Abstract
Natural killer T (NKT) cells are a unique population of lymphocytes that coexpress a semiinvariant T cell and natural killer cell receptors, which are particularly abundant in the liver. To investigate the possible effect of these cells on the development of the liver stages of malaria parasites, a glycolipid, α-galactosylceramide (α-GalCer), known to selectively activate Vα14 NKT cells in the context of CD1d molecules, was administered to sporozoite-inoculated mice. The administration of α-GalCer resulted in rapid, strong antimalaria activity, inhibiting the development of the intrahepatocytic stages of the rodent malaria parasites Plasmodium yoelii and Plasmodium berghei. The antimalaria activity mediated by α-GalCer is stage-specific, since the course of blood-stage-induced infection was not inhibited by administration of this glycolipid. Furthermore, it was determined that IFN-γ is essential for the antimalaria activity mediated by the glycolipid. Taken together, our results provide the clear evidence that NKT cells can mediate protection against an intracellular microbial infection.
Natural killer T cells (NKT cells) are a unique subset of lymphocytes that express markers of NK cells and a semiinvariant T cell receptor (TCR) (1–3). In mice, NKT cells express the NK cell marker NK1.1 and a TCR with an invariant Vα chain, encoded by the Vα14 and Jα281 gene segments, in association with a highly skewed set of Vβ chains, most frequently Vβ8.2 (4). The TCR of NKT cells recognizes the MHC class I-like molecule CD1d (5–8). NKT cells have been implicated in the rejection of bone marrow allografts (9) and in the control of the development of autoimmune diseases (10–12).
A limited number of studies have suggested a role for NKT cells in the immune response to infectious agents. In Mycobacteria infection, one study has recently shown that NKT cells contribute to the granulomatous reaction caused by mycobacterial cell walls (13). Another study has shown that this infection increases the density of CD4+ NKT cells in the liver and shifts their cytokine production from IL-4 to IFN-γ (14). In MHC class II-deficient mice, CD4+ NKT cells (a subset of NKT cells) appear to regulate the development of cell-mediated immunity to Toxoplasma gondii infection (15). After Listeria infection, NKT cells have been implicated in recruiting monocytes to the sites of infection by rapidly secreting IL-4 (16). However, a recent study demonstrated that in vivo treatment of mice with anti-CD1 monoclonal antibody decreases the lethality of Listeria infection in mice, suggesting a pathogenic role mediated by NKT cells (17).
NKT cells were also shown to play a role in the initiation of certain antitumor responses (18). A glycolipid, α-galactosylceramide (α-GalCer, KRN7000), a natural product isolated from marine sponges, displays a profound antitumor activity in mice (19), and was shown to bind to CD1d by surface plasmon resonance studies (20, 21). In the context of CD1d molecules, α-GalCer activates murine Vα14-expressing NKT cells in vitro and in vivo (22, 23), causing inhibition of the development of hepatic metastasis of melanomas (24).
In the liver of normal mice, 20–30% of their lymphoid cells consist of NKT cells (1–3). Because malaria parasites in their infective stage, namely sporozoites, rapidly invade, differentiate, and multiply within hepatocytes, after being introduced into mammalian hosts by mosquito bite, we decided to determine the possible role of NKT cells in the development of these liver stages of these parasites. Protective antimalaria immunity in mice has been shown to be mediated not only by antibodies but also by various populations of T cells, including CD8+, CD4+, and γδ T cells, all shown to inhibit the development of the parasite's liver stages (25–27). In addition, NK cells were recently shown to be capable of mediating sporozoite-induced immunity (28). However, the potential role played by NKT cells, one of the major T cell populations of the liver, targeted against the intrahepatocytic development of malaria parasites, has up to now remained unknown and is the subject of the current communication.
Materials and Methods
Parasites and Their Use for Challenge.
Plasmodium berghei (NK65 strain) and Plasmodium yoelii (17X NL strain) sporozoites and blood forms were obtained as previously described (26). For sporozoite and blood form challenge, 75 P. yoelii or 5 × 103 P. berghei sporozoites and 1 × 103 P. yoelii or P. berghei-infected red blood cells, respectively, were injected intravenously into the tail vein of the mice. Parasitemia was determined by microscopic examination of Giemsa-stained daily thin blood smears, from days 3 to 14 after the challenge. Complete protection was defined as the absence of parasitemia.
