Abstract
Background: We previously reported that a randomized controlled trial of antenatal micronutrient supplements in rural Nepal decreased the risk of low birth weight by ≈15%.
Objective: The objective was to examine the effects of micronutrient supplementation on growth and body composition in children of supplemented mothers through school age.
Design: Mothers received 1 of 5 micronutrient supplements daily: folic acid, folic acid + iron, folic acid + iron + zinc, multiple micronutrients, or a control. All of the supplements contained vitamin A. Children born during this trial were revisited at age 6–8 y to measure height, weight, midupper arm circumference, waist circumference, and triceps and subscapular skinfold thicknesses. Arm fat and muscle area were estimated by using standard formulas, and height-for-age, weight-for-age, and body mass index–for-age z scores were calculated by using the World Health Organization growth standard.
Results: Of the 3771 surviving children, 3324 were revisited and consented to anthropometric measurements. Maternal supplementation with folic acid + iron + zinc resulted in an increase in mean height (0.64 cm; 95% CI: 0.04, 1.25) and a reduction in mean triceps skinfold thickness (−0.25 mm; 95% CI: −0.44, −0.06), subscapular skinfold thickness (−0.20 mm; 95% CI: −0.33, −0.06), and arm fat area (−0.18 cm2; −0.34, −0.01). No significant differences were found between groups in mean weight or body mass index–for-age z scores, waist circumference, or arm muscle area. Other micronutrient combinations including a multiple micronutrient formulation failed to show a growth benefit.
Conclusion: Antenatal supplementation with zinc may benefit child growth, particularly in areas where a deficiency of this nutrient is common.
INTRODUCTION
Maternal and child undernutrition remains a major public health problem worldwide and is an underlying cause of 3.5 million deaths each year (1). In many developing regions of the world, growth restriction begins in utero and continues through infancy and early childhood (2). Impaired growth is a consequence of undernutrition, with an increasing recognition that micronutrient deficiencies may also play a role in this process. Recently, much research on whether micronutrient interventions delivered during pregnancy can improve birth weight and reduce early infant mortality in developing countries has been conducted (3–12), and a meta-analysis concluded that multiple micronutrient supplementation provides little benefit over a traditionally recommended iron + folic acid supplement (13). Few of these studies have provided any data on the longer-term growth, health, and survival of the children born during these trials. One study from Nepal found that a multiple micronutrient supplement provided during pregnancy resulted in sustained increases in infant weight, triceps skinfold thickness (TSF), head, chest, and midupper arm circumference (MUAC) through 2 y of age. These children were heavier than those who received the iron + folic acid control by a mean of 204 g (95% CI: 27, 381) (14). In Peru, children of women who had received an iron + folic acid + zinc supplement during pregnancy had greater weight, chest circumference, and calf muscle area at 4 mo of age than did children of women who received the iron + folic acid control (15). However, an antenatal zinc supplementation trial from Bangladesh found that there was no difference in the growth of infants through 6 mo of age (16).
We conducted a micronutrient supplementation trial in 4998 pregnant women in rural Nepal from 1999 to 2001. The women were randomly assigned to 4 different combinations of micronutrients (including a multiple micronutrient supplement) with vitamin A or vitamin A alone as the control. We found that the combination of iron + folic acid increased mean birth weight by ≈40 g and reduced the risk of low birth weight by 16% relative to the control. The multiple micronutrient supplement provided no added increase in birth weight beyond that seen with iron + folic acid (3, 17). The aim of the present analysis was to examine the effects of supplementation on child growth and body composition at 6–8 y of age.
SUBJECTS AND METHODS
From 1999 to 2001, we conducted a community-based randomized controlled trial of antenatal micronutrient supplements in rural Nepal as part of the Nepal Nutrition Intervention Project–Sarlahi. In 2006–2008, we revisited children who were born during this trial at the age of 6–8 y. Details of the original trial were previously described (3). Briefly, the study area consisted of 30 village development communities that were further divided into 426 smaller communities, referred to as sectors, which served as the unit of randomization. Women living within the study area with a confirmed positive pregnancy test and who provided informed consent were enrolled in the trial.
Pregnant women were randomly assigned to receive 1 of 5 daily micronutrient supplements: 1) control, 2) folic acid (400 μg), 3) folic acid + iron (60 mg ferrous fumarate), 4) folic acid + iron + zinc (30 mg zinc sulfate), or 5) multiple micronutrients containing folic acid, iron, zinc, and an additional 11 vitamins and minerals (10 μg vitamin D, 10 mg vitamin E, 1.6 mg thiamine, 1.8 mg riboflavin, 20 mg niacin, 2.2 mg vitamin B-6, 2.6 μg vitamin B-12, 100 mg vitamin C, 65 μg vitamin K, 2.0 mg Cu, and 100 mg Mg). All supplements were given with 1000 μg retinol equivalents (RE) of preformed vitamin A (retinyl palmitate) and compared with a vitamin A alone control. At baseline, household socioeconomic status, maternal height, weight, smoking, and alcohol consumption were assessed. A total of 4130 infants were born during the trial, and field staff were alerted at the time of birth and made every effort to arrive at the household on the day of delivery to measure newborn weight, length, and head and chest circumferences. The mean (±SD) gestation of the mothers at enrollment into the study was 11 ± 5 wk, and compliance was high (median: 88%) and did not vary by treatment group (3).
