Abstract
Background: Soy foods contain several components (isoflavones and amino acids) that potentially affect bone. Few long-term, large clinical trials of soy as a means of improving bone mineral density (BMD) in late postmenopausal women have been conducted.
Objective: Our goal was to evaluate the long-term effect of dietary soy protein and/or soy isoflavone consumption on skeletal health in late postmenopausal women.
Design: We conducted a randomized, double-blind, placebo-controlled clinical trial in 131 healthy ambulatory women aged >60 y. Ninety-seven women completed the trial. After a 1-mo baseline period, subjects were randomly assigned into 1 of 4 intervention groups: soy protein (18 g) + isoflavone tablets (105 mg isoflavone aglycone equivalents), soy protein + placebo tablets, control protein + isoflavone tablets, and control protein + placebo tablets.
Results: Consumption of protein powder and isoflavone pills did not differ between groups, and compliance with the study powder and pills was 80–90%. No significant differences in BMD were observed between groups from baseline to 1 y after the intervention or in BMD change between equol and non-equol producers. However, there were significant negative correlations between total dietary protein (per kg) and markers of bone turnover (P < 0.05).
Conclusions: Because soy protein and isoflavones (either alone or together) did not affect BMD, they should not be considered as effective interventions for preserving skeletal health in older women. The negative correlation between dietary protein and bone turnover suggests that increasing protein intakes may suppress skeletal turnover. This trial was registered at clinicaltrials.gov as NCT00668447.
INTRODUCTION
Osteoporosis is a disease that primarily affects older women. Because of the risks of using hormone replacement therapy (1), many postmenopausal women are insisting on natural treatments (eg, soy foods) for chronic diseases (eg, osteoporosis). Soy contains several components that could potentially benefit skeletal health, such as isoflavones and low sulfur amino acid composition.
Soy foods contain isoflavones, which are naturally occurring plant compounds similar to mammalian estrogens. The major isoflavones in soy are daidzin and genistin (as glycosides) and their corresponding aglycone forms, daidzein and genistein. The phenolic ring is a critical structural element of these compounds that binds to estrogen receptors and exerts estrogen-like effects in cells (2). Isoflavones selectively bind to and activate estrogen receptor- β more so than estrogen receptor- α (3, 4). Thus, the clinical effects of isoflavones may be similar to selective estrogen receptor modulators, ie, beneficial effects on bone and heart without detrimental effects on breast and uterine tissue. Soy isoflavones may be helpful for older women because the pathogenesis of osteoporosis involves not only increased bone resorption, because of estrogen deficiency and decreased calcium absorption, but also age-related decreases in bone formation because of decreased osteoblast renewal (5). Furthermore, Prestwood et al (6) suggested that the skeleton of women older than 70 y is more sensitive to low-dose estrogen treatment than is that of younger women.
Although there are studies showing potential beneficial effects of soy isoflavones on bone mineral density (BMD) and/or markers of bone turnover in peri- or postmenopausal women (7–14), there are no known publications addressing this question in older postmenopausal women. Interestingly, many human intervention trials have failed to find beneficial effects of soy isoflavones and/or protein on bone (15–22). In a recent meta analysis, Liu et al (23) did not find significant beneficial effects of soy isoflavones on BMD changes in women from 10 nutrition trials of 1 y duration. These authors identified only a slight trend toward improvement at the spine (P = 0.08) in those receiving the high doses of isoflavones. Accordingly, we report here a 1-y nutrition intervention study to evaluate the effect of soy protein and soy isoflavone alone and in combination on BMD and bone turnover in late postmenopausal women.
SUBJECTS AND METHODS
Study overview
We conducted a 1-y, double-blind, randomized, placebo controlled, prospective, 2 × 2 factorial intervention trial. Although 131 women older than 60 y began the clinical trial, only 97 women successfully completed the trial. After a 1-mo lead in period (designed to stabilize calcium intake), the subjects were randomly assigned into 1 of 4 intervention groups: soy protein + isoflavone tablets, soy protein + placebo tablets, control protein + isoflavone tablets, and control protein + placebo tablets. We subsequently counseled the subjects every 3 mo on incorporating the protein into their diet while maintaining their total dietary calcium close to the Recommended Dietary Allowance. The primary outcome was BMD at the beginning and end of 1 y of intervention; secondary outcomes included markers of bone turnover and equol production. We tested the following hypotheses: 1) soy protein or soy isoflavones alone will have a beneficial effect on bone in older women compared with the control and placebo treatment, 2) soy protein plus isoflavones will have an additional effect on bone turnover and BMD compared with the other interventions, and 3) within the group receiving the isoflavones, equol producers would have more positive effects on BMD than would the non-equol producers.
Study subjects
The institutional review board at the University of Connecticut Health Center approved the study, and all women gave written informed consent before their screening evaluation. Participants in this study included women older than 60 y (mean ± SD: 73.1 ± 5.9 y; range at entrance: 60–93 y). Women were excluded if they had any disease that could affect bone metabolism (including Paget disease, primary hyperparathyroidism, osteomalacia, untreated hyperthyroidism, or multiple myeloma); had cancer of any kind (except basal or squamous cell of skin) in past 5 y; had used of any of the following medications within the past 2 y (calcitonin, calcitriol, heparin, phenytoin, or phenobarbital); had ever used bisphosphonates, corticosteroids for a long time (>6 mo), methotrexate, or fluoride; had an estimated creatinine clearance <50 mL/min; had a history of chronic liver disease or evidence of liver disease on screening; had a history of hip fracture; had known vertebral fracture within the past year; and were vegans. Subjects initially underwent a screening visit that included BMD testing. Those women with a BMD T score < −3.0 at the hip or spine during the screening visit were excluded. The screening visit was followed 1 mo later by the baseline visit, at which time they received the intervention products (protein powders and tablets). After the second visit, the subjects were seen every 3 mo during the 1-y intervention period. Transvaginal ultrasound was conducted at the beginning and end of the 1-y intervention to assess endometrial thickness (a potential consequence of isoflavone administration).
Nutrition intervention
At the screening visit, we instructed the women on how to achieve a total dietary calcium intake of 1200–1500 mg/d (from food and supplements). For those women who did not consume enough dairy foods to achieve this level, we provided calcium citrate caplets that included vitamin D (315 mg Ca and 200 IU vitamin D; Mission Pharmacal Company, San Antonio, TX). The calcium and vitamin D contents of the calcium citrate caplets approximated one serving of dairy products. One month later, the subjects returned for their baseline visit, at which point they were assigned randomly to 1 of 4 treatment groups: soy protein + isoflavone tablets, soy protein + placebo tablets, control protein + isoflavone tablets, and control protein + placebo tablets.