Mice.
Six- to 8-week-old BALB/c, C57BL/6, and 129/sv wild-type and mutant mice deficient in IL-12, perforin, tumor necrosis factor (TNF)-α, IFN-γ, IFN-γ receptor, and Fas ligand were obtained from The Jackson Laboratory. We established Vα14 NKT-deficient mice by specific deletion of the Jα281 gene segment with homologous recombination and aggregation chimera techniques (18). Vα14/Vβ8.2 NKT-transgenic mice on a RAG1−/− C57BL/6 background, which overexpress Vα14/Vβ8.2 NKT cells but do not have NK, T, and B cells, were generated by mating RAG1−/−/Vβ8.2tg mice and RAG1−/−/Vα14tg mice as previously described (22). CD1-deficient mice were generated from embryonic stem cells of 129 origin and used after seven or eight backcrosses to BALB/c or C57BL/6 (6). In all cases, +/+ or +/− littermates were used as controls.
Chemicals.
α-GalCer [(2S,3S,4R)-1-O-(α-d-galactopyranosyl)-N-hexacosyl-2-amino-1,3,4-octadecanetriol] was synthesized by Kirin Brewery (Gunma, Japan). The original solution was dissolved with 0.5% polysorbate-20 (Nikko Chemical, Tokyo) in 0.9% NaCl solution and diluted with the vehicle (0.5% polysorbate-20 in 0.9% NaCl solution) just before use.
Results
α-GalCer Administration Results in Inhibition of Development of Liver Stages of Murine Malaria.
Because α-GalCer-activated NKT cells rapidly produce large amounts of IFN-γ, which is known to inhibit intrahepatocytic development of malaria parasites (29), we assessed the antimalaria activity elicited by the administration of this glycolipid to mice. BALB/c and C57BL/6 mice were injected intraperitoneally with a single dose of α-GalCer, which ranged from 10 ng to 1 μg. Two days later these mice were challenged with 105 P. yoelii sporozoites, and 42 h after the challenge, the liver was removed from these mice and the amount of parasite-specific rRNA was measured by a semiquantitative competitive reverse transcriptase-PCR (RT-PCR) assay (30).
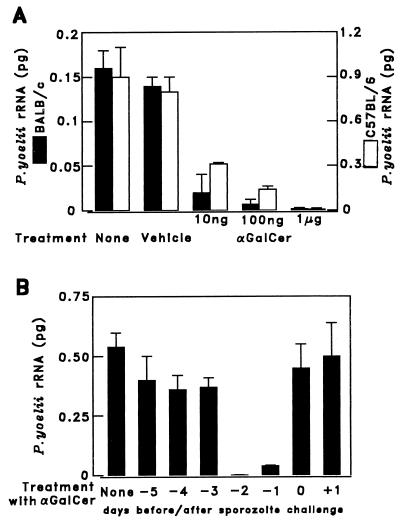
Administration of a single dose of 1 μg of α-GalCer exerted a strong antiparasite effect, in both strains of mice, inhibiting almost completely the parasite-specific rRNA, compared with controls (Fig. 1A). This antimalaria activity was also assessed by another outcome, namely parasitemia. Mice treated with αGalCer developed complete protection, failing to develop parasitemia upon challenge with 75 P. yoelii sporozoites (Table 1).
Figure 1.
Administration of α-GalCer to mice inhibits the development of P. yoelii liver stages. (A) BALB/c and C57BL/6 mice were injected i.p. with different doses (10 ng, 100 ng, or 1 μg) of α-GalCer in 0.025% polysorbate-20, control vehicle (0.025% polysorbate-20 in PBS), or PBS. Two days later the mice were challenged i.v. with 1 × 105 P. yoelii sporozoites, and after 42 h their livers were removed and their rRNA was measured by semiquantitative competitive RT-PCR (30). (B) A single dose of 1 μg of α-GalCer was administered to five mice between 5 days before (−5) and 1 day after (+1) sporozoite challenge. The parasite burden in the liver was measured, as in A. The results represent one of two similar experiments expressed as the mean ± SD of five mice.
