromised by unequal birth weights. In the study by Dijkhuizen et al (29), iron supplementation between 4 and 10 mo had no effect on growth, but initial iron status was not taken into account as in the other studies (18–20) that did find effects on growth. Thus, the present study confirms earlier studies showing that medicinal iron decreases the growth of iron-replete infants.
We present evidence that infants in the FeMed group may have received considerably more than the intended 7 mg ferrous sulfate/d. With all due caution in interpreting disappearance data, the consistency of the data suggests that many infants really did receive >7 mg each day, perhaps as much as 11 mg/d on average. In the FeCer group, in contrast, our data indicate that the iron intake was close to the intended 7 mg/d. The difference in actual dose of iron received, but also the fact that medicinal iron was ingested as a single dose whereas cereal iron was ingested in multiple small amounts, may explain why an effect on growth was observed only in the FeMed group and not in the FeCer group. The mechanism by which iron decreases the rate of growth is not known. Regardless of the mechanism, the implication is that medicinal iron should be used with caution and preferably only in infants with depleted iron stores. In infants at high risk of IDA, ie, those born with low iron stores, the use of medicinal iron in a prophylactic mode may be justified on the grounds that a modest effect on growth is the lesser of 2 evils compared with IDA, with its potential for lasting effects on cognitive development. Lesser doses of iron than the 7 mg/d used in the present study seem not to have been explored in breastfed infants but might protect against ID while lacking effects on growth.
An important finding of the present study was that breastfed infants are susceptible to early severe ID. This confirms what has been reported before for many industrialized countries (1, 11–14, 16), but contradicts the sometimes held notion that breastfeeding protects infants from ID. In the control group, 2 infants developed IDA and 6 developed ID without anemia (prevalence: 14.3%). However, the more important statistic was the total number of infants with IDA before 6 mo of life, ie, 4 of 171 infants or 2.3%. The importance of early IDA lies in the fact that it is unlikely to be recognized by current child health care practices and therefore could exist for months before dietary sources provide sufficient iron to correct it.
Our study provides evidence that a low birth iron endowment places breastfed infants at risk of early ID. Using plasma ferritin at 1 mo as proxy for the iron endowment, 3 of the 4 infants who had IDA by 6 mo of age were born with a low iron endowment, ie, plasma ferritin below the fifth percentile (87 μg/L) at 1 mo. In the fourth infant (subject 09905), plasma ferritin was likely to have been spuriously elevated at 1 mo. Others have reported that some infants are born with low iron stores and may be at increased risk of ID (6–8). Tamura et al (8) showed that infants born with low iron endowments are at risk of impaired neurodevelopmental outcome, the actual cause of which might have been unrecognized severe ID in infancy rather than the low iron endowment per se. Although in some cases the cause of low iron endowment may be known (6), most often it is not. The strong correlation of plasma ferritin at 1 mo with plasma ferritin as far away as 2 y of age suggests that genetic factors play a role in determining the size of iron stores. Hay et al (12) also reported tracking of serum ferritin between 6 and 12 mo but not between 6 and 24 mo or between 12 and 24 mo. Hernell and Lönnerdal (30) reported “strong tracking” (no data given) of serum ferritin and other indicators of iron status.
A surprise was the finding that iron supplementation from 4 to 9 mo was associated with a lower prevalence of mild ID during the second year of life. There also was a tendency toward higher iron stores. ID during the second year of life was always mild and was not accompanied by evidence of limited tissue iron availability. However, in light of the findings of Lozoff et al (31), effects of mild ID on infant and toddler behavior cannot be ruled out.
In the control group, most of the infants received infant cereal after 4 mo and ≈50% received some formula, both of which are rich sources of fortification iron. However, these infants had lower iron stores than did infants in the 2 intervention groups. This suggests that the intake of iron in the control infants was less than the 7 mg/d received by the intervention groups.
The present study had limitations. Because of the limited sample size, the study did not have the power to determine unequivocally whether provision of 7 mg Fe/d prevents ID. Nevertheless, there clearly was less ID among infants receiving iron. A strength of the study was that an estimate of the birth iron endowment was provided and that infants were observed longitudinally from 1 to 24 mo of age.
In conclusion, the present study showed that the regular provision of iron to breastfed infants is feasible and is effective at increasing iron stores and decreasing the prevalence of ID. Medicinal iron and iron-fortified cereal were about equally effective. Medicinal iron but not cereal led to a decrease in growth, albeit a small one. Several infants developed IDA early in life and most of these infants had evidence of a low birth iron endowment. It may be possible to detect infants at risk of early severe ID by screening for low plasma ferritin early in life.