All subjects received dietary counseling every 3 mo at the University of Connecticut's General Clinical Research Center from the research dietitian; we carefully assessed their nutrient intake and monitored their compliance with the protocol. The subjects completed 4-d dietary records every 3 mo during the 1-y intervention. These records were carefully reviewed with each subject for completeness and accuracy and were then analyzed with the Food Processor II Nutrient Analysis Program (ESHA Research Inc, Salem, OR). We asked each subject to suspend taking any nutritional or herbal supplement that would interfere with our primary outcomes. Other soy foods were prohibited during the clinical trial. At each 3-mo visit, the subject returned her unused protein powders and tablets, we accounted for the amount she consumed, and a new 3-mo supply was dispensed. All subjects and investigators were blinded to the protein and the tablet intervention.
Soy and placebo products
Both control and soy proteins were isolates, meaning that they were of the highest concentration of protein (85–90% by wt) to minimize the volume that each woman ingested. The soy protein was an alcohol-washed, soy protein isolate containing 90% protein and negligible isoflavone (0.2 mg/g product; Pro Fam 930, 066–930). The soy protein and isoflavones were kindly provided to us by Archer Daniels Midland, Co, Decatur, IL. The control protein was a mix consisting of 50% protein from sodium caseinate, 25% from whey protein, and 25% from egg white protein (Century Foods International, Sparta, WI). The use of a mix of proteins as a control provides a more balanced level of amino acids and better reflects the mix of proteins that humans typically consume, while avoiding the unique characteristics of one source of protein. The calculated amino acid composition of the soy and the control protein is provided in Table 1. Subjects consumed 20 g protein powder (containing 18 g protein) daily by incorporating it into their commonly consumed beverages or foods. To maintain the dietary protein intake constant, the subject was counseled to decrease her intake of other sources of protein from primarily animal sources by ≈ 3 oz/d (90 g/d)—the approximate equivalent of the protein powder.
TABLE 1.
Essential amino acid content of soy protein and control mix protein
| Soy isolate | Control mix | |
| mg/20 g protein | mg/20 g protein | |
| Isoleucine | 980 | 1085 |
| Leucine | 1600 | 1894 |
| Lysine | 1320 | 1527 |
| Threonine | 760 | 915 |
| Valine | 940 | 1232 |
| Methionine + cystine | 520 | 892 |
| Tryptophan | 240 | 261 |
| Phenylalanine + tyrosine | 1880 | 1451 |
All subjects ingested 3 study tablets daily that contained soy isoflavones or placebo, which were both formulated to appear identical (kindly provided by Archer Daniels Midland, Co). Each soy isoflavone tablet contained 35 mg isoflavone aglycone equivalents from primarily genistein, glycitein, and daidzein and their β-glycosides. The aglycone equivalent value was determined by multiplying the total isoflavones by 0.61. The placebo pills contained a blend of 10-DE maltodextrin (90%) and caramel color (10%) to match the color of the isoflavones; both components were food-grade. The composition of the tablets is shown in Table 2.
TABLE 2.
Analyzed isoflavone content of the isoflavone and placebo tablets used in this study
| Isoflavones | Placebo | |
| mg/tablet | mg/tablet | |
| Daidzin | 22.5 | 0 |
| Glycitin | 3.7 | 0 |
| Genistin | 28.5 | 0 |
| Daidzein | 0.5 | 0 |
| Glycitein | 0.3 | 0 |
| Genistein | 0.3 | 0 |
| Other | 1.2 | 0 |
| Total | 57.0 | 0 |
Biochemical assessment
During the screening visit, we collected blood and urine for measurement of screening chemistries (serum total or ionized calcium, albumin, thyroid-stimulating hormone, alkaline phosphatase, complete blood count with differential, urinary calcium, and creatinine) measured by routine clinical chemistry. The women completed a brief physical examination that included BMD testing.
Women who were eligible after the screening visit returned for the baseline visit 1 mo later. We then measured serum markers of bone formation [bone-specific alkaline phosphatase (BAP)] and markers of bone resorption [urine N-telopeptide crosslinks of collagen normalized for creatinine (NTX/CRT)]. During the 1-mo period between the screen and the baseline visit, the subjects were instructed to consume between 1200 and 1500 mg Ca (from both food and supplemental sources) to help stabilize bone and calcium homeostasis. Blood and urine samples were collected between 0700 and 1000 after the subjects fasted for 10 to 12 h and divided into 0.5-mL aliquots and stored at –70 ° C. Ionized calcium was measured within 2 h of collection. Urinary NTX measurements were performed by using the Osteomark NTX Direct Response assay (Ostex International, Seattle, WA); the intraassay variability was <10% for measures of bone resorption. BAP was measured by enzyme-linked immunosorbent assay (CIS Bio International, Bagnols/Cèze, France); the average intraassay variability was <5%.
Bone density measurement
Areal bone mineral content (g/cm2) of the proximal femur, lumbar spine, wrist, and total body were measured by dual-energy X-ray absorptiometry by using a Lunar DPX-IQ (Lunar Radiation Inc, Madison, WI). The accuracy for bone mineral content measurement reported by the manufacturer has an SEE of 0.8%, with a correlation of bone mineral content to known calcium hydroxyapatite of 0.99. The long-term stability of this instrument is 0.5%, as determined by daily measurement of a Hologic spine phantom. The CVs of bone density measures analyzed with 4.6f software were as follows: femoral neck, 1.5%; femoral total, 1.1%; trochanter, 1.7%; ultradistal radius, 3.4%; 33% radius, 1.6%; total radius, 1.3%; lumbar spine, 1.8%; total body, 0.7%.