Table 1.
Administration of α-GalCer to mice protects them against sporozoite-induced but not blood form-induced P. yoelii or P. berghei infection
| Parasite stage | Parasite species | Mouse strain | Injected with | No. of mice protected/no. of challenged | Percentage protection (no parasitemia) |
|---|---|---|---|---|---|
| Sporozoites | P. yoelii | C57BL/6 | α-GalCer | 14/15 | 93 |
| Vehicle | 0/10 | 0 | |||
| — | 0/15 | 0 | |||
| BALB/c | α-GalCer | 13/15 | 87 | ||
| Vehicle | 0/10 | 0 | |||
| — | 0/15 | 0 | |||
| P. berghei | C57BL/6 | α-GalCer | 9/10 | 90 | |
| Vehicle | 0/5 | 0 | |||
| — | 0/10 | 0 | |||
| Blood forms | P. yoelii | C57BL/6 | α-GalCer | 0/10 | 0 |
| — | 0/10 | 0 | |||
| BALB/c | α-GalCer | 0/10 | 0 | ||
| — | 0/10 | 0 | |||
| P. berghei | C57BL/6 | α-GalCer | 0/10 | 0 | |
| — | 0/10 | 0 |
However, when mice were challenged with 1,000 P. yoelii- or P. berghei-infected red blood cells, all of the α-GalCer-treated mice failed to become protected and developed parasitemia (Table 1). Thus the antimalaria activity of α-GalCer administration is stage specific, affecting only the preerythrocytic developmental stages of malaria parasites.
The optimal timing of the administration of α-GalCer for its antiplasmodial effect was determined by injecting a single i.p. dose of 1 μg of α-GalCer at different times, from 5 days before to 1 day after sporozoite challenge. As shown in Fig. 1B, the maximal antimalaria effect was displayed when α-GalCer was administered 1 or 2 days before sporozoite challenge.
Antimalaria Activity of α-GalCer Requires CD1 and NKT Cells.
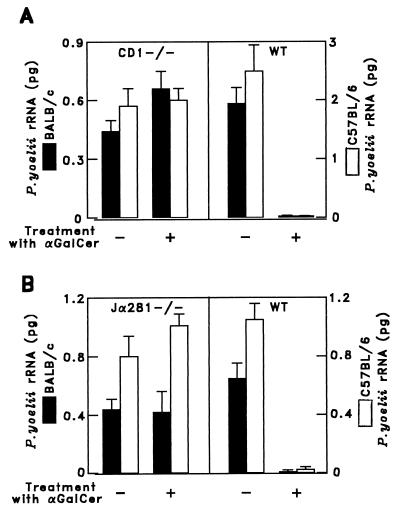
To ascertain whether CD1d molecules are required for the antiparasitic activity of α-GalCer, this glycolipid was injected into CD1d-deficient BALB/c and C57BL/6 mice, which lack CD1d-restricted NKT cells (6, 8). The antiparasitic activity of α-GalCer required the presence of CD1d-restricted NKT cells. Administration of this glycolipid to CD1d-deficient mice of either strain failed entirely to protect them against malaria. This was confirmed by the RT-PCR assay, by measuring plasmodial rRNA (Fig. 2A), and by monitoring parasitemia (Table 2). Further direct evidence of the involvement of Vα14NKT cells in the α-GalCer-mediated antimalaria activity was obtained by administering this glycolipid to mice with a deletion of their Jα281 gene segment (Jα281−/−) (16). This resulted in the complete failure of α-GalCer administration to protect NKT-deficient BALB/c or C57BL6 mice from sporozoite-induced malaria (Fig. 2B). These results were corroborated by α-GalCer administration to TCRα-deficient mice, which failed to become protected (data not shown).
Figure 2.
The antimalaria activity of α-GalCer requires CD1d-positive Vα14 NKT cells. Five CD1-deficient and wild-type (WT) mice on BALB/c and C57BL/6 background (A) and Vα14 NKT-deficient mice (Jα281−/−) or WT mice of these strains (B) were injected i.p. with 1 μg of α-GalCer. Two days later the mice were challenged with P. yoelii sporozoites, and their parasite burden in the liver was assessed as in Fig. 1. The results represent one of two similar experiments expressed as mean values ± SD.