Our continued presence in the area has allowed additional assessments of vital events and stability of residence in this population of children. In addition, 3669 of the children participated in a larger randomized placebo-controlled trial of iron and folic acid with or without zinc, which enrolled ≈25,000 children <36 mo of age in 2001–2004, neither of which were found to significantly improve survival (18, 19). In the study population of concern for this analysis, the median age of enrollment was 12 mo (range: 6–35 mo) and the median duration of supplementation was 12 mo (range: 1–25 mo). Children also received vitamin A supplements (100,000 IU for children <6 mo of age and 200,000 IU for children ≥6 mo of age) twice per year through the Nepal National Vitamin A Program or from study staff.
Follow-up assessments
In 2006–2008, at 6–8 y of age, children were tracked by using their last known address, which was updated if the family had moved within the study area. Children who had moved to either the district center or Kathmandu were also tracked and visited by study teams. The home-based follow-up assessments were conducted by 2 teams of specialized fieldworkers. The initial visit included informed consent, a socioeconomic status interview addressed to the head of household, and a questionnaire about the child’s literacy and educational attainment. A second visit was conducted by a specialized team of trained anthropometrists who measured height, weight, waist circumference, TSF thickness, subscapular skinfold (SSF) thickness, and MUAC, each in triplicate by using standard procedures (20). Height was measured with the child in the standing position and the head positioned in the Frankfurt plane using a portable Harpenden stadiometer (Harpenden, Crosswell, United Kingdom). Weight was measured with an electronic scale (model 881; Seca, Hamburg, Germany). MUAC was measured by using a standard insertion tape at the midpoint of the left arm with the arm hanging relaxed. TSF thickness was measured with Holtain precision calipers (Holtain Ltd, Crymych, United Kingdom) at the same location, and SSF thickness was measured below the lateral angle of the shoulder blade. Waist circumference was measured with a long insertion tape (model 200; Seca) at the natural waist, midway between the 10th rib and the iliac crest, with the subject standing erect with their arms hanging at their sides and weight equally distributed over both legs.
The medians of all anthropometric measurements were used for analysis. To estimate the risk of stunting and underweight and to make comparisons with internationally recognized reference populations, weight-for-age (WAZ), height-for-age (HAZ), and body mass index (BMI; in kg/m2)–for-age (BMIZ) z scores were calculated against the World Health Organization (WHO) growth reference for children <5 y of age (21) and school-age children (22). Arm fat area (AFA) and arm muscle area (AMA) were calculated by using the following equations:
where TSF and MUAC are in cm (20).
The follow-up study received ethical approval from the Institutional Review Boards at Johns Hopkins School of Public Health, Baltimore, MD, in the United States and at the Institute of Medicine, Tribhuvan University, Kathmandu, in Nepal.
Statistical analyses
Baseline and follow-up characteristics, including socioeconomic status indicators, maternal literacy and education, maternal smoking, and alcohol consumption during pregnancy, and paternal occupation, were compared across treatment group. To examine the balance across groups, the chi-square test was used. Outcome variables were checked for normality, and biologically implausible extreme values for any anthropometric were excluded from the analysis. This resulted in 2 height and 3 weight measurements being excluded. Children born during the maternal supplementation trial who were surviving and whose parents provided consent were enrolled into the child iron and zinc supplementation trial. Neither supplement had an effect on child mortality (18, 19), and growth outcomes have yet to be published. Despite the fact that children were re-randomized into the childhood intervention groups, the distribution was unequal (P < 0.001; Table 1). The effect of this intervention as a potential confounder was tested by using the Wald test by adding dummy variables for the child’s receipt of either iron or zinc. The terms were not found to be statistically significant (P > 0.05 for all) and did not substantially change the β coefficients of the antenatal intervention terms. Interaction terms between the prenatal and postnatal treatment groups were also tested and not found to be statistically significant (P > 0.1 for all) and were therefore not adjusted for in the final models.
TABLE 1.
Household and individual characteristics by maternal supplementation group for participating children aged 6–8 y in rural Nepal, 2006–2008
| Maternal supplementation group |
|||||
| Control (n = 735)1 | Folic acid (n = 658) | Folic acid + iron (n = 674) | Folic acid + iron + zinc (n = 708) | Multiple micronutrient (n = 749) | |
| n (%) | |||||
| Ethnicity2 | |||||
| Pahadi | 204 (27.8) | 205 (31.2) | 197 (29.2) | 218 (30.8) | 184 (24.6) |
| Madheshi | 531 (72.2) | 453 (68.8) | 477 (70.8) | 490 (69.2) | 565 (75.4) |
| Family asset ownership (any vs none) | |||||
| Goats | 454 (62.1) | 431 (65.5) | 424 (62.9) | 489 (69.3) | 485 (64.8) |
| Cattle | 501 (68.4) | 463 (70.4) | 483 (71.7) | 495 (70.1) | 510 (68.2) |
| Land | 532 (73.5) | 515 (78.6) | 533 (79.6) | 519 (73.7) | 593 (80.0) |
| Radio | 233 (31.8) | 220 (33.4) | 221 (32.8) | 239 (33.9) | 252 (33.7) |
| Literacy | |||||
| Child | 129 (17.9) | 100 (15.2) | 98 (14.6) | 140 (19.9) | 109 (14.7) |
| Mother | 156 (21.4) | 120 (18.3) | 145 (21.6) | 146 (20.7) | 147 (19.8) |
| Religion/caste3 | |||||
| Hindu-Brahmin | 64 (8.8) | 47 (7.1) | 36 (5.3) | 47 (6.7) | 40 (5.4) |
| Hindu-Chetri | 57 (7.8) | 28 (4.3) | 58 (8.6) | 55 (7.8) | 46 (6.2) |
| Hindu-Vaishya | 412 (56.4) | 450 (68.4) | 467 (69.3) | 464 (65.7) | 496 (66.4) |
| Hindu-Shudra | 99 (13.5) | 84 (12.8) | 62 (9.2) | 102 (14.4) | 107 (14.3) |
| Muslim | 98 (13.4) | 43 (6.5) | 47 (7.0) | 31 (4.4) | 55 (7.4) |
| Buddhist or Christian | 2 (0.3) | 6 (0.9) | 4 (0.6) | 7 (1.0) | 3 (0.4) |
| Child supplement group34 | |||||
| Iron | 174 (23.6) | 143 (21.7) | 167 (24.8) | 139 (19.7) | 223 (29.8) |
| Iron + zinc | 203 (27.6) | 182 (27.6) | 225 (33.3) | 125 (17.6) | 95 (12.7) |
| Zinc | 158 (21.5) | 162 (24.6) | 165 (24.3) | 253 (35.8) | 189 (25.2) |
| Placebo | 184 (25.0) | 153 (23.2) | 106 (15.6) | 179 (25.2) | 228 (30.4) |
| Nonparticipant | 16 (2.2) | 18 (2.9) | 11 (1.9) | 12 (1.7) | 14 (1.9) |
Total sample size. Data were missing for animal ownership (n = 6), radio ownership (n = 6), land ownership (n = 30), child literacy (n = 30), maternal literacy (n = 24), and religion/caste (n = 7).