Serum isoflavones and equol
Serum isoflavones and equol were measured by using HPLC-Coularray as described previously (24). In brief, serum (500 μL) was buffered with 500 μL of 100 mmol/L ammonium acetate (pH 4.6), mixed with 50 μL internal standard (4-hydroxybenzophenone; 110 μmol/L), and subjected to enzymatic hydrolysis (overnight, 37 ° C) using 200 U β-glucuronidase and 15 U sulfatase prepared in 100 μL ammonium acetate (pH 4.6). After incubation, proteins were precipitated by using acetonitrile (1 mL), the sample was delipidated by using hexane (3 mL), and the isoflavones were extracted 3 times by using 3 mL methyl tert-butyl ether. Extracts were dried under nitrogen gas, reconstituted in mobile phase A, and injected on the HPLC system. The sample was separated at 1 mL/min on a C18 Luna (2), 250 × 4.6 mm (5 μm; Phenomenex; Torrance, CA) by using 25 mmol potassium phosphate buffer/L (pH 2.7) as mobile phase A and methanol:acetonitrile:mobile phase A (50:30:20) as mobile phase B. The gradient was delivered as follows: 50% B to 65% B from 0 to 20 min, a linear gradient to 75% B from 20 to 30 min, and a linear gradient to 100% from 30 to 35 min. Initial conditions were returned over 2 min, and the system was equilibrated at 50% B for 12 min before subsequent injection. Analytes were detected by using potential settings of 325, 450, 575, and 700 mV and were quantitated on their dominant channel. Serum isoflavone concentrations were calculated by using area ratios for standards and the internal standard, and the lower limit of quantification was ≈20 nmol/L for each analyte. Equol producers were defined as those individuals who had a 12-mo serum concentration of S-equol >20 nmol/L (5 μg/L) according to the method of Setchell and Cole (25). The intraassay and interassay CVs were <8% for all analytes.
Statistical analysis
Baseline and clinical characteristics are reported as means ± SDs stratified by treatment group. All subsequent data are presented as means ± SEMs. One-factor analysis of variance was used to test the difference in baseline characteristics between the treatment groups and to also test nutrient intake, serum isoflavones, serum equol, and endometrial thickness at 12 mo. Then, a 2-factor analysis of variance was used to evaluate differences between groups in BMD change from baseline to 12 mo. The changes in bone markers were evaluated at baseline, 3 mo, and 12 mo by using the same statistical procedure. In those subjects who received the isoflavone supplement, an independent t test was used to evaluate the difference in BMD change between equol and non-equol producers. Data from all subjects were pooled, and partial correlations examined the relation between total protein intake and percentage change in bone markers from baseline to 12 mo. We also assessed the association between serum isoflavones and BMD (change from baseline) by using Pearson’s correlation statistic in those participants receiving isoflavones. All analyses were done by using SPSS version 14.0 (SPSS Inc, Chicago, IL). A P value <0.05 was considered statistically significant.
RESULTS
Subjects
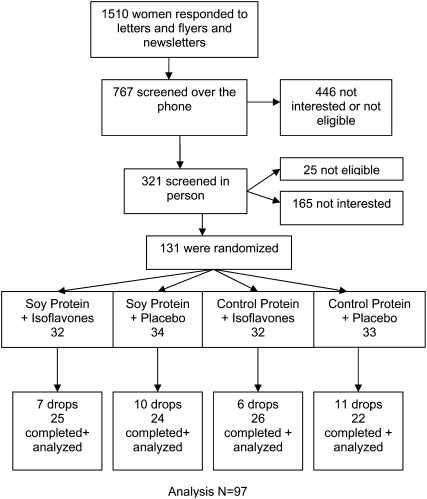
There were 1510 individuals who responded to the advertisements for this study, of whom 767 passed the initial telephone screen. The profile of the study subjects and their treatment assignment are presented in Figure 1. After randomization, 34 women voluntarily withdrew from the study. One-third of the withdrawals were due to nonstudy-related issues (eg, hospitalization for other medical conditions and change in family situation or lifestyle), one-third were due to study-related issues (eg, time commitment and gastrointestinal changes), and approximately one-third were due to other or unspecified reasons. The subjects’ baseline characteristics (age, anthropometric measures, lifestyle, nutrition, BMD, and markers of bone turnover) are presented in Table 3. No differences in subject characteristics at baseline were observed between groups, as expected.
FIGURE 1.
Inclusion and follow-up of the study population. Analysis n = 97.
TABLE 3.
Baseline characteristics of the study participants1
| Soy protein + isoflavones (n = 32) | Soy protein + placebo (n = 34) | Control protein + isoflavones (n = 32) | Control protein + placebo (n = 33) | P (ANOVA) | |
| Age (y) | 73.0 ± 5.72 | 74.0 ± 6.2 | 72.3 ± 5.7 | 72.9 ± 6.1 | 0.71 |
| Height (cm) | 157.7 ± 6.4 | 159.9 ± 6.1 | 159.6 ± 6.1 | 159.1 ± 5.2 | 0.44 |
| Weight (kg) | 72.3 ± 14.8 | 70.5 ± 10.8 | 70.3 ± 12.2 | 71.8 ± 12.6 | 0.90 |
| BMI (kg/m2) | 29.3 ± 6.9 | 27.6 ± 4.3 | 27.8 ± 5.4 | 28.4 ± 4.8 | 0.59 |
| Smokers [n (%)] | 0.63 | ||||
| Never | 12 (40) | 19 (61) | 15 (50) | 16 (52) | |
| Current/previous | 18 (60) | 12 (39) | 15 (50) | 15 (48) | |
| Drinks alcohol (%) | 22 (76) | 21 (68) | 21 (70) | 13 (43) | 0.05 |
| Time since last menses (y) | 23.5 ± 9.9 | 24.3 ± 10.8 | 22.6 ± 9.3 | 22.8 ± 8.1 | 0.91 |
| Endometrial thickness (mm) | 2.97 ± 0.81 | 3.14 ± 0.81 | 2.82 ± 0.73 | 3.32 ± 1.25 | 0.50 |
| Total no. of observations | 16 | 11 | 17 | 13 | |