Table 2.
Administration of α-GalCer to CD1d-deficient mice fails to protect them against sporozoite-induced P. yoelii malaria infection
| Mice | Injected with | No. of mice protected/no. of challenged | Percentage protection (no parasitemia) |
|---|---|---|---|
| C57BL/6 | |||
| CD1−/− | α-GalCer | 0/6 | 0 |
| — | 0/5 | 0 | |
| CD1+/− | α-GalCer | 5/5 | 100 |
| — | 0/4 | 0 | |
| CD1+/+ | α-GalCer | 5/5 | 100 |
| — | 0/5 | 0 | |
| BALB/c | |||
| CD1−/− | α-GalCer | 0/6 | 0 |
| — | 0/5 | 0 | |
| CD1+/+ | α-GalCer | 4/4 | 100 |
| — | 0/4 | 0 |
Antimalaria Activity Mediated by α-GalCer Administration Does Not Require IL-12 or NK Cells.
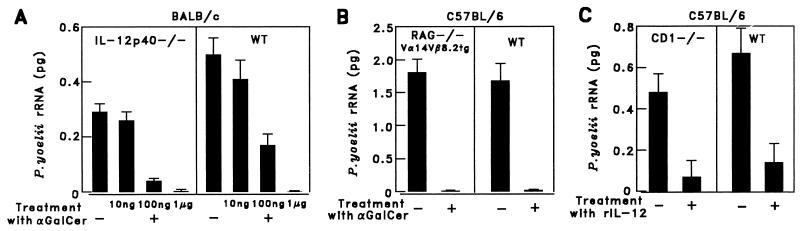
To determine whether IL-12 contributes to the antiplasmodial activity of α-GalCer-activated NKT cells, this glycolipid was administered to IL-12-deficient mice that lacked the p40 subunit of IL-12 (31). We found that the NKT-mediated antiplasmodial activity of α-GalCer does not require IL-12, inasmuch as this glycolipid inhibited liver stage development in IL-12-deficient mice, as determined by RT-PCR (Fig. 3A). The minimal dose of α-GalCer required to inhibit more than 90% of the malaria parasite burden in the liver was almost the same in IL-12-deficient and wild-type mice (Fig. 3A). This was corroborated by monitoring parasitemia after injection of 75 P. yoelii sporozoites into α-GalCer-treated or untreated IL-12-deficient and wild-type mice. As shown in Table 3, αGalCer treatment prevented patency (the appearance of blood stages of the malaria parasites) in IL-12-deficient as well as in wild-type mice, documenting that α-GalCer can elicit “complete protection” against malaria, in the absence of IL-12.
Figure 3.
The antimalaria activity of α-GalCer does not require IL-12 or NK cells, whereas IL-12 displays its antimalaria activity in CD1−/− mice. (A) Five IL-12-deficient (IL-12p40−/−) and WT mice on a BALB/c background were injected i.p. with a single dose (10 ng, 100 ng, or 1 μg) of α-GalCer in 0.025% polysorbate-20 or PBS. (B) Vα14/Vβ8.2 transgenic mice on a RAG-1−/− C57BL/6 background (RAG−/−Vα14Vβ8.2tg) and WT controls were injected i.p. with 1 μg of α-GalCer. (C) Five CD1-deficient and WT mice on the C57BL/6 background were injected i.p. with 0.3 μg of rIL-12. Two days (A and B) or one day (C) later, the mice were challenged with 1 × 105 P. yoelii sporozoites, and the parasite burden in the liver was assessed as in the legend of Fig. 1. The results represent one of two similar experiments.
Table 3.
