Abstract
Background
The incidence of malignancies in the head and neck region is rising. Head and neck tumors are the eighth most frequent type of malignancy in German men, forming 3.3% of the total. As a result, the demand for functionally successful and esthetically pleasing reconstructions has increased.
Methods
Review based on a selective analysis of the pertinent literature and the guidelines of the German Association of Oral and Maxillofacial Surgery as well as the authors’ clinical and scientific experience.
Results
Microsurgical flap transfer has become the most important type of reconstruction, with a more than 90% rate of success, i.e., complete integration of the transplant in the recipient site. The most frequent complications are thromboses of the vein or artery of the pedicle. For each specific defect constellation, the most appropriate donor sites have been identified. Some donor sites are used for more than one defect. The principal risk factors for flap loss are prior operations on the neck, atherosclerosis, and previous radiation treatment. New developments include the use of perforator flaps, which can be anastomosed to very small vessels in the face, and wrist-carriers, which offer complete independence from head and neck vessels.
Conclusion
The treatment, rehabilitation, and follow-up care of patients with tumors of the head and neck must be carried out by an interdisciplinary team. Full awareness of the available options for reconstruction helps the radiation therapist, oncologist, psychooncologist, general practitioner, and dentist to coordinate their efforts and advise their often mutilated and sometimes suicidal patients effectively.
Keywords: plastic surgery, head and neck cancer, microsurgery, tissue transplantation, tumor surgery
Plastic surgical reconstruction is one of the main tasks facing the oral and maxillofacial surgeon, the otorhinolaryngologist, and the plastic surgeon. It is a major component of practically all areas of these three surgical specialties but is most commonly required in reconstructive procedures after ablative tumor surgery. When grafting is performed with microvascular anastomosis, the reported rates of success (i.e., a complete "take" of the flap) are about 90% to 95% (1). Because of a rise not only in tumor incidence, but also in patients’ expectations regarding their quality of life after surgery, the need for functionally and esthetically high-quality reconstruction is now greater than ever.
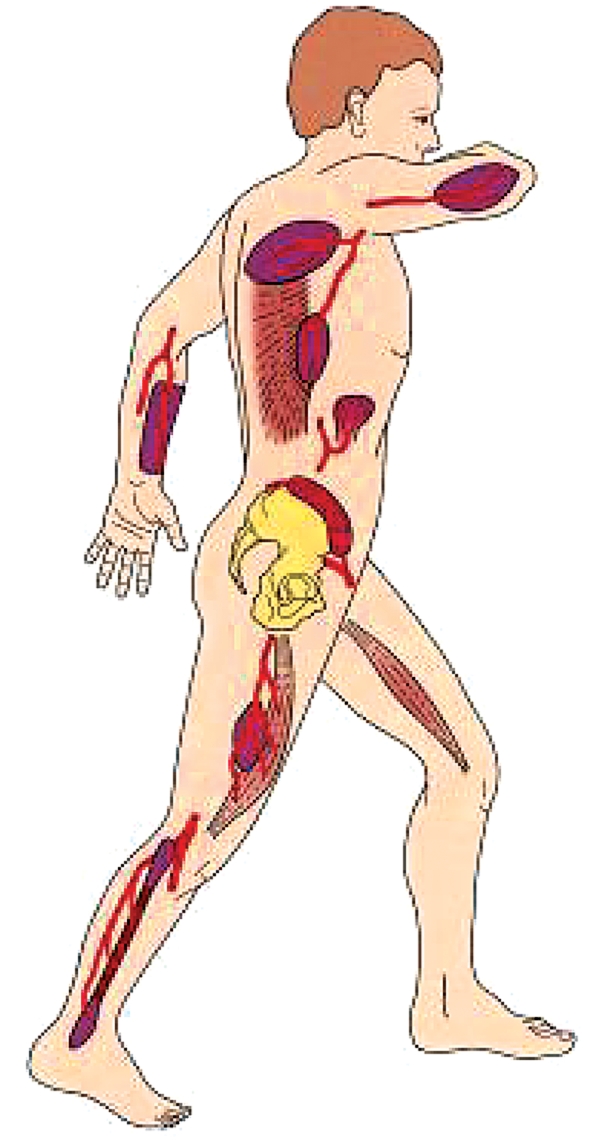
Among the many donor sites that have been described since microsurgical tissue transfer was first introduced via anatomical studies, the most useful ones have proven to be those in which a relatively unvarying anatomy enables technically simple harvesting of the flap (figure 1). An overview of the main donor sites for microvascular tissue transfer and their indications and disadvantages is provided in the table. Operative times are considerably shorter when the flap tissue can be harvested simultaneously with surgery at the affected area of the head and neck, i.e., when the patient does not need to be repositioned intraoperatively.