| No. of observations >6 | 0 | 0 | 0 | 0 | |
| Nutrient intake3 | |||||
| Protein (g) | 62.5 ± 13.7 | 65.4 ± 16.7 | 67.7 ± 25.5 | 57.0 ± 21.9 | 0.30 |
| Calcium (mmol) | 19.3 ± 7.7 | 19.3 ± 6.7 | 19.2 ± 8.0 | 17.8 ± 8.1 | 0.88 |
| Vitamin D (IU) | 183 ± 162 | 119 ± 69 | 153 ± 104 | 157 ± 86 | 0.22 |
| Energy (MJ) | 5.96 ± 1.41 | 6.73 ± 1.96 | 5.80 ± 1.71 | 5.40 ± 1.71 | 0.06 |
| Bone variables | |||||
| Femoral neck BMD | 0.809 ± 0.098 | 0.861 ± 0.088 | 0.810 ± 0.085 | 0.800 ± 0.108 | 0.05 |
| Femoral neck T score | −1.4 ± 0.8 | −1.0 ± 0.7 | −1.4 ± 0.7 | −1.5 ± 0.9 | 0.05 |
| Total femur BMD | 0.888 ± 0.137 | 0.922 ± 0.093 | 0.869 ± 0.112 | 0.872 ± 0.144 | 0.30 |
| Total femur T score | −0.9 ± 1.1 | −0.6 ± 0.8 | −1.1 ± 0.9 | −1.1 ± 1.2 | 0.30 |
| Trochanter BMD | 0.747 ± 0.148 | 0.769 ± 0.093 | 0.733 ± 0.116 | 0.729 ± 0.135 | 0.56 |
| Trochanter T score | −0.4 ± 1.6 | −0.2 ± 0.8 | −0.5 ± 1.1 | −0.6 ± 1.21 | 0.56 |
| Ward triangle BMD | 0.641 ± 0.123 | 0.700 ± 0.096 | 0.674 ± 0.114 | 0.647 ± 0.114 | 0.13 |
| Ward triangle T score | −2.1 ± 0.9 | −1.6 ± 0.7 | −1.8 ± 0.9 | −2.0 ± 0.9 | 0.13 |
| Lumbar spine L2-L4 BMD | 1.127 ± 0.160 | 1.218 ± 0.196 | 1.102 ± 0.207 | 1.110 ± 0.208 | 0.07 |
| Lumbar spine L2-L4 T score | −0.6 ± 1.3 | 0.1 ± 1.6 | −0.8 ± 1.7 | −0.7 ± 1.7 | 0.07 |
| Forearm 33% radius BMD | 0.585 ± 0.073 | 0.596 ± 0.068 | 0.583 ± 0.089 | 0.579 ± 0.086 | 0.85 |
| Forearm 33% radius T score | −1.8 ± 1.0 | −1.6 ± 0.9 | −1.8 ± 1.2 | −1.9 ± 1.2 | 0.85 |
| Ultradistal radius BMD | 0.311 ± 0.062 | 0.319 ± 0.050 | 0.306 ± 0.048 | 0.311 ± 0.060 | 0.80 |
| Ultradistal radius T score | −1.8 ± 1.7 | −1.6 ± 1.4 | −2.0 ± 1.3 | −1.8 ± 1.6 | 0.80 |
| Total radius BMD | 0.461 ± 0.067 | 0.465 ± 0.054 | 0.461 ± 0.068 | 0.458 ± 0.075 | 0.98 |
| Total radius T score | −1.8 ± 1.4 | −1.7 ± 1.1 | −1.8 ± 1.4 | −1.9 ± 1.5 | 0.98 |
| Total-body BMD | 1.106 ± 0.085 | 1.129 ± 0.071 | 1.092 ± 0.075 | 1.086 ± 0.103 | 0.18 |
| Total-body T score | −0.2 ± 1.1 | 0.05 ± 0.8 | −0.4 ± 0.9 | −0.5 ± 1.3 | 0.18 |
| Markers of bone turnover | |||||
| Serum BAP (U/L) | 24.7 ± 8.8 | 21.2 ± 4.7 | 24.1 ± 7.5 | 26.8 ± 8.6 | 0.09 |
| Urinary NTX/CRT (nmol BCE · mmol−1· L creatinine−1) | 37.5 ± 16.8 | 33.1 ± 14.8 | 40.0 ± 19.6 | 37.5 ± 14.9 | 0.54 |
NTX/CRT, urinary N-telopeptide crosslinks of collagen normalized for creatinine; BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; BCE, bone collagen equivalents.
Mean ± SD (all such values).
Nutrient intake from food sources only; supplements not included.
Intervention compliance
Consumption of protein powder and isoflavone pills did not differ between the 4 groups. On average, the participants consumed 16.1 ± 0.4 g soy (or control) protein supplement daily containing 14.3 ± 3.6 g protein (79% compliance). The actual average isoflavone pill intake was 2.7 ± 0.35 (containing 94 ± 12 mg aglycone equivalents), which reflected 90% compliance.
As anticipated, genistein and daidzein were present in the serum from the women supplemented with isoflavones (Table 4). Of the 51 total women who received isoflavones tablets, all but 3 had measurable concentrations of isoflavones in their fasting serum. In the 46 women who received the placebo tablets, only 4 women had measurable serum concentrations of isoflavones. Of the 51 subjects supplemented with isoflavones, 25 were equol producers (49%) and 26 were non-equol producers (51%).
TABLE 4.
Nutrient intake, serum isoflavones, serum equol, and endometrial thickness at 12 mo by treatment group
| Soy protein + isoflavones (n = 25) | Soy protein + placebo (n = 24) | Control protein + isoflavones (n = 26) | Control protein + placebo (n = 22) | P (ANOVA) | |
| Nutrient intake | |||||
| Protein (g/d) | |||||
| Food sources | 65.3 ± 2.21 | 65.3 ± 2.4 | 65.0 ± 3.2 | 67.9 ± 2.9 | 0.87 |
| Supplement | 15.1 ± 0.5 | 14.5 ± 0.8 | 13.9 ± 0.8 | 13.6 ± 0.7 | 0.46 |
| Total | 80.4 ± 2.3 | 79.8 ± 2.7 | 78.9 ± 3.6 | 81.5 ± 3.0 | 0.94 |
| Calcium (mmol/d) | |||||
| Food sources | 20.6 ± 1.5 | 21.3 ± 1.5 | 20.4 ± 1.5 | 21.0 ± 1.0 | 0.96 |
| Supplement | 14.4 ± 1.3 | 12.0 ± 1.2 | 13.4 ± 1.4 | 12.1 ± 0.8 | 0.48 |
| Total | 35.0 ± 1.2 | 33.3 ± 1.4 | 34.0 ± 1.4 | 33.1 ± 1.0 | 0.72 |
| Vitamin D (IU) | |||||
| Food sources | 137 ± 13 | 143 ± 20 | 157 ± 19 | 161 ± 15 | 0.71 |
| Supplement | 366 ± 34 | 305 ± 32 | 340 ± 35 | 308 ± 21 | 0.48 |
| Total | 503 ± 27 | 448 ± 36 | 502 ± 32 | 469 ± 26 | 0.52 |
| Energy (MJ)2 | 6.08 ± 0.22 | 6.52 ± 0.24 | 6.30 ± 0.27 | 6.32 ± 0.27 | 0.66 |
| Serum isoflavones (μmol/L) | |||||
| Daidzein | 1.18 ± 0.23 | 0.21 ± 0.11 | 0.87 ± 0.17 | 0.13 ± 0.13 | 0.01 |
| Genistein | 0.90 ± 0.20 | 0.03 ± 0.02 | 0.88 ± 0.18 | 0.01 ± 0.04 | 0.01 |
| Equol from producers (n = 25) | 0.145 ± 0.046 | — | 0.191 ± 0.061 | — | 0.72 |
| Endometrial thickness (mm) | 3.38 ± 0.32 | 4.00 ± 0.54 | 2.88 ± 0.21 | 3.19 ± 0.33 | 0.16 |
| No. of observations >6 | 0 | 1 | 0 | 0 | |
| Endometrial thickness change (mm) | 0.41 ± 0.22 | 0.86 ± 0.54 | 0.06 ± 0.23 | −0.16 ± 0.43 | 0.24 |
| No. of observations | 16 | 11 | 17 | 13 |
Mean ± SD (all such values).