Administration of α-GalCer to IL-12-deficient mice protects them against sporozoite-induced P. yoelii malaria infection
| Mice | Injected with | No. of mice protected/no. of challenged | Percentage protection (no parasitemia) |
|---|---|---|---|
| BALB/c | |||
| IL-12p40−/− | α-GalCer | 6/6 | 100 |
| — | 0/6 | 0 | |
| IL-12p40+/+ | α-GalCer | 6/6 | 100 |
| — | 0/6 | 0 |
To exclude the possibility that NK cells might be essential to the antimalaria activity of α-GalCer, we administered this glycolipid to transgenic mice that overexpressed Vα14 NKT cells on a RAG1−/− background, with no NK, T, or B cells (22). In these transgenic Vα14 NKT mice, α-GalCer inhibited the development of liver stages to the same extent as in wild-type mice (Fig. 3B).
IL-12-Mediated Inhibition of Liver Stages Occurs in CD1-Deficient Mice.
The administration of recombinant IL-12 resulted in inhibition of the development of liver stages in CD1-deficient, as well as in wild-type, mice (Fig. 3C). This indicates that the antimalaria activity of IL-12 is independent of CD1 and NKT cells and is likely to be mediated by other mechanisms.
Antimalaria Activity of α-GalCer Is Primarily Mediated by IFN-γ.
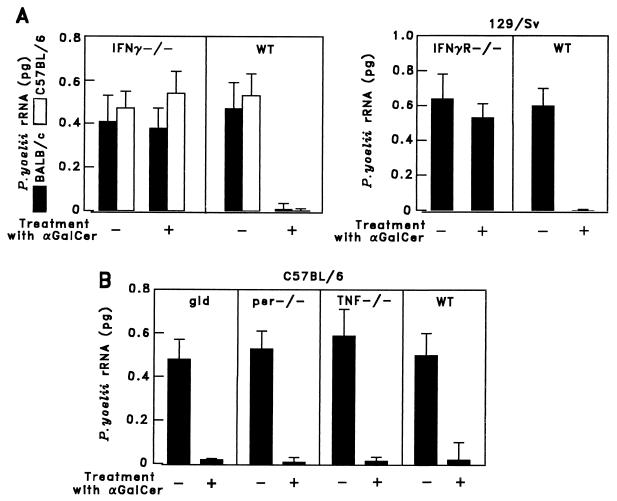
To determine the potential effector molecules that mediate the antiparasite activity of α-GalCer, we injected this glycolipid into knock-out mice lacking IFN-γ, IFN-γ receptor, TNF-α, Fas/Fas ligand, or perforin. This was followed 2 days later by sporozoite challenge. α-GalCer was unable to inhibit parasite development in the liver of mice lacking IFN-γ or the IFN-γ receptor (Fig. 4A). However, none of the other molecules tested, Fas/Fas ligand, perforin, or TNF-α, appeared to be required for the inhibitory effect of α-GalCer on plasmodial liver stages (Fig. 4B). Therefore IFN-γ appears to be a major mediator of the in vivo antiplasmodial activity of α-GalCer.
Figure 4.
The antimalaria activity of α-GalCer is primarily mediated by IFN-γ. Five mice lacking IFN-γ or IFN-γ receptor (A) and mice lacking Fas ligand (gld), perforin (per−/−), or TNF-α (TNF−/−) and their respective WT controls (B) were injected i.p. with 1 μg of α-GalCer. Two days later the mice were challenged with P. yoelii sporozoites, and the parasite burden in the liver was assessed as in Fig. 1. The results represent one of two similar experiments.
Comparison of the Number of IFN-γ-Secreting Cells of Vα14 NKT-Deficient and IL-12-Deficient Mice Treated with α-GalCer.
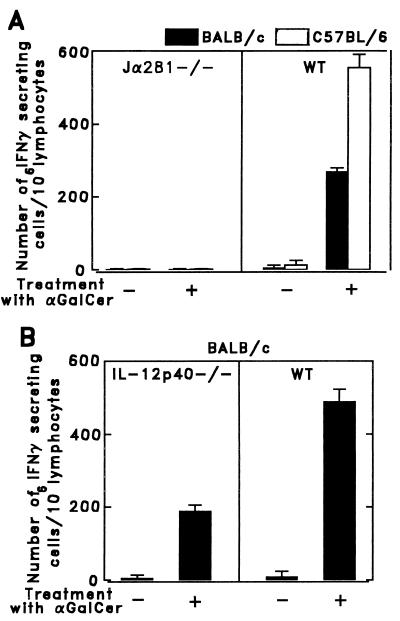
Because IFN-γ appeared to be a major mediator of the antimalaria activity elicited by NKT cells, we determined the capacity of lymphoid cells in the liver of Vα14 NKT-deficient α-GalCer-treated mice to secrete this cytokine, using an enzyme-linked immunospot (ELISPOT) assay (32). Liver lymphocytes were obtained according to the method described by Goossens et al. (33). The cells from α-GalCer-treated NKT-deficient Jα281−/− mice failed to secrete IFN-γ, compared with the liver cells of wild-type mice, which secreted IFN-γ (Fig. 5A). These results confirm previous data showing that α-GalCer selectively activates Vα14 NKT cells (24) and are in agreement with our results on the lack of protection of α-GalCer-treated Vα14 NKT-deficient mice (Fig. 2B).