Acknowledgments
We thank Charles Rebouche and Joyce Dunlap for performing the laboratory analyses and gratefully acknowledge Hannes Ledolter for assistance with the statistical analysis.
The authors' responsibilities were as follows—EEZ: designed the study, supervised the execution of the data analysis, and wrote the manuscript; SEN: designed the study, managed the data, and conducted the data analysis; and JMJ: performed and/or supervised the subject recruitment and all the study procedures. The funding sources had no influence on the design or interpretation of the study. Neither Gerber nor Mead Johnson were involved in designing the study or analyzing the data. None of the authors had a conflict of interest.
APPENDIX A
Infants with iron deficiency anemia (IDA; n = 4) and infants with unusual courses (n = 2).
Subject 10117
This male infant with a birth weight of 3147 g was born at term. At 1 mo of age, he had an extremely low ferritin concentration of 23 μg/L and a hemoglobin concentration of 94 g/L. The mother reported that the pregnancy was uncomplicated. The mother denied having anemia and took vitamin and iron supplements during pregnancy. At 2 mo of age, the infant's plasma ferritin concentration had fallen to 6 μg/L and the hemoglobin concentration was 93 g/L; treatment with iron was recommended, but for unknown reasons was not implemented. At 3 mo of age, the ferritin concentration was 4 μg/L and the hemoglobin concentration was 96 g/L. Iron treatment was started. After 1 mo (at age 4 mo), the plasma ferritin concentration was 13 μg/L and the hemoglobin concentration was 103 g/L; after more treatment, the plasma ferritin concentration was 19 μg/L and the hemoglobin concentration was 112 g/L at age 5.5 mo. The infant started to receive cereal at this point and formula 1 mo later. At 9 mo of age, after iron treatment had been discontinued for some time, the plasma ferritin concentration was 10 μg/L and the hemoglobin concentration was 108 g/L. At subsequent checks, the plasma ferritin concentration was <10 μg/L on 2 occasions (at 18 and 24 mo), but the hemoglobin concentration remained ≥112 g/L. This infant was not randomly assigned to treatment, and iron status data are not included in Table 2.
Subject 09905
This male infant was born at term with a birth weight of 2892 g. At 1 mo of age, he had a plasma ferritin concentration of 222 μg/L and a hemoglobin concentration of 103 g/L. Because the C-reactive protein concentration was 37.1 μg/L, the plasma ferritin concentration (222 μg/L) was most likely elevated but was not classified as such by our rules (see Data analysis). The infant was closely monitored because of early anemia. At 2 mo of age, the plasma ferritin concentration was 91 μg/L and the hemoglobin concentration was 98 g/L. At 3 mo of age, the plasma ferritin concentration was 42 μg/L and the hemoglobin concentration was 103 g/L. At 4 mo of age, the ferritin concentration was 17 μg/L and the hemoglobin concentration was 112 g/L. The infant was randomly assigned to the control group. At 5.5 mo of age, the plasma ferritin concentration was 5 μg/L and the hemoglobin concentration was 98 g/L. The infant started receiving cereal. When IDA was confirmed 1 mo later (plasma ferritin: 4 μg/L; hemoglobin: 90 g/L), iron treatment was initiated. Within 1 mo the plasma ferritin concentration rose to 24 μg/L (hemoglobin was not measured), but at 9 mo the plasma ferritin concentration was again 8 μg/L and the hemoglobin concentration was 113 g/L. Supplemental formula was started. At none of the subsequent checks did this infant have ID.
Subject 09943
This female infant was born at term with a weight of 3657 g. The plasma ferritin concentration at 1 mo of age was 65 μg/L and the hemoglobin concentration was 142 g/L. Because plasma ferritin was low, the measurements were repeated at 2 mo of age (plasma ferritin: 58 μg/L; hemoglobin: 115 g/L). At 4 mo of age, the plasma ferritin concentration was 32 μg/L and the hemoglobin concentration was 116 g/L; the infant was randomly assigned to the control group. At 5.5 mo of age, the plasma ferritin concentration was 12 μg/L and the hemoglobin concentration was 128. At 7.5 mo of age, the plasma ferritin concentration was 5 μg/L and the hemoglobin concentration was 121; the infant started receiving cereal. At 9 mo of age, the plasma ferritin concentration was 4 μg/L and the hemoglobin concentration was 123 g/L. It was recommended that greater amounts of iron-fortified foods be fed, but the plasma ferritin concentration remained low (3 μg/L) at 12 mo with a hemoglobin concentration of 119 g/L. At 15 mo of age, the plasma ferritin concentration was 1 μg/L and the hemoglobin concentration was 114 g/L. This infant left the study at this point without having received any formula.