Energy from food sources only, not supplement.
Overall, our subjects gained 0.5 kg, on average, during the intervention. No differences in initial or ending body weight were observed between groups. Average calcium and protein intakes (from diet and supplements) also did not differ between groups (Table 4).
Bone variables
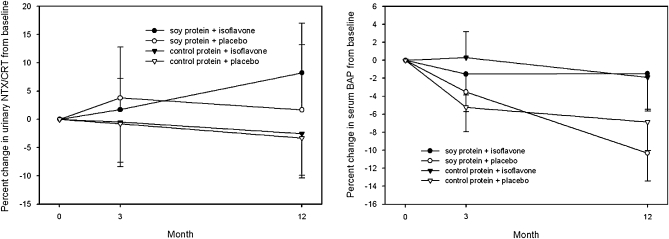
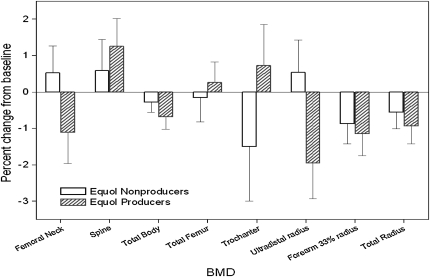
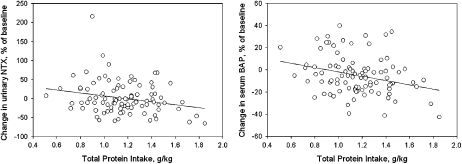
No significant differences in BMD from baseline to 1 y after intervention were observed between groups (Table 5). No differences in the markers of bone turnover were observed between the 4 groups over the duration of the study (expressed as a percentage of baseline), as evident in Figure 2. In the women who received isoflavones, no significant differences in BMD were observed between equol and non-equol producers (Figure 3). However, there was a significant correlation between change in NTX/CRT (P = 0.023, r = −0.23) and dietary protein per kilogram and change in BAP (P = 0.005, r = −0.28) and dietary protein per kilogram (Figure 4), which suggests that protein intake was related to bone turnover. No significant associations between serum isoflavones and change in BMD were observed between groups.
TABLE 5.
Bone characteristics at 12 mo by treatment group1
| Soy protein + isoflavones (n = 25) | Soy protein + placebo (n = 24) | Control protein + isoflavones (n = 26) | Control protein + placebo (n = 22) |
P for main effects (ANOVA) |
P for interaction | ||
| Soy protein | Isoflavone | ||||||
| Femoral neck BMD | 0.821 ± 0.0182 | 0.842 ± 0.020 | 0.804 ± 0.020 | 0.795 ± 0.025 | 0.130 | 0.775 | 0.490 |
| BMD change3 | 0.001 ± 0.008 | 0.001 ± 0.005 | −0.006 ± 0.005 | −0.003 ± 0.005 | 0.317 | 0.835 | 0.818 |
| Femoral neck T score | −1.3 ± 0.2 | −1.2 ± 0.8 | −1.5 ± 0.2 | −1.5 ± 0.2 | 0.130 | 0.775 | 0.490 |
| Total femur BMD | 0.901 ± 0.025 | 0.892 ± 0.022 | 0.860 ± 0.022 | 0.866 ± 0.032 | 0.188 | 0.948 | 0.776 |
| BMD change3 | 0.003 ± 0.006 | −0.011 ± 0.006 | −0.003 ± 0.004 | −0.008 ± 0.006 | 0.830 | 0.093 | 0.430 |
| Total femur T score | −0.8 ± 0.2 | −0.9 ± 0.2 | −1.2 ± 0.2 | −1.1 ± 0.3 | 0.188 | 0.948 | 0.776 |
| Trochanter BMD | 0.751 ± 0.030 | 0.734 ± 0.022 | 0.720 ± 0.021 | 0.720 ± 0.031 | 0.407 | 0.744 | 0.743 |
| BMD change3 | −0.004 ± 0.010 | −0.018 ± 0.008 | −0.002 ± 0.008 | −0.016 ± 0.009 | 0.819 | 0.102 | 0.946 |
| Trochanter T score | −0.4 ± 0.3 | −0.5 ± 0.2 | −0.6 ± 0.2 | −0.6 ± 0.3 | 0.407 | 0.744 | 0.743 |
| Ward triangle BMD | 0.660 ± 0.025 | 0.681 ± 0.023 | 0.674 ± 0.024 | 0.635 ± 0.024 | 0.501 | 0.695 | 0.205 |
| BMD change3 | 0.009 ± 0.012 | −0.003 ± 0.010 | 0.004 ± 0.008 | −0.012 ± 0.007 | 0.478 | 0.129 | 0.813 |
| Ward triangle T score | −1.9 ± 0.2 | −1.8 ± 0.2 | −1.8 ± 0.2 | −2.1 ± 0.2 | 0.501 | 0.695 | 0.205 |
| Lumbar spine L2-L4 BMD | 1.111 ± 0.032 | 1.191 ± 0.045 | 1.140 ± 0.051 | 1.103 ± 0.045 | 0.489 | 0.611 | 0.170 |
| BMD change3 | 0.004 ± 0.009 | 0.001 ± 0.008 | 0.018 ± 0.009 | 0.010 ± 0.007 | 0.181 | 0.500 | 0.826 |
| Lumbar spine L2-L4 T score | −0.7 ± 0.3 | −0.8 ± 0.3 | −0.5 ± 0.4 | −0.8 ± 0.4 | 0.489 | 0.611 | 0.170 |
| Forearm 33% radius BMD | 0.565 ± 0.015 | 0.591 ± 0.016 | 0.565 ± 0.017 | 0.579 ± 0.018 | 0.722 | 0.242 | 0.720 |
| BMD change3 | −0.004 ± 0.004 | −0.004 ± 0.003 | −0.008 ± 0.003 | −0.006 ± 0.003 | 0.383 | 0.690 | 0.653 |
| Forearm 33% radius T score | −2.1 ± 0.2 | −1.7 ± 0.2 | −2.1 ± 0.2 | −1.9 ± 0.3 | 0.722 | 0.242 | 0.720 |
| Ultradistal radius BMD | 0.306 ± 0.013 | 0.312 ± 0.012 | 0.301 ± 0.010 | 0.310 ± 0.012 | 0.770 | 0.504 | 0.907 |
| BMD change3 | −0.002 ± 0.002 | −0.004 ± 0.003 | −0.004 ± 0.003 | −0.003 ± 0.003 | 0.836 | 0.864 | 0.582 |
| Ultradistal radius T score | −2.0 ± 0.4 | −1.8 ± 0.3 | −2.1 ± 0.3 | −1.8 ± 0.3 | 0.770 | 0.504 | 0.907 |
| Total radius BMD | 0.449 ± 0.014 | 0.462 ± 0.013 | 0.451 ± 0.013 | 0.458 ± 0.015 | 0.940 | 0.480 | 0.840 |
| BMD change3 | −0.001 ± 0.002 | −0.002 ± 0.002 | −0.006 ± 0.002 | −0.005 ± 0.002 | 0.056 | 0.848 | 0.808 |
| Total radius T score | −2.0 ± 0.3 | −1.8 ± 0.3 | −2.0 ± 0.3 | −1.9 ± 0.3 | 0.940 | 0.480 | 0.840 |
| Total-body BMD | 1.094 ± 0.019 | 1.121 ± 0.014 | 1.085 ± 0.014 | 1.085 ± 0.019 | 0.183 | 0.423 | 0.419 |
| BMD change3 | −0.007 ± 0.004 | −0.003 ± 0.003 | −0.004 ± 0.003 | −0.005 ± 0.003 | 0.969 | 0.678 | 0.510 |