Pahadis originate from the hills of Nepal, and Madheshis originate from the plains of northern India.
P < 0.001 across groups (chi-square test).
Children were invited to participate in a randomized controlled trial of iron and/or zinc supplementation during childhood. All children in the study area between the ages of 1 and 36 mo were eligible for enrollment, and 3458 children included in this analysis participated, with enrollment at a median age of 23 mo.
Differences in mean anthropometric indicators were compared across treatment groups by using generalized estimation equation (GEE) linear models with exchangeable correlation, to account for the fact that communities, not individuals, were randomly assigned (23). Adjusted models all control for the age of the child at follow-up. Models were checked by examining plots of the leverage versus the square of the residuals. Sensitivity analyses were conducted by removing points with a high residual or high leverage and reanalyzing the data. Any points that had undue influence on the estimation of the coefficients were removed, resulting in 2 high height measurements from the folic acid + iron + zinc group being removed. Odds ratios were calculated for dichotomous outcome variables using GEE logistic regression models with a logit link and exchangeable correlation. To reveal latent effects of supplementation on weight and height beyond those previously reported at birth (3), models controlling for weight or length measurements at birth were also examined. The incremental gains in weight and height between birth and follow-up were also calculated and examined by using GEE linear models. Interactions were examined between the maternal intervention group and maternal height and BMI at baseline, sex of the child, birth weight, and gestational age at birth by stratifying on those factors. These factors were chosen a priori because of interactions noted previously in this population (17) and others (4, 8, 12). Interactions with a P value <0.1 were considered statistically significant. Data were analyzed by using STATA version 10 (StataCorp, College Station, TX).
RESULTS
Of the targeted 3900 children, 129 had died, 160 had moved, 82 could not be reached at home, and 5 did not provide consent to participate, which resulted in 3524 children enrolled in the follow-up study representing 94% of living children (Figure 1). The follow-up rate did not differ between groups (P = 0.55). Nonparticipating children were more likely to be of Pahadi ethnicity (54% compared with 26%; P < 0.001), have a literate mother (37% compared with 24%; P < 0.001), and own a radio (42% compared with 32%; P < 0.01) and were less likely to own cattle (60% compared with 70%; P < 0.001).
FIGURE 1.
Enrollment and losses to follow-up of children through 6–8 y of age by maternal supplement group. The initial sample size included live born children. All children of mothers who participated in the initial trial and who survived to 6 mo of age were eligible for follow-up. Losses to follow-up include children who moved out of the study area, were not at home despite repeated household visits, or refused to participate. The follow-up rate is based on the percentage of living children who were met by at least one team.