Figure 5.
Relative number of α-GalCer-activated liver lymphoid cells secreting IFN-γ in Vα14 NKT-deficient mice, IL-12-deficient mice, and their respective controls injected i.p. with 1 μg of α-GalCer. One day after α-GalCer injection, lymphocytes were obtained from the liver according to the method described by Goossens et al. (33), and the relative number of cells secreting IFN-γ was determined by an enzyme-linked immunospot assay (32). Results are shown for Vα14 NKT-deficient (Jα281−/−) (A), IL-12-deficient (IL-12p40−/−) (B), and their respective control (WT) mice. The results reflect two separate experiments and are expressed as mean values ± SD of triplicate cultures.
The lack of a requirement for IL-12 for the antimalaria activity mediated by α-GalCer led us to determine the number of IFN-γ-secreting liver lymphoid cells of α-GalCer-treated, IL-12-deficient mice. As shown in Fig. 5B, the liver lymphoid cells from α-GalCer-treated, IL-12-deficient mice were able to produce IFN-γ. However, the number of IFN-γ-secreting cells was 2.5 times smaller than that of α-GalCer-treated, wild-type mice. These data suggest that despite lower numbers of T cells producing IFN-γ in IL-12-deficient mice, the amounts of IFN-γ secreted in these mice are sufficient to cause antimalaria activity. The second, less likely, possibility is that in the absence of IL-12, α-GalCer-activated NKT cells may use not only IFN-γ but also additional mediators.
Discussion
We have demonstrated that the administration of a single relatively small dose of α-GalCer to mice results in a striking inhibition of development of the liver stages of the rodent malaria parasites P. yoelii and P. berghei. This inhibition was assessed by two distinct criteria: the decrease of malaria-specific rRNA in the liver of α-GalCer-treated, sporozoite-inoculated mice, compared with controls, and parasitemia or its absence, indicating that complete protection against malaria resulted from the administration of this glycolipid to sporozoite-injected mice.
A key finding of our research was that this protection elicited by α-GalCer required NKT cells and CD1 molecules, because inhibition of parasite development failed to occur in CD1−/− and Jα281−/− mice. Earlier studies suggested the involvement of NK cells in the activity elicited by α-GalCer (34). However, we observed that the absence of NK cells in transgenic Vα14+/+ RAG1−/− mice failed to alter the inhibitory effect of α-GalCer administration (Fig. 3B). Taken together, our results strongly indicate that NKT cells mediate the antimalaria activity elicited by α-GalCer in the absence of NK, T, and B cells.
Although α-GalCer does not require IL-12 for its antimalaria activity, the administration of IL-12 to sporozoite-inoculated mice has a strong inhibitory effect on the development of liver stages, as observed earlier by others (35). We also found that the antimalaria activity of IL-12 does not require CD1 or NKT cells, indicating that activated NKT cells and IL-12 have a different mode of action on plasmodial liver stages.
While the precise molecular mechanism of the antimalaria effect of α-GalCer remains to be fully clarified, we established that IFN-γ secretion and the presence of an IFN-γ receptor are essential for the observed protection. The deficiency of certain other effector molecules, such as perforin, TNF-α, or Fas/Fas ligand, did not interfere with the antimalaria effect of α-GalCer.