Subject 09950
This male infant was born at term with a weight of 3657 g. At 1 mo of age, the plasma ferritin concentration was 34 μg/L and the hemoglobin concentration was 109 g/L. At 4 mo of age, the plasma ferritin concentration was 5 μg/L and the hemoglobin concentration was 115 g/L. The infant was randomly assigned to the control group. At 5.5 mo of age, the plasma ferritin concentration was μg/L 4 and the hemoglobin concentration was 104 g/L. The infant met the criteria for IDA, and iron treatment was initiated. The infant started to receive supplemental formula at 6.5 mo of age. At 7.5 mo of age, the plasma ferritin concentration was 15 μg/L and the hemoglobin concentration was 106 g/L; cereal was started. At 9 mo of age, the plasma ferritin concentration was 22 μg/L and the hemoglobin concentration was 119 g/L. Thereafter, this infant continued to maintain good iron status, except at 24 mo of age, when the plasma ferritin concentration was 9 μg/L and the hemoglobin concentration was 132 g/L.
Subject 10068
This male infant was born at term with a birth weight of 3203 g. At 1 mo of age, the plasma ferritin concentration was 51 μg/L and the hemoglobin concentration was 138 g/L. At 4 mo of age, the plasma ferritin concentration was 9 μg/L and the hemoglobin concentration was 109 g/L. The infant was randomly assigned to the FeMed group. At 5.5 mo of age, the plasma ferritin concentration was 7 μg/L and the hemoglobin concentration was 99 g/L. The mother reported that the infant was frequently spitting up the medicinal iron. Treatment with a larger daily dose of iron was recommended, but at 6.5 mo of age the plasma ferritin concentration was still 7 μg/L and the hemoglobin concentration was 99 g/L. After further consultation with the parents of the need for iron treatment and initiation of cereal, the plasma ferritin concentration was 16 μg/L and the hemoglobin concentration was 110 g/L at 7.5 mo. Subsequently, this infant remained in good iron status and free of anemia.
Subject 09906
This male infant was born at term with a weight of 4196 g. At 1 mo of age, the plasma ferritin concentration was 109 μg/L (hemoglobin not available). At 4 mo of age, the plasma ferritin concentration was 23 μg/L and the hemoglobin concentration was 124 g/L. The infant was randomly assigned to the FeCer group. Supplemental formula was started at 6.5 mo of age. At 7.5 mo of age, the plasma ferritin concentration was 19 μg/L and the hemoglobin concentration was 103 g/L. At 9 mo of age, the plasma ferritin concentration was 9 μg/L and the hemoglobin concentration was 106 g/L. At this point, the infant's physician started supplementation with medicinal iron. At 12 mo of age, the plasma ferritin concentration was still only 5 μg/L and the hemoglobin concentration was 106 g/L; however, the plasma ferritin concentration gradually increased to 14.5 μg/L at 24 mo with a hemoglobin concentration of 119 g/L.