Figure 1.
Important donor sites for microvascular flaps to the oral and maxillofacial region, from cranial to caudal: lateral brachial flap, scapular flap, latissimus dorsi flap, antebrachial flap, small bowel flap, iliac crest flap, gracilis flap, anterolateral thigh / vastus lateralis flap, fibular flap
Table. Donor sites for microsurgical flaps and their characteristics.
| Donor site | Defect | Indication | Disadvantages |
| Intestine | |||
| Jejunum flap | 1–6 | Extensive, flat mucosal defects | Poor tolerance of mechanical stress |
| Forearm | |||
| Radial flap Ulnar flap | 1–6 (7–11) | Flat intraoral mucosal defects | Cosmetically noticeable donor site |
| Arm | |||
| Lateral brachial flap | 8 (2–7) | Intraoral soft-tissue defects (voluminous) | Technically demanding anastomosis |
| Shoulder | |||
| Scapular flap Parascapular flap | 9, 10, 17 (8) | Extensive, deep skin defects on the face | Intraoperative repositioning of the patient |
| Back | |||
| Latissimus dorsi flap | 8–11 | Volume-demanding (perforating) defects | Positioning |
| Groin | |||
| Inguinal flap | 7 | Extensive subcutaneous fatty tissue defects | Variable, fine vessels |
| Abdomen | |||
| Rectus abdominis flap | 9–11 (8) | Volume-demanding large defects | Volume of flap, occasional hernia formation |
| Thigh | |||
| Anterolateral thigh/ Vastus lateralis flap | 8–11 (1–6*) | Volume-demanding, sometimes flat soft-tissue defects | Variable vascular anatomy (cutaneous branch) |
| Calf | |||
| Peroneal flap | 1–6 | Flat intraoral soft-tissue defects | Technically demanding flap harvesting Angiography needed |
| Foot | |||
| Dorsalis pedis flap | 1–6 | Flat intraoral soft-tissue defects | Impairment of function at donor site |
| Pelvis | |||
| Iliac crest flap | 13 (15, 16) | Purely osseous defects in the toothbearing region of the mandible | Possibly, protracted symptoms at donor site Voluminous island of skin |
| Shoulder | |||
| Scapular flap | 12 (15, 16) | Extensive bony and soft-tissue defects of the maxilla | Intraoperative repositioning of the patient Limited bone height |
| Calf | |||
| Fibular flap | 14–16 (12, 13) | Long bony defects of the mandible, Combined bony and soft-tissue defects | Limited bone height Angiography needed |
1 = velum, 2 = planum buccale, 3 = lateral floor of mouth, 4 = anterior floor of mouth, 5 = hemiglossectomy defect, 6 = lateral pharyngeal wall, 7 = defect of hard palate, 8 = subtotal/total glossectomy, 9 = perforating defect, 10 = facial skin / neck, 11 = scalp, 12 = maxillary defect, 13 = mandibular defect (hemimandible), 14 = mandibular defect (subtotal/total), 15 = combined bony and soft-tissue defect, 16 = perforating bony and soft-tissue defect, 17 = fatty tissue substitute; *with primary thinning.
Microsurgical anastomosis of the flap vessels can only be performed, however, if suitable vessels are available to be connected to them at the site of the tissue defect. Extensive prior surgery of the neck, infections, generally poor vascular status, or prior radiotherapy may thus limit the applicability of microsurgical anastomosis, which is often the sole option for reconstruction (2).
While anastomosis of the flap vessels to preirradiated vessels in the oral and maxillofacial area may still be possible in the hand of experienced surgeons (3), the special operative techniques needed in the "vessel-depleted neck" carry a higher surgical risk. An adverse event, often in the form of thrombosis of a vascular pedicle, can lead to total loss of the transplanted tissue, necessitating the immediate harvesting of a second flap for microsurgical transplantation. The special techniques needed in the vessel-depleted neck include the following:
Use of the thoraco-acromial vessel system
Use of interposition flaps or vascular loops
In isolated venous deficits, use of the cephalic vein (4).
Nonetheless, many mutilated patients still remain untreated because such operations require not only a highly experienced surgeon, but also compliance and the ability to tolerate extensive surgery on the part of the patient. In recent years, there have been further technical developments that should make an adequate reconstruction possible even under the most difficult conditions.
The most important criterion for success is the complete survival ("take") of the microsurgically grafted tissue. Because complete healing is easy to observe, the success of particular methods of microsurgical reconstruction with particular types of flap was judged by the percentage of flap survival in the early years of this surgical subspecialty. More recent studies have shown, however, that success cannot be judged by flap survival alone: the patient’s own assessment of the functional and esthetic result must be considered as well (5).