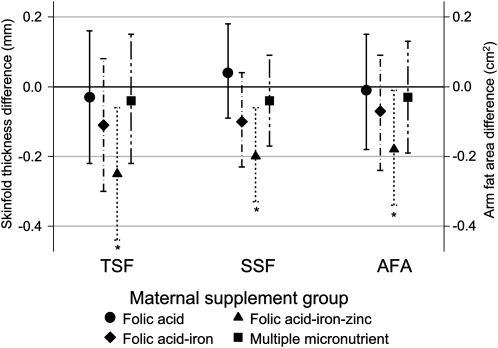
The mean (±SD) age at follow-up was 7.5 ± 0.4 y. There were no differences in socioeconomic status factors by supplement group at follow-up (Table 1). The mean (±SD) height was 113.5 ± 5.5 cm, weight was 18.0 ± 2.3 kg, BMI was 14.0 ± 1.0, MUAC was 15.4 ± 1.1 cm, and waist circumference was 51.2 ± 3.0 cm, which did not differ significantly by supplement group (Table 2). Mean (±SD) TSF and SSF thicknesses were 5.9 ± 1.5 and 4.8 ± 1.0 mm, respectively. After adjustment for the design effect and child age, both TSF and SSF thicknesses were lower (TSF: −0.25 mm; 95% CI: −0.44, −0.06; P = 0.010; SSF: −0.20 mm; 95% CI: −0.33, −0.06; P = 0.004), with a corresponding lower arm fat area (−0.18 cm2; 95% CI: −0.34, −0.01; P = 0.034), in children whose mothers had received an antenatal folic acid + iron + zinc supplement than in children whose mothers had received the control supplement (Figure 2). There was no difference in arm muscle area between the intervention groups. There was a modest, nonsignificant increase in mean height among children in the folic acid + iron + zinc group after adjustment for child age at follow-up, who were 0.49 cm (95% CI: −0.13, 1.12; P = 0.12) taller relative to the controls. The difference became statistically significant after further adjustment for length at birth (0.65 cm; 95% CI: 0.04, 1.26; P = 0.036). To examine whether there were latent effects on growth, the incremental gains in weight and height from birth to follow-up were also compared across supplement groups. Children in all groups gained an average of 15.29 ± 2.20 kg and grew 65.53 ± 5.48 cm. Children in the folic acid + iron+ zinc group grew at a faster rate, growing 0.71 cm (95% CI: 0.09, 1.33; P = 0.026) greater in height relative to the controls between birth and follow-up after controlling for the child’s age at follow-up. The average weight gain across all groups was 15.29 ± 2.20 kg. There were no significant differences relative to the controls after adjustment for child age: folic acid, 0.04 kg (95% CI: −0.25, 0.33); folic acid + iron, 0.00 (95% CI: −0.29, 0.29); folic acid + iron + zinc, 0.12 (95% CI: −0.16, 0.41); and multiple micronutrients, −0.11 (95% CI: −0.39, 0.17).
TABLE 2.
Anthropometric measures and body-composition indicators by maternal supplementation group for participating children aged 6–8 y in rural Nepal, 2006–20081
| Maternal supplementation group |
|||||
| Child anthropometric measures | Control (n = 701)2 | Folic acid (n = 630) | Folic acid + iron (n = 641) | Folic acid + iron + zinc (n = 663) | Multiple micronutrient (n = 721) |
| Height (cm) | 113.27 ± 5.37 | 113.61 ± 5.28 | 113.33 ± 5.32 | 113.93 ± 5.793 | 113.36 ± 5.72 |
| Weight (kg) | 17.96 ± 2.19 | 18.12 ± 2.13 | 18.05 ± 2.32 | 18.12 ± 2.36 | 17.99 ± 2.40 |
| BMI (kg/m2) | 13.96 ± 1.08 | 14.01 ± 1.05 | 14.01 ± 1.06 | 13.92 ± 1.02 | 13.96 ± 1.03 |
| Midupper arm circumference (cm) | 15.38 ± 1.11 | 15.45 ± 1.10 | 15.39 ± 1.16 | 15.39 ± 1.12 | 15.42 ± 1.11 |
| Triceps skinfold thickness (mm) | 5.91 ± 1.45 | 5.90 ± 1.47 | 5.84 ± 1.43 | 5.70 ± 1.473 | 5.90 ± 1.51 |
| Subscapular skinfold thickness (mm) | 4.82 ± 1.08 | 4.89 ± 1.09 | 4.75 ± 1.02 | 4.64 ± 0.874 | 4.81 ± 1.06 |
| Sum of skinfold thicknesses (mm) | 10.73 ± 2.36 | 10.80 ± 2.34 | 10.58 ± 2.28 | 10.34 ± 2.164 | 10.71 ± 2.36 |
| Waist circumference (cm) | 51.23 ± 3.02 | 51.34 ± 2.94 | 51.27 ± 3.14 | 51.19 ± 2.93 | 51.22 ± 3.03 |
| Arm fat area (cm2) | 4.30 ± 1.24 | 4.31 ± 1.24 | 4.25 ± 1.27 | 4.16 ± 1.223 | 4.30 ± 1.26 |
| Arm muscle area (cm2) | 14.61 ± 2.04 | 14.78 ± 2.02 | 14.70 ± 2.23 | 14.78 ± 2.08 | 14.72 ± 2.12 |
| Height-for-age z score5 | −1.93 ± 0.89 | −1.89 ± 0.83 | −1.90 ± 0.88 | −1.83 ± 0.92 | −1.92 ± 0.90 |
| Weight-for-age z score5 | −2.11 ± 0.88 | −2.06 ± 0.82 | −2.07 ± 0.92 | −2.08 ± 0.90 | −2.11 ± 0.90 |
| BMI-for-age z score5 | −1.23 ± 0.87 | −1.19 ± 0.86 | −1.19 ± 0.86 | −1.27 ± 0.84 | −1.23 ± 0.83 |
All values are means ± SDs.
Total number of children who provided anthropometric measurements. Of these children, data were missing for weight (n = 5), height (n = 6), BMI (n = 6), midupper arm circumference (n = 2), triceps skinfold thickness (n = 4), subscapular skinfold thickness (n = 4), and waist circumference (n = 12).
Significantly different from control, with adjustment for the age of the child at follow-up and the design effect by using a generalized estimation equation linear regression model: 3P < 0.05, 4P < 0.01.
Calculated by using the World Health Organization growth reference for school-age children (22).
FIGURE 2.
Differences (95% CIs) in triceps skinfold (TSF) thickness, subscapular skinfold (SSF) thickness, and arm fat area (AFA) among children aged 6–8 y in each maternal supplementation group compared with the control group. Values were adjusted for the design effect and child age at follow-up by using a generalized estimation equation linear regression model. *Significantly different from control, P < 0.05.