REFERENCES
- 1.Saarinen UM, Siimes MA, Dallman PR. Iron absorption in infants: high bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J Pediatr 1977;91:36–9 [DOI] [PubMed] [Google Scholar]
- 2.Hicks PD, Zavaleta N, Chen Z, Absrams SA, Lönnerdal B. Iron deficiency, but not anemia, upregulates iron absorption in breast-fed Peruvian infants. J Nutr 2006;136:2435–8 [DOI] [PubMed] [Google Scholar]
- 3.Food and Nutrition Board, Institute of Medicine Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press, 2000 [Google Scholar]
- 4.Dallman PR, Siimes MA, Stekel A. Iron deficiency in infancy and childhood. Am J Clin Nutr 1980;33:86–118 [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Chaparro CM. Iron status of breast-fed infants. Proc Nutr Soc 2007;66:412–22 [DOI] [PubMed] [Google Scholar]
- 6.Georgieff MK, Landon MB, Mills MM, et al. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr 1990;117:455–61 [DOI] [PubMed] [Google Scholar]
- 7.Georgieff MK, Wewerka SW, Nelson CA, de Regnier R-A. Iron status at 9 months of infants with low iron stores at birth. J Pediatr 2002;141:405–9 [DOI] [PubMed] [Google Scholar]
- 8.Tamura T, Goldenberg RL, Hou J, et al. Cord serum ferritin concentrations and mental psychomotor development of children at five years of age. J Pediatr 2002;140:165–70 [DOI] [PubMed] [Google Scholar]
- 9.Makrides M, Leeson R, Gibson RA, Simmer K. A randomized controlled clinical trial of increased dietary iron in breast-fed infants. J Pediatr 1998;133:559–62 [DOI] [PubMed] [Google Scholar]
- 10.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138:679–87. [DOI] [PubMed] [Google Scholar]
- 11.Lind T, Lönnerdal B, Persson L-A, Stenlund H, Tennefors K, Hernell O. Effects of weaning cereals with different phytate contents on hemoglobin, iron stores, and zinc: a randomized intervention in infants from 6 to 12 mo of age. Am J Clin Nutr 2003;78:168–75 [DOI] [PubMed] [Google Scholar]
- 12.Hay G, Sandstad B, Whitelaw A, Borch-Iohnsen B. Iron status in a group of Norwegian children aged 6-24 months. Acta Paediatr 2004;93:592–8 [PubMed] [Google Scholar]
- 13.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–54 [PubMed] [Google Scholar]
- 14.Michaelsen KF, Milman N, Samuelson G. A longitudinal study of iron status in healthy Danish infants: effects of early iron status, growth velocity and dietary factors. Acta Paediatr 1995;84:1035–44 [DOI] [PubMed] [Google Scholar]
- 15.Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. Am J Clin Nutr 2009;89:525–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64:S34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet 1994;343:1252–4 [DOI] [PubMed] [Google Scholar]
- 18.Dewey KG, Dommellöf M, Cohen RJ, Rivera LL, Hernell O, Lönnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in Sweden and Honduras. J Nutr 2002;132:3249–55 [DOI] [PubMed] [Google Scholar]
- 19.Majumdar I, Paul P, Talib H, Ranga S. The effect of iron therapy on the growth of iron-replete and iron-deplete children. J Trop Pediatr 2003;49:84–8 [DOI] [PubMed] [Google Scholar]
- 20.Lind T, Seswandhana R, Persson L-A, Lönnerdal B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 2008;97:770–5 [DOI] [PubMed] [Google Scholar]
- 21.Ziegler EE, Jiang T, Romero E, Vinco A, Frantz JA, Nelson SE. Cow's milk and intestinal blood loss in late infancy. J Pediatr 1999;135:720–6 [DOI] [PubMed] [Google Scholar]
- 22.Fomon SJ, Nelson SE. Size and growth. In: Fomon SJ, ed Nutrition of normal infants. St. Louis, MO: Mosby; 1993:36–84 [Google Scholar]
- 23.Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med 1989;114:368–77 [PubMed] [Google Scholar]
- 24.Domellöf M, Dewey KG, Lönnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency should be reevaluated. J Nutr 2002;132:3680–6 [DOI] [PubMed] [Google Scholar]
- 25.Feelders RA, Vreugdenhil G, Eggermont AMM, Kuiper-Kramer PA, van Eijk HG, Swaak AJG. Regulation of iron metabolism in the acute-phase response: interferon γ and tumour necrosis factor α induce hypoferraemia, ferritin production and a decrease in circulating transferrin receptors in cancer patients. Eur J Clin Invest 1998;28:520–7 [DOI] [PubMed] [Google Scholar]
- 26.Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics 2008;122:S36–42 [DOI] [PubMed] [Google Scholar]
- 27.Dee DL, Sharma AJ, Cogswell ME, Grummer-Strawn LM, Fein SB, Scanlon KS. Sources of supplemental iron among breastfed infants during the first year of life. Pediatrics 2008;122:S98–104 [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Dewey KG, Lönnerdal B, et al. Comparison of plasma ferritin concentration with the ratio of plasma transferrin receptor to ferritin in estimating body iron stores: results of 4 intervention trials. Am J Clin Nutr 2008;87:1892–8 [DOI] [PubMed] [Google Scholar]
- 29.Dijkhuizen MA, Wieringa FT, West CE, Martuti S, Muhilal Effects of iron and zinc supplementation in Indonesian infants on micronutrient status and growth. J Nutr 2001;131:2860–5 [DOI] [PubMed] [Google Scholar]
- 30.Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr 2002;76:858–64 [DOI] [PubMed] [Google Scholar]
- 31.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobsen SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr 2008;152:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]