| Total-body T score | −0.4 ± 0.2 | −0.1 ± 0.2 | −0.5 ± 0.2 | −0.5 ± 0.2 | 0.183 | 0.423 | 0.419 |
| Markers of bone turnover | |||||||
| Serum BAP (U/L) | 23.7 ± 1.65 | 18.8 ± 1.07 | 23.3 ± 1.27 | 25.2 ± 2.03 | 0.051 | 0.333 | 0.030 |
| Urinary NTX/CRT (nmol BCE · mmol−1· L creatinine−1) | 35.9 ± 2.44 | 30.2 ± 2.74 | 35.3 ± 3.61 | 35.0 ± 3.21 | 0.497 | 0.320 | 0.367 |
NTX/CRT, urinary N-telopeptide crosslinks of collagen normalized for creatinine; BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; BCE, bone collagen equivalents.
Mean ± SEM (all such values).
Raw change in BMD from baseline in the 97 valid completers.
FIGURE 2.
Mean (±SEM) changes in markers of bone turnover [urinary N-telopeptide crosslinks of collagen normalized for creatinine (NTX/CRT) and bone-specific alkaline phosphatase (BAP)] expressed as a percentage change from baseline in the 97 women who completed the study. There were no significant differences from baseline or between the 4 groups by ANOVA.
FIGURE 3.
Mean (±SEM) percentage change in bone mineral density (BMD) between equol (n = 25) and non-equol (n = 26) producers among women who received the isoflavone tablets. There were no significant differences between equol and non-equol producers at any of the bone sites by an independent t test.
FIGURE 4.
Significant correlations between total dietary protein and urinary N-telopeptide crosslinks of collagen normalized for creatinine (NTX) at 12 mo (P = 0.023, r = −0.23) and bone-specific alkaline phosphatase (BAP) (P = 0.005, r = −0.28) expressed as a percentage change from baseline in the 97 women who completed the study.
Adverse events and subject safety
No significant differences in endometrial thickness at baseline or 1 y after the intervention were observed between groups. One woman had an endometrial thickness >6 mm. Other complaints included gastrointestinal disturbance (n = 10), differences in mammogram or breast tenderness (n = 2), new-onset cardiac symptoms (n = 2), increases in blood pressure (n = 4), respiratory infections (n = 3), and unrelated medical conditions (n = 4). The rates of adverse events did not differ between groups.
DISCUSSION
The primary purpose of this prospective intervention trial was to identify the potential skeletal benefit of incorporating soy protein and/or soy isoflavones into the diets of healthy late postmenopausal women. After 1 y of intervention, in comparison with the control group, we observed no measurable improvement in BMD or in markers of bone turnover between the women consuming soy protein or isoflavones alone or in combination. Likewise, the ability to synthesize equol from dietary isoflavone precursors was unrelated to BMD. The subjects’ compliance with both the protein and isoflavone intervention in this study was excellent, as assessed by serum isoflavone concentrations at 12 mo.
There is tremendous interest in the use of soy foods to protect the skeleton. Much of the enthusiasm is based on the cross-sectional studies that suggest that high consumption of soy products is associated with increased bone mass or decreased bone resorption or fractures (26–33). Only a few epidemiologic studies failed to find a positive association with bone health (34, 35). Whereas population studies have shown an association, they are limited in that they cannot establish causality, which requires an intervention trial.
Overall, the intervention trials that examined the effect of soy isoflavones on BMD or markers of bone turnover are less conclusive than the epidemiologic studies. Potter et al (7) initially showed a significant increase in both bone mineral content and density in the lumbar spine in postmenopausal women who consumed 90 mg isoflavones for 6 mo. Subsequently, a second 6-mo intervention trial by Alekel et al (8) also showed that 80 mg isoflavones/d attenuated bone loss at the lumbar spine in perimenopausal women. A series of short-term human intervention trials (≤6 mo) reported positive changes (13, 36–38), as did longer-term studies (9, 10, 12, 14). Most recently, Atteritano et al (39), in a study of young postmenopausal women selected for osteopenia, found an increase in spine and hip BMD with genistein compared with a loss in the placebo group.