It is noteworthy that the kinetics of antimalaria activity of α-GalCer resemble those of recombinant IFN-γ, which display a strong in vivo antimalaria activity when administered on the same day as sporozoite challenge (29). There is approximately a 1- to 2-day delay in the onset of the antimalaria activity of α-GalCer compared with that of IFN-γ. Most likely, after α-GalCer injection it takes 1–2 days for NKT cells to secrete the amounts of IFN-γ required to eliminate the liver stages. The very short-lasting antimalaria activity of α-GalCer may be explained by some unique characteristics of NKT cells recently demonstrated by Eberl and MacDonald (36). They have shown that in vivo anti-CD3ɛ antibody treatment causes activation of liver NKT cells, followed by rapid depletion of this cell population, indicating that liver NKT cells are highly sensitive to activation-induced cell death. We hypothesize that NKT cells activated by α-GalCer may also undergo rapid cell death, causing the short-lasting effect of α-GalCer.
Timing of the administration of α-GalCer is critical for its effect in the mouse model because of the very short persistence (i.e., 2 days) of the liver stages of rodent malaria parasites. However, the liver stages of the human malaria parasites Plasmodium falciparum and Plasmodium vivax persist for 7–10 days, providing a longer time and thus more opportunity for α-GalCer to exert its anti-liver stage activity. In addition, P. vivax, the most prevalent human malaria parasite, frequently develops latent liver stage “hypnozoites,” which remain dormant in the liver for many weeks or months after the initial infection, causing relapses of the infection. Primaquine, the only drug available that can eradicate these liver stages, frequently has adverse effects on humans (37), and primaquine-resistant strains of P. vivax have been reported (37, 38). Thus repeated administration of αGalCer, which, independently of its dosage, does not induce morbidity in mice or monkeys (24), might be used to prevent P. vivax relapses under certain conditions.
We observed strict stage specificity of the antimalaria effect resulting from α-GalCer administration, affecting only the parasite's liver stages but not blood stages (Table 1). Others have recently reported that acute rodent malaria may activate NKT cells. They found that parasitemia in mice results in a very considerable increase of NKT cells in the liver, reaching maximal numbers at the time of peak parasitemia. These purified liver NKT cells, collected on the 10th day of infection, long after the hepatocytic phase, were shown to display a clear inhibitory effect on the development of liver stages. This effect could be partially reversed by adding an anti-IFN-γ antibody to the system (39).
As for sporozoite-induced immunity, we recently found that α-GalCer administration increases the efficacy of a suboptimal immunizing dose, revealing a possible adjuvant effect. We observed that the simultaneous injection of α-GalCer with 104 irradiated P. yoelii sporozoites, a dose that otherwise fails to protect mice, induced complete protection against malaria (G.G.-A., Y.K., and M. Tsuji, unpublished data). Whether α-GalCer can also increase and prolong the protective effect of other malaria antigens (peptides, recombinant proteins, DNA, or recombinant viruses) that elicit protective responses against liver stages remains to be investigated.
In conclusion, by using the NKT cell ligand, α-GalCer, our studies present clear evidence of a protective role of activated NKT cells against an intracellular microbial infection, murine malaria. These findings may be relevant to human malaria, particularly because it has been demonstrated that α-GalCer can also stimulate human NKT cells (40), which represent a large percentage of T lymphocytes present in the human liver (41).
Acknowledgments
We thank R. S. Nussenzweig for critical review of the manuscript. This work was supported by National Institutes of Health Grants AI40656 and AI36526, a Basque Country Postdoctoral Fellowship (to G.G.-A.), and a C. V. Starr Foundation Postdoctoral Fellowship (to O.B.-R.). C.d.O., a graduate student from the laboratory of H. del Portillo (University of São Paulo), was funded by fellowships from the Brazilian agencies Comissao de Aperfeiçoamento de Pessoal de Nivel Superior and Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo.