Microvascular tissue transfer
This technique began to be used to cover defects in the oral and maxillofacial area in the early 1980’s (6). Currently, many different types of flap are used, with varying characteristics. Each type of flap has a spectrum of indications that may be broad or narrow; for special types of defect, multiple flaps can be used. Finding the optimal solution to a given reconstructive problem involves a determination of the most suitable tissue, the most favorable donor site, and the physiologically least stressful operation for the patient. In 2005, Thoma and Sprague defined evidence-based microsurgery as the union of all relevant research findings with the available clinical data and patient values (7).
Microsurgeons traditionally work and publish alone or in small teams. Thus, hardly any multicenter studies are available to provide high-level evidence. A PubMed literature search on the terms "microsurgery," "free flap," "microsurgical procedures," and "free tissue transfers" did not reveal a single randomized controlled study comparing different types of microsurgical flap. A review of the reconstructive treatment of the types of typical defect in this area that will be presented here must, therefore, be based on the evidence level of the currently available retrospective case series (8).
Defects in mobile segments of the oral cavity
The reconstructive treatment of flat defects requiring little volume supplementation in the mobile segments of the oral cavity is performed with thin and flexible flaps. Dorsalis pedis flaps and, more recently, jejunum flaps in particular have been found useful for this type of application.
Small-bowel flaps and dorsalis pedis flaps
On July 30, 1959, Seidenberg was the first to replace a cancerous esophageal segment with a small-bowel flap; this operation was simultaneously the first successful microsurgical tissue transfer in a human being (9). The experimental basis for free small-bowel grafting had already been laid in 1907 by Carrel, who also recognized the applicability of the bowel for covering defects in the oral cavity (10). The functional results were rated as good in view of the continued elasticity of the tissue as well as the production of mucus, particularly in larger-sized flaps and in anatomically difficult sites such as the palatal veil and the hypopharynx. The indications for dorsalis pedis flaps are limited to special situations at present because of the difficulty of harvesting the flap and the unfavorable location of the donor site.
Forearm flaps
The radial flap, which entered the literature in 1981 (11), is likewise characterized by outstanding modeling ability, thinness, technically simple flap harvesting, and a long, wide-caliber vascular pedicle. This type of flap can be harvested concurrently with surgery in the oral and maxillofacial area without any problem, and is therefore the most commonly used type of microvascular flap used in this area. Two disadvantages are the sacrifice of the radial artery and the location from which the flap is taken, which is an esthetically exposed and functionally important area of the body that must be covered with a split- or full-thickness skin flap. Disturbances of wound healing have also been reported in a number of publications, with frequencies ranging from 30% to 50%. These are due to the unfavorable characteristics of the resection bed as a recipient area for a skin flap (12).
Deep and extensive soft-tissue defects
Scapular/parascapular flaps
This type of septo-cutaneous skin and fatty tissue flap from the shoulder region, supplied by the cutaneous branch of the circumflex scapular artery (13), has been found to be particularly useful as a soft-tissue flap to cover extraoral defects because of its favorable skin coloration, and also as a vascularized fatty tissue flap to compensate for contour defects. Scapular flaps can also be used as osteo-cutaneous flaps for mandibular reconstruction. The thin bone is particularly suitable for reconstruction of maxillary defects (14). The main disadvantage of this type of flap is the need to reposition the patient intraoperatively. Because of the topographical location of the donor area, the flap cannot be harvested simultaneously during surgery in the head and neck region.
Lateral brachial flaps
This type of septo-cutaneous flap is also relatively thin (15). It is harvested from the lateral side of the arm and its vascular pedicle consists of the terminal branches of the deep brachial artery. This type of flap, very similar to facial skin in both texture and color, was already found in the initial clinical case series to be suitable for coverage of tissue defects in the head and neck area as well as inside the mouth. Its particular uses include total and partial tongue reconstruction and the coverage of defects in other areas of the oral cavity where deep and extensive resections have been performed.
Latissimus dorsi and rectus abdominis flaps
A latissimus dorsi flap was used as early as 1896 to cover the tissue defect after mastectomy for breast cancer (16). The first microvascular transfer of the flap for reconstruction in the head and neck area was performed in 1976. Because of the extraordinarily large quantity of tissue this flap provides, it can be used to create multiple skin islands and to cover extensive soft tissue defects. Further indications include the repair of defects of the scalp or of the skull base and orbit, to separate the intracranial cavity from the paranasal sinuses, as shown in the case illustration (box) and in figures 2 and 3. Reinnervation of the grafted muscle tissue by the thoracodorsal nerve is important for the restoration of facial motor function, as well as in reconstruction of the tongue.