However other human trials with skeletal-related outcomes have not been supportive (15–22, 40–43). Most recently, Brink et al (22) found in a 1-y randomized, double-blind, placebo-controlled, clinical trial that the consumption of isoflavone-enriched food did not affect BMD in a group of early postmenopausal women. In a recent meta-analysis of 10 randomized controlled clinical trials (23), isoflavones at a dosage similar to ours, produced no change in BMD over a 1-y period in women.
To attempt to explain the divergent results, we hypothesized that those individuals who were able to produce the bioactive compounds (eg, equol) from isoflavones would show the largest benefit. After oral ingestion, isoflavone glucosides are hydrolyzed by small-intestinal β-glucosidases to produce aglycones. Aglycones may be absorbed intact or further fermented by intestinal microflora in the large intestine to yield equol (44). Equol is readily absorbed, enters the circulation, and is excreted in the urine (45). It is estimated that 30–50% of humans produce equol from soy isoflavones (46); a relatively high proportion of Chinese are equol producers. Setchell et al (47) showed that, in humans, S-equol has a high affinity for estrogen receptor β [K(i) = 0.73 nmol/L], whereas R-equol is far less active. Equol production may be associated with a reduced risk of chronic diseases (46). However, we were unable to find differences in BMD dependant on equol production (Figure 3). Perhaps the dosage of oral isoflavone was not high enough to raise serum isoflavones or equol to the level needed for a biological effect. Our oral dose of isoflavone was selected because it is at the higher range of what can be obtained from food alone.
Soy proteins are relatively low in the sulfur-containing amino acids (compared with meat proteins) and, as such, are hypothesized to be potentially beneficial to the calcium economy and the skeleton (48). Because fewer sulfur-containing amino acids are found in soy foods, the result is a slight decrease in urinary calcium (49), which was assumed to occur because of better retention of calcium in bone. However, our data do not support this contention because we did not observe differences in bone variables between the control protein mix from animal sources and the soy protein.
The data from all groups were pooled to evaluate the association between total dietary protein (from all sources) and changes in the markers of bone turnover. Indeed, inverse correlations were observed between total dietary protein and change in NTX/CRT and BAP (Figure 4). Regardless of the source of protein, higher protein intakes were associated with lower bone turnover—a process generally considered favorable to bone. Our cross-sectional observation is entirely consistent with intervention trials. For example, in a short-term dual-stable calcium isotope kinetic study, we (50) showed that increasing dietary protein does not increase bone resorption. In fact, during the high-protein diet, there was a significant reduction in the fraction of urinary calcium of bone origin and a trend toward a reduction in the rate of bone turnover. The trend toward a reduced bone turnover observed in the short term highly controlled clinical trial (50) may be applicable to free-living subjects under less-stringent experimental conditions. In any case, there was no evidence of an increase in bone turnover or skeletal catabolism in our subjects because they increased their overall protein intake from 63 g/d (baseline mean) to 80 g/d (12-mo mean).
This study had limitations. We experienced a 25% dropout rate, similar to other trials (16). The duration of the intervention was limited to 1 y. To address the adequacy of our sample size, we calculated a 95% CI for the variable where the P value of the 12-mo change in BMD was the lowest. From Table 5, this happens for the main effect of soy protein on the change in total-radius BMD, P = 0.056. The 95% CI for the true effect of soy protein at this site is −0.00005 g/cm2, 0.0082 g/cm2. Thus, it is unlikely that soy protein increases total-radius BMD (or averts its decline) by >0.0082 g/cm2. This represents a change of ≈2%, which is well within the error of the measurement and would generally not be considered clinically significant. Therefore, it is unlikely, even with a larger sample size, that we could find a clinically meaningful effect of soy on BMD. Furthermore, we did not see any hints of change in the markers of bone turnover, which corroborates the stability of BMD. This study also had its strengths. We enrolled older women who are rarely studied in long-term nutrition trials and who are at high risk of bone loss. The 2 × 2 design allowed us to separate the potential effect of soy protein from that of soy isoflavones.
In summary, the addition of dietary soy protein and or isoflavone did not affect the change in BMD (or markers of bone turnover) over a 1-y period in a group of late postmenopausal women.
Acknowledgments
We are indebted to all of the subjects who volunteered in the clinical trial. We also thank Rosanne Lipcius, Sally Lynch, Pamela Fall, Deborah Dauser, and Lisa K Pesce for their technical assistance in conducting the clinical aspects of this study.
The authors’ responsibilities were as follows—AMK, KMP, and JEK: overall design and execution of the study; RHA: nutritional management of the subjects; KMM, SJW, and AK: statistical analysis and management of the data; and RSB and DEA: analysis of the isoflavones and equol. All authors were involved in the study design, execution of the protocol, analysis of the data, or writing of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Anderson GL, Judd HL, Kaunitz AM, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA 2003;290:1739–48 [DOI] [PubMed] [Google Scholar]
- 2.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252–63 [DOI] [PubMed] [Google Scholar]
- 3.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem 2003;51:7632–5 [DOI] [PubMed] [Google Scholar]
- 4.Margeat E, Bourdoncle A, Margueron R, Poujol N, Cavailles V, Royer C. Ligands differentially modulate the protein interactions of the human estrogen receptors alpha and beta. J Mol Biol 2003;326:77–92 [DOI] [PubMed] [Google Scholar]
- 5.Prestwood K, Kenny AM. Osteoporosis: pathogenesis, diagnosis and treatment in older adults. Loeser RF, ed Clinics in geriatric medicine: musculoskeletal and connective tissue disorders. Philadelphia, PA: WB Saunders Co, 1998:577–99 [PubMed] [Google Scholar]
- 6.Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA 2003;290:1042–8 [DOI] [PubMed] [Google Scholar]
- 7.Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW., Jr Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr 1998;68:1375S–9S [DOI] [PubMed] [Google Scholar]