The overall mean (±SD) HAZ, WAZ, and BMIZ values were −1.90 ± 0.88, −2.09 ± 0.89, and −1.22 ± 0.85, respectively, and did not differ significantly between treatment groups (Table 2). The prevalence of stunting (HAZ < −2 z scores) was 45.6% and underweight (WAZ < −2 z scores) was 52.9% (Table 3). Children in the folic acid + iron + zinc group had a lower risk of stunting than did the control children (odds ratio: 0.79; 95% CI: 0.61, 1.03; P = 0.080) after control for child age and length-for-age z scores at birth. There was no difference in the risk of underweight or low BMI (< −2 BMIZ) by allocation group.
TABLE 3.
Risk of stunting and underweight by maternal supplementation group for participating children aged 6–8 y based on the World Health Organization growth references for school-age children in rural Nepal, 2006–20081
| Maternal supplementation group |
|||||||||
| Control (n = 701)2 | Folic acid (n = 630)2 |
Folic acid + iron (n = 641)2 |
Folic acid + iron + zinc (n = 663)2 |
Multiple micronutrient (n = 721)2 |
|||||
| Child indicator | n (%) | n (%) | Odds ratio (95% CI)3 | n (%) | Odds ratio (95% CI)3 | n (%) | Odds ratio (95% CI)3 | n (%) | Odds ratio (95% CI)3 |
| Stunting4 | 322 (46.0) | 297 (47.2) | 1.06 (0.82, 1.38) | 288 (45.0) | 1.12 (0.86, 1.45) | 270 (40.8) | 0.79 (0.61, 1.03) | 351 (48.7) | 1.24 (0.96, 1.59) |
| Underweight5 | 369 (52.7) | 325 (51.7) | 1.05 (0.80, 1.38) | 349 (54.6) | 1.28 (0.98, 1.68) | 336 (50.8) | 0.98 (0.75, 1.27) | 395 (54.8) | 1.27 (0.97, 1.65) |
| Low BMI6 | 117 (16.7) | 108 (17.2) | 1.09 (0.78, 1.50) | 114 (17.8) | 1.11 (0.80, 1.53) | 127 (19.2) | 1.25 (0.92, 1.72) | 126 (17.5) | 1.17 (0.86, 1.60) |
z Scores were calculated by using the World Health Organization 2007 growth reference for school-age children (22).
Total number of children who provided anthropometric measurements. Of these children, data were missing for height-for-age (n = 6), weight-for-age (n = 5), and BMI-for-age (n = 7) z scores.
The values were compared between treatment groups and the control group, with adjustment for the design effect by using a generalized estimation equation binomial regression model with exchangeable correlation adjusted for child age at follow-up and either stunting or underweight at birth.
Height-for-age z score < −2.
Weight-for-age z score < −2.
BMI z score < −2.
After several maternal and child factors were controlled for (Table 4), it became apparent that the effect of folic acid + iron + zinc on linear growth was highest among children born to mothers who were stunted (<145 cm during pregnancy) and among women with higher BMIs (≥18.5). The folic acid, folic acid + iron + zinc, and multiple micronutrient effects on height were strongest among children born to women with higher BMIs during pregnancy. For example, children born to women with a low BMI (<18.5) who received the multiple micronutrient supplements were 1.02 cm shorter (95% CI: −1.87, −0.17) than the control children, whereas children born to women with a higher BMI who received the same supplement were 0.41 taller (95% CI: −0.30, 1.12) than the control children (P for interaction = 0.005). In addition, children born to stunted mothers who received folic acid + iron+ zinc were an average of 1.57 cm taller (95% CI: 0.23, 2.90) than the control children. The children born to nonstunted mothers who received the folic acid + iron+ zinc supplement were on average 0.43 cm taller (95% CI: −0.21, 1.07) (P for interaction = 0.094). Furthermore, the effect of the folic acid + iron + zinc supplement was greater among children born to women with a higher BMI in early pregnancy (P = 0.096).
TABLE 4.
Differences in child height (cm) by maternal supplementation group for participating children aged 6–8 y relative to the control, stratified by maternal and child factors, 2006–20081
| Maternal supplementation group |
|||||
| Control2 | Folic acid3 | Folic acid + Iron3 | Folic acid + Iron + zinc3 | Multiple Micronutrient3 | |
| Overall (n = 3181) | 113.27 ± 5.38 | 0.27 (−0.34, 0.89) | −0.00 (−0.61, 0.61) | 0.64 (0.04, 1.25) | −0.12 (−0.72, 0.48) |
| Maternal height during pregnancy | |||||
| <145 cm (n = 458) | 110.61 ± 5.38 | 1.05 (−0.31, 2.41) | 0.63 (−0.73, 1.98) | 1.574 (0.23, 2.90) | 0.72 (−0.57, 2.02) |
| ≥145 cm (n = 2592) | 113.88 ± 5.15 | −0.01 (−0.65, 0.63) | −0.18 (−0.82, 0.46) | 0.434 (−0.21, 1.07) | −0.26 (−0.88, 0.37) |
| Maternal BMI during pregnancy | |||||
| <18.5 kg/m2 (n = 1164) | 113.32 ± 5.29 | −0.344 (−1.21, 0.54) | −0.554 (−1.43, 0.34) | 0.124 (−0.74, 0.98) | −1.023 (−1.87, -0.17) |
| ≥18.5 kg/m2 (n = 1885) | 113.36 ± 5.35 | 0.564 (−0.18, 1.30) | 0.344 (−0.38, 1.07) | 1.044 (0.30, 1.78) | 0.413 (−0.30, 1.12) |
| Sex | |||||
| Male (n = 1601) | 113.92 ± 5.37 | 0.15 (−0.64, 0.94) | 0.24 (−0.53, 1.01) | 0.37 (−0.39, 1.13) | 0.32 (−0.45, 1.08) |
| Female (n = 1580) | 112.68 ± 5.32 | 0.39 (−0.40, 1.19) | −0.24 (−1.04, 0.57) | 0.92 (0.10, 1.73) | −0.50 (−1.28, 0.27) |
| Gestational age at birth | |||||
| <37 wk (n = 669) | 113.47 ± 5.57 | 0.54 (−0.68, 1.76) | −0.02 (−1.23, 1.19) | 0.56 (−0.68, 1.80) | −0.12 (−1.30, 1.07) |
| ≥37 wk (n = 2506) | 113.21 ± 5.32 | 0.21 (−0.45, 0.88) | 0.02 (−0.64, 0.67) | 0.69 (0.04, 1.34) | −0.04 (−0.68, 0.60) |
| Birth weight | |||||
| <2.5 kg (n = 1087) | 111.79 ± 4.94 | 0.74 (−0.20, 1.69) | −0.11 (−1.11, 0.88) | 0.64 (−0.32, 1.59) | 0.20 (−0.75, 1.16) |
| ≥2.5 kg (n = 2094) | 114.16 ± 5.48 | 0.03 (−0.67, 0.73) | −0.00 (−0.68, 0.68) | 0.65 (−0.04, 1.34) | −0.29 (−0.95, 0.38) |
One hundred thirty-one children were missing data on maternal height during pregnancy, 132 on maternal BMI during pregnancy, and 6 on gestational age.