Abbreviations
- NK cells
natural killer cells
- NKT cells
NK T cells
- α-GalCer
α-galactosylceramide
- TNF
tumor necrosis factor
- RT-PCR
reverse transcriptase–PCR
- TCR
T cell receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bendelac A, Rivera M N, Park S H, Roark J H. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Vicari A P, Zlotnik A. Immunol Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 4.Lantz O, Bendelac A. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Lantz O, Quimby M E, Yewdell J W, Bennink J R, Brutkiewicz R R. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 6.Mendiratta S K, Martin W D, Hong S, Boesteanu A, Joyce S, Van Kaer L. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Scherer D C, Singh N, Mendiratta S K, Serizawa I, Koezuka Y, Van Kaer L. Immunol Rev. 1999;169:31–44. doi: 10.1111/j.1600-065x.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y H, Chiu N M, Mandal M, Wang N, Wang C R. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Moore M W, Dennert G. J Immunol. 1994;152:4407–4416. [PubMed] [Google Scholar]
- 10.Mieza M A, Itoh T, Cui J Q, Makino T Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 11.Hammond K L, Poulton L D, Palmisano L J, Silveira P A, Godfrey D I, Baxter A G. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehuen A. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apostolou I, Takahama Y, Belman C, Kawano T, Huerre M, Marchal G, Cui J, Taniguchi M, Nakauchi H, Fourne J-J, et al. Proc Natl Acad Sci USA. 1999;96:5141–5146. doi: 10.1073/pnas.96.9.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emoto M, Emoto Y, Buchwalow I B, Kaufmann S H E. Eur J Immunol. 1999;29:650–659. doi: 10.1002/(SICI)1521-4141(199902)29:02<650::AID-IMMU650>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Denkers E Y, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. J Exp Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flesch I E, Wandersee A, Kaufmann S H. J Immunol. 1997;159:7–10. [PubMed] [Google Scholar]
- 17.Szalay G, Ladel C H, Blum C, Brossay L, Kaufmann S H. J Immunol. 1999;162:6955–6958. [PubMed] [Google Scholar]
- 18.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 20.Naidenko O V, Maher J K, Ernst W A, Sakai T, Modlin R L, Kronenberg M. J Exp Med. 1999;190:1069–1080. doi: 10.1084/jem.190.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai T, Naidenko O V, Iijima H, Kronenberg M, Koezuka Y. J Med Chem. 1999;42:1836–1841. doi: 10.1021/jm990054n. [DOI] [PubMed] [Google Scholar]
- 22.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 23.Burdin N, Brossay L, Koezuka Y, Smiley S T, Grusby M J, Cui J, Taniguchi M, Hayakawa K, Kronenberg M. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 24.Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, Shimosaka A, Koezuka Y. Cancer Res. 1998;58:1202–1207. [PubMed] [Google Scholar]
- 25.Nardin E H, Nussenzweig R S. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji M, Romero P, Nussenzweig R S, Zavala F. J Exp Med. 1990;172:1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig R S, Zavala F, Tonegawa S. Proc Natl Acad Sci USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doolan D L, Hoffman S L. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 29.Ferreira A, Schofield L, Enea V, Schellekens H, Van der Meide P, Collins W E, Nussenzweig R S, Nussenzweig V. Science. 1986;232:881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 30.Briones M, Tsuji M, Nussenzweig V. Mol Biochem Parasitol. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- 31.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 32.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 33.Goosens P L, Jouin H, Marchal G, Milon G. J Immunol Methods. 1990;132:137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- 34.Carnaud C, Lee D, Donnars O, Park S-H, Beavis A, Koezuka Y, Bendelac A. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 35.Hoffman S L, Crutcher J M, Puri S K, Ansari A A, Villinger F, Franke E D, Singh P P, Finkelman F, Gately M K, Dutta G P, Sedegah M. Nat Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 36.Eberl G, MacDonald H R. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 37.Whitby M. J Antimicrob Chemother. 1997;40:749–752. doi: 10.1093/jac/40.6.749. [DOI] [PubMed] [Google Scholar]
- 38.Collins W E, Jeffery G M. Am J Trop Med Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- 39.Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, Cazenave P A. J Immunol. 2000;164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 40.Spada F M, Koezuka Y, Porcelli S A. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inshihara S, Nieda M, Kitayama J, Osada T, Yabe T, Ishikawa Y, Nagawa H, Muto T, Juji T. Eur J Immunol. 1999;29:2406–2413. doi: 10.1002/(SICI)1521-4141(199908)29:08<2406::AID-IMMU2406>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]