- 8.Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr 2000;72:844–52 [DOI] [PubMed] [Google Scholar]
- 9.Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003;88:4740–7 [DOI] [PubMed] [Google Scholar]
- 10.Lydeking-Olsen E, Beck-Jensen JE, Setchell KD, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss–a 2 year randomized, placebo-controlled trial. Eur J Nutr 2004;43:246–57 [DOI] [PubMed] [Google Scholar]
- 11.Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res 2002;17:1904–12 [DOI] [PubMed] [Google Scholar]
- 12.Marini H, Minutoli L, Polito F, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med 2007;146:839–47 [DOI] [PubMed] [Google Scholar]
- 13.Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J Clin Nutr 2007 [DOI] [PubMed] [Google Scholar]
- 14.Huang HY, Yang HP, Yang HT, Yang TC, Shieh MJ, Huang SY. One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect. J Nutr Biochem 2006;17:509–17 [DOI] [PubMed] [Google Scholar]
- 15.Dalais FS, Ebeling PR, Kotsopoulos D, McGrath BP, Teede HJ. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin Endocrinol (Oxf) 2003;58:704–9 [DOI] [PubMed] [Google Scholar]
- 16.Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 2004;292:65–74 [DOI] [PubMed] [Google Scholar]
- 17.Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause 2004;11:290–8 [DOI] [PubMed] [Google Scholar]
- 18.Roughead ZK, Hunt JR, Johnson LK, Badger TM, Lykken GI. Controlled substitution of soy protein for meat protein: effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J Clin Endocrinol Metab 2005;90:181–9 [DOI] [PubMed] [Google Scholar]
- 19.Spence LA, Lipscomb ER, Cadogan J, et al. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr 2005;81:916–22 [DOI] [PubMed] [Google Scholar]
- 20.Balk E, Chung M, Chew P, et al. Effects of soy on health outcomes. Evid Rep Technol Assess (Summ) 2005;126:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong JM, Martin BR, Jackson GS, et al. Soy isoflavones do not affect bone resorption in postmenopausal women: a dose-response study using a novel approach with 41Ca. J Clin Endocrinol Metab 2007;92:577–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr 2008;87:761–70 [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone 2009:44:948–53 [DOI] [PubMed] [Google Scholar]
- 24.Craft NE, Mitchell T. Comparison of plasma isoflavone extraction. FASEB J 2007;21:701.27 [Google Scholar]
- 25.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–93 [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi T, Onouchi T, Takahashi M, Ito H, Orimo H. Effect of soy protein on bone metabolism in postmenopausal Japanese women. Osteoporos Int 2000;11:721–4 [DOI] [PubMed] [Google Scholar]
- 27.Kim MK, Chung BC, Yu VY, et al. Relationships of urinary phyto-oestrogen excretion to BMD in postmenopausal women. Clin Endocrinol (Oxf) 2002;56:321–8 [DOI] [PubMed] [Google Scholar]
- 28.Kritz-Silverstein D, Goodman-Gruen DL. Usual dietary isoflavone intake, bone mineral density, and bone metabolism in postmenopausal women. J Womens Health Gend Based Med 2002;11:69–78 [DOI] [PubMed] [Google Scholar]
- 29.Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab 2001;86:5217–21 [DOI] [PubMed] [Google Scholar]
- 30.Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol 2001;97:109–15 [DOI] [PubMed] [Google Scholar]
- 31.Greendale GA, FitzGerald G, Huang MH, et al. Dietary soy isoflavones and bone mineral density: results from the study of women's health across the nation. Am J Epidemiol 2002;155:746–54 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med 2005;165:1890–5 [DOI] [PubMed] [Google Scholar]
- 33.Ikeda Y, Iki M, Morita A, et al. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J Nutr 2006;136:1323–8 [DOI] [PubMed] [Google Scholar]
- 34.Kardinaal AF, Morton MS, Bruggemann-Rotgans IE, van Beresteijn EC. Phyto-oestrogen excretion and rate of bone loss in postmenopausal women. Eur J Clin Nutr 1998;52:850–5 [DOI] [PubMed] [Google Scholar]
- 35.Nagata C, Inaba S, Kawakami N, Kakizoe T, Shimizu H. Inverse association of soy product intake with serum androgen and estrogen concentrations in Japanese men. Nutr Cancer 2000;36:14–8 [DOI] [PubMed] [Google Scholar]
- 36.Harkness LS, Fiedler K, Sehgal AR, Oravec D, Lerner E. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J Womens Health (Larchmt) 2004;13:1000–7 [DOI] [PubMed] [Google Scholar]
- 37.Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: a four-week study. J Am Coll Nutr 2002;21:97–102 [DOI] [PubMed] [Google Scholar]
- 38.Roudsari AH, Tahbaz F, Hossein-Nezhad A, Arjmandi B, Larijani B, Kimiagar SM. Assessment of soy phytoestrogens' effects on bone turnover indicators in menopausal women with osteopenia in Iran: a before and after clinical trial. Nutr J 2005;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atteritano M, Mazzaferro S, Frisina A, et al. Genistein effects on quantitative ultrasound parameters and bone mineral density in osteopenic postmenopausal women. Osteoporos Int (Epub ahead of print 24 February 2009) [DOI] [PubMed] [Google Scholar]
- 40.Wangen KE, Duncan AM, Merz-Demlow BE, et al. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab 2000;85:3043–8 [DOI] [PubMed] [Google Scholar]
- 41.Zittermann A, Geppert J, Baier S, et al. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr 2004;43:100–8 [DOI] [PubMed] [Google Scholar]
- 42.Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236–42 [DOI] [PubMed] [Google Scholar]
- 43.Vupadhyayula PM, Gallagher JC, Templin T, Logsdon SM, Smith LM. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women-a 2-year randomized, double-blind, placebo-controlled trial. Menopause (Epub ahead of print 21 January 2009) [DOI] [PubMed] [Google Scholar]
- 44.de Pascual-Teresa S, Hallund J, Talbot D, et al. Absorption of isoflavones in humans: effects of food matrix and processing. J Nutr Biochem 2006;17:257–64 [DOI] [PubMed] [Google Scholar]
- 45.Song KB, Atkinson C, Frankenfeld CL, et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr 2006;136:1347–51 [DOI] [PubMed] [Google Scholar]
- 46.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–70 [DOI] [PubMed] [Google Scholar]
- 47.Setchell KD, Clerici C, Lephart ED, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 2005;81:1072–9 [DOI] [PubMed] [Google Scholar]
- 48.Massey LK. Dietary animal and plant protein and human bone health: a whole foods approach. J Nutr 2003;133:862S–5S [DOI] [PubMed] [Google Scholar]
- 49.Schuette SA, Hegsted M, Zemel MB, Linkswiler HM. Renal acid, urinary cyclic AMP, and hydroxyproline excretion as affected by level of protein, sulfur amino acid, and phosphorus intake. J Nutr 1981;111:2106–16 [DOI] [PubMed] [Google Scholar]
- 50.Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 2005;90:26–31 [DOI] [PubMed] [Google Scholar]