All values are means ± SDs.
All values are β coefficients (95% CIs) and were compared with the control group, with adjustment for child length at birth, child age at follow-up, and the design effect by using a generalized estimation equation linear regression model.
P for interaction < 0.1.
DISCUSSION
We report here the findings of a long-term follow-up study of a cohort of children randomly exposed in utero to micronutrient supplements or a control containing only vitamin A to examine the effect on child growth and body composition. Maternal supplementation with folic acid + iron + zinc resulted in an increase in height and a reduction in TSF and SSF thicknesses and arm fat area but not muscle area. The effect was greatest in children born to mothers who were stunted or who had a higher BMI in early pregnancy. These effects seen in the folic acid + iron + zinc group were not evident in any of the other treatment groups, including the multiple micronutrient group, which also contained folic acid, iron, and zinc. There were no significant differences in attained weight, waist circumference, MUAC, or BMI between any of the treatment groups. We wished to also examine whether there were benefits of maternal micronutrient intake during pregnancy on child growth beyond the modest increases seen at birth. It is possible that in this population, in which 15% of the women were stunted (<145 cm) and 38% had a BMI < 18.5, maternal constraint may have limited the in utero growth potential that could have been achieved through supplementation. We explored this in 2 ways: 1) by examining models that controlled for weight and length at birth and 2) by calculating the change in weight or length between birth and follow-up. From these models, we were able to see that children in the folic acid + iron + zinc group experienced greater postnatal linear growth than did their peers in the control group, particularly if their mother was stunted herself. There was no difference between groups in postnatal weight gain, however.
There are few other antenatal micronutrient trials with substantial postnatal follow-up against which to compare our results. A trial of antenatal zinc supplementation in a poor population from urban Bangladesh resulted in no improvements in growth between birth and 6 mo of age (16). One study from Peru found that folic acid + iron + zinc supplementation had no effect on birth anthropometric measures, but, by 4–12 mo of age, children had greater weight, chest circumference, and calf muscle area than did the iron + folic acid group (15). It is possible that the Bangladeshi trial may not have followed the infants long enough to have observed a noticeable, lagged effect on growth. In a study of rhesus monkeys, zinc deprivation during pregnancy through 1 y postpartum was found to reduce linear growth and increase subcutaneous fat deposition in animals beginning at the age of weaning (24). The difference in skinfold thicknesses in this animal study was greatest between 7 and 9 mo of age; values in the zinc-deficient group returned to control values by 1 y, yet losses in femur length were maintained through the end of the 1 y follow-up period. This echoes our results, but at a younger age. We are not aware of any studies that have examined the effect of prenatal zinc supplementation on the risk of stunting in later childhood, but our findings are supported by numerous randomized trials of zinc supplementation among children that have shown a significant reduction in stunting (25). In particular, these studies have noted that the effect of zinc supplementation appears to be greater in children of low initial WAZ and HAZ values. Our findings that the effect size was greatest among women who were stunted during pregnancy mirror this observation.
The particular mechanisms through which prenatal zinc supplementation may have influenced postnatal growth patterns are unknown. It is possible that the offspring of women who had been supplemented had greater reserves of zinc at birth that could have been directed toward more rapid postnatal growth. Studies of infants born small-for-gestational age have found improvements in linear growth with supplemental zinc (26). Zinc is essential for cellular differentiation, metabolism, and growth. It is important for DNA synthesis and the hormonal regulation of cell division (27). Furthermore, it plays an important role in the regulation of insulin-like growth factor I and its receptor (27) and may act as an activator of insulin-like growth factor I activity within osteoblasts, promoting bone growth (28).
We previously noted a mean increase in birth weight of 64 g (95% CI: 12, 115 g) among children in the multiple micronutrient group (3). This increase in size at birth has apparently not been maintained through the school-age years. In contrast with our findings, a similar multiple micronutrient trial conducted in a neighboring district in Nepal showed that children in the multiple micronutrient group were an average of 77 g (24–130 g) heavier at birth (4) and >200 g heavier (27–381 g) than the iron + folic acid control group at the age of 2 y (14). Although there were no overall differences in anthropometric measures between the multiple micronutrient and the control group, there was evidence of a significant interaction with maternal BMI. Children born to women with a low BMI were ≈1 cm shorter than control children, as compared with children born to women with a high BMI who were 0.4 cm taller than control children. This suggests that the maternal BMI during pregnancy can modify the effect of the multiple micronutrient supplement on growth—an interaction that has been noted by other groups (4, 8, 12), which may indicate that although supplementation may have reduced maternal micronutrient deficiencies, the underlying macronutrient deficiencies among women of low BMI may have limited offspring growth potential. It is unclear why the differences observed in the folic acid + iron + zinc group were not seen in the multiple micronutrient group, which contained all of the same nutrients. We previously postulated that interactions between iron and zinc had contributed to a null birth weight effect in the folic acid + iron + zinc group that was overcome with the addition of other nutrients in the multiple micronutrient supplement (3), which would not explain the current findings. Although the multiple micronutrient supplement improved maternal serum zinc concentrations, it failed to reduce indicators of subclinical infection in the mothers during pregnancy—an effect that was seen with the folic acid + iron + zinc supplement, which suggests that an inhibitory interaction with the additional nutrients in the supplement affected other outcomes as well (29). Thus, it is possible that there were interactions with other nutrients contained in the multiple micronutrient supplement that may have inhibited the effects of zinc on linear growth, although there is limited data to answer this question (30). The possibility that this is a chance difference, however, cannot be ruled out.
The differences in height and skinfold thicknesses among children in the folic acid + iron + zinc group relative to the control group were modest, and it is interesting to note that there was no reduction in waist circumference but there was a reduction in arm fat area. It is possible that this may reflect a greater effect on peripheral rather than on central adiposity. Yet, it is clear from the WAZ, HAZ, and BMIZ values and corresponding stunting and underweight data within our 6–8-y-old study population that the prevalence of undernutrition is high. Therefore, is a reduction in adiposity desirable? Adipose tissue is mobilized during infection and therefore fat stores are likely to be protective in a poor rural area such as this (31). There were no differences in symptoms of neonatal morbidity between treatment groups (32), which suggests that this reduction in adiposity, if present in early life, in the folic acid + iron + zinc group posed no adverse effects. On the other hand, South Asians have a greater percentage body fat for a given BMI than other populations (33)—a phenotype that has been documented at birth (34). In a similar South Asian population, skinfold thicknesses have been found to be positively associated with insulin resistance (35) and inversely associated with HDL cholesterol (36) among children in Pune, India. Interventions that are likely to improve linear growth without increasing adiposity may perhaps be of particular relevance among populations undergoing the rapid nutrition transition (37).
The differences in height, TSF thickness, and SSF thickness seen among children whose mothers had received folic acid + iron + zinc corresponded to effect sizes of 0.11, 0.18, and 0.20 SD, respectively, in relation to those whose mothers served as controls, receiving only vitamin A during pregnancy and the early postpartum period. To place this into perspective, a meta-analysis of supplemental zinc provided directly to prepubertal children estimated effect sizes of 0.35 and 0.31 SD for height and weight, respectively, with durations of supplementation and follow-up ranging from 2 to 15 mo (25). Although small, the change we observed may represent differences in linear growth and shifts in adipose accrual over the first ≈7 y of life that, on the population level, may be beneficial in an otherwise chronically undernourished population. However, future evaluation of the long-term health benefit of these modest nutritional changes is needed.
This study is the first of its kind to present growth data from a follow-up of children randomly exposed to micronutrients during gestation. Its strengths include its large sample size, a high rate of follow-up over a long period of time, and extensive collection of anthropometric data among a study population in which 40% of infants were born with a low birth weight (3), where their mothers were at risk of multiple micronutrient deficiencies during pregnancy (38), and where childhood malnutrition and infection are common. Our study also has some important limitations to consider. More sophisticated measures of body composition than anthropometric measures could have improved our estimates on the changes in body composition. Second, a study of maternal zinc supplementation alone or in combination with folic acid may have allowed us to better understand the effects of zinc without the possibility of interactions with iron. Yet, we are limited by the original study design and supplement combinations when looking at treatment effects. Last, given that the effect sizes are modest and that there were no associations in the multiple micronutrient group, the possibility that the observed differences in the folic acid + iron + zinc supplement were due to chance cannot be ruled out. However, the randomized design of the original trial and high follow-up rate suggest the association to be causal. Confirmation of these results among offspring of other cohorts randomly assigned to zinc versus other regimens would help to clarify the generalizability of this effect.
In conclusion, this study provides unique insights into the effects of intrauterine micronutrient supplementation exposure through maternal supplementation on long-term growth. However, it also raises questions particularly relating to the mechanisms and lagged consequences of zinc deficiency during pregnancy. Whether the small observed reduction in adiposity with iron, folic acid, and zinc supplementation translate into meaningful clinical differences as these children age will be of importance to study in the future. Interventions that are likely to improve linear growth without increasing the potential for excess fat deposition are particularly important for countries undergoing the nutrition transition, where the prevalence of chronic disease is increasing. Iron + folic acid supplementation is already recommended for women during pregnancy; thus, the addition of zinc to this regimen may provide additional benefits, but further research in other populations is warranted.
Acknowledgments
We thank Sharada Ram Shrestha, Jane Schmitz, Darrell Mast, Andre Hackman, Gwendolyn Clemens, and all of the members of the Nepal study team, including the field managers, supervisors, and interviewers, who contributed to the successful implementation of the study.
The authors’ responsibilities were as follows—CPS: conceived of the study design and implementation, conducted the data analysis and interpretation, and wrote the manuscript; PC (principal investigator of the original trial and co-principal investigator of the follow-up study): conceived and designed the follow-up and edited the manuscript; SLC: developed the study procedures, managed the implementation of both the original trial and the follow-up, and edited the manuscript; KPW (principal investigator of the larger follow-up study): helped interpret and edit the manuscript; and SKK (project director in Nepal): contributed to the study design and procedures and implementation. None of the authors had any conflicts of interest to declare.
REFERENCES
- 1.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 2.de Onis M. Child growth and development. Semba RD, Bloem MW, eds Nutrition and health in developing countries. Totowa, NJ: Humana Press, 2001:71–91 [Google Scholar]
- 3.Christian P, Khatry SK, Katz J, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet 2005;365:955–62 [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan U, Gonzalez-Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: a randomized controlled trial in a semirural community in Mexico. Am J Clin Nutr 2003;77:720–5 [DOI] [PubMed] [Google Scholar]
- 6.Friis H, Gomo E, Nyazema N, et al. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr 2004;80:178–84 [DOI] [PubMed] [Google Scholar]
- 7.Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr 2005;59:1081–9 [DOI] [PubMed] [Google Scholar]
- 8.Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group, Shankar AH, Jahari AB, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet 2008;371:215–27 [DOI] [PubMed] [Google Scholar]
- 9.Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med 2007;356:1423–31 [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Ray M, Dua T, Radhakrishnan G, Kumar R, Sachdev HP. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring: a double-blind, randomized, placebo-controlled trial. Arch Pediatr Adolesc Med 2007;161:58–64 [DOI] [PubMed] [Google Scholar]
- 11.Zagre NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster-randomized, double-blind, controlled programmatic study in rural Niger. Food Nutr Bull 2007;28:317–27 [DOI] [PubMed] [Google Scholar]
- 12.Roberfroid D, Huybregts L, Lanou H, et al. Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr 2008;88:1330–40 [DOI] [PubMed] [Google Scholar]
- 13.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2006;4:CD004905. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya A, Saville N, Shrestha BP, de L Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet 2008;371:492–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iannotti LL, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. Am J Clin Nutr 2008;88:154–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osendarp SJ, van Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet 2001;357:1080–5 [DOI] [PubMed] [Google Scholar]
- 17.Christian P, West KP, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr 2003;78:1194–202 [DOI] [PubMed] [Google Scholar]
- 18.Tielsch JM, Khatry SK, Stoltzfus RJ, et al. Effect of daily zinc supplementation on child mortality in southern Nepal: a community-based, cluster randomised, placebo-controlled trial. Lancet 2007;370:1230–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tielsch JM, Khatry SK, Stoltzfus RJ, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet 2006;367:144–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson RS. Principles of nutritional assessment. 2nd ed New York, NY: Oxford University Press, 2005 [Google Scholar]
- 21.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76–85 [DOI] [PubMed] [Google Scholar]
- 22.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30 [PubMed] [Google Scholar]
- 24.Golub MS, Gershwin ME, Hurley LS, Saito WY, Hendrickx AG. Studies of marginal zinc deprivation in rhesus monkeys. IV. Growth of infants in the first year. Am J Clin Nutr 1984;40:1192–202 [DOI] [PubMed] [Google Scholar]
- 25.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2002;75:1062–71 [DOI] [PubMed] [Google Scholar]
- 26.Castillo-Duran C, Weisstaub G. Zinc supplementation and growth of the fetus and low birth weight infant. J Nutr 2003;133:1494S–7S [DOI] [PubMed] [Google Scholar]
- 27.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr 2000;130:1500S–8S [DOI] [PubMed] [Google Scholar]
- 28.Matsui T, Yamaguchi M. Zinc modulation of insulin-like growth factor's effect in osteoblastic MC3T3-E1 cells. Peptides 1995;16:1063–8 [DOI] [PubMed] [Google Scholar]
- 29.Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP., Jr Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am J Clin Nutr 2006;83:788–94 [DOI] [PubMed] [Google Scholar]
- 30.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr 2000;130:1378S–83S [DOI] [PubMed] [Google Scholar]
- 31.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol 1998;(suppl 27):177–209 [DOI] [PubMed] [Google Scholar]
- 32.Christian P, Darmstadt GL, Wu L, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Arch Dis Child 2008;93:660–4 [DOI] [PubMed] [Google Scholar]
- 33.Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc 2004;63:387–96 [DOI] [PubMed] [Google Scholar]
- 34.Yajnik CS, Fall CH, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord 2003;27:173–80 [DOI] [PubMed] [Google Scholar]
- 35.Bavdekar A, Yajnik CS, Fall CH, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes 1999;48:2422–9 [DOI] [PubMed] [Google Scholar]
- 36.Joglekar CV, Fall CH, Deshpande VU, et al. Newborn size, infant and childhood growth, and body composition and cardiovascular disease risk factors at the age of 6 years: the Pune Maternal Nutrition Study. Int J Obes (Lond) 2007;31:1534–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein AD, Thompson AM, Waters A. Childhood growth and chronic disease: evidence from countries undergoing the nutrition transition. Matern Child Nutr 2005;1:177–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang T, Christian P, Khatry SK, Wu L, West KP., Jr Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr 2005;135:1106–12 [DOI] [PubMed] [Google Scholar]