Box. Case illustration.
A 5-year-old girl was found to have an embryonal rhabdomyosarcoma in the right orbit. She was treated with radiation and chemotherapy according to the CWS protocol (Cooperative Weichteilsarkom-Studie = Cooperative Soft Tissue Sarcoma Study). The tumor continued to grow, as seen in figure 2. When the patient was 8 years old, she underwent an orbital exenteration, which was performed in the midst of a second treatment with chemotherapy. The emptied orbit was lined with a microsurgical latissimus dorsi flap, and the reconstruction was optimized with a dermal fat flap and a nonvascularized bone flap into the orbit. After the latissimus dorsi flap had shrunk, a frontalis suspension was performed to elevate the denervated upper eyelid, and the patient was then provided with an artificial eye (figure 3). She has now been free of residual or recurrent tumor for 7 years and is followed up in the interdisciplinary tumor clinic.
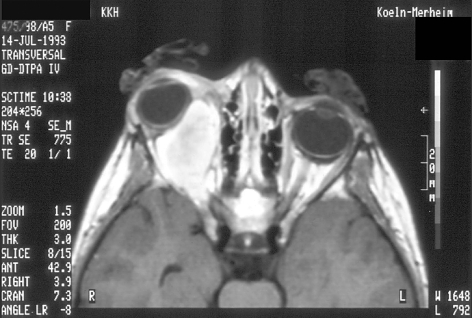
Figure 2.
This axial MRI of the patient at age 8 (see case illustration, above) shows progressive and infiltrative growth of the right orbital rhabdomyosarcoma after combined radio- and chemotherapy.
Figure 3.
After orbital exenteration and placement of a microsurgical latissimus dorsi flap into the emptied orbit, the patient has been provided with an artificial eye. She has now been free of residual or recurrent tumor for 7 years and is developing normally for her age.
The rectus abdominis flap, which has the necessary structural features for a combined skin and mucous membrane reconstitution, can be used similarly. The peritoneum that is transferred along with it is replaced in the ensuing period by the local mucosa (17). The often copious subcutaneous fat of the abdomen, however, substantially limits the usefulness of this type of flap for reconstruction in the oral and maxillofacial area.
Defects involving the bone of the mandible and maxilla
After microvascular bone transfer, unlike nonvascularized bone grafting, the osteocytes in the flap survive because of the reconstitution of the medullary and periosteal circulation. The bony tissue remains well perfused independently of the nature of the underlying tissue, so that possible contamination is better tolerated and healing can occur even in a preirradiated area or in the aftermath of osteomyelitis. Bone that has been grafted by microvascular transfer does not become atrophic, or does so only to a mild degree, even years after the procedure; only remodeling takes place, for functional reasons (18).
Bony reconstruction is technically demanding and must be adapted to the patient’s occlusion. The oral and maxillofacial surgeon’s additional training in dentistry finds application in the proper positioning of the bone flap for the dental implants that will be inserted later.
Iliac crest flaps
Since its initial description (19), this type of flap has been found particularly useful for the reconstruction of defects involving up to half of the mandible. It is the flap of choice for such defects because of the large amount of bone that it provides, as well as the opportunity for individual modeling. It is especially useful for replacement of nonatrophic, young mandibular bone as well as for superposition grafting in cases of extreme atrophy.
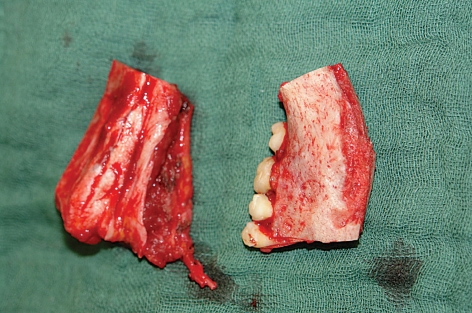
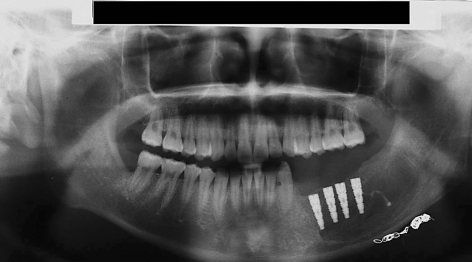
The reconstruction of a tooth-bearing segment of the mandible by iliac crest grafting in a 15-year-old patient who had undergone embolization of a mandibular arteriovenous malformation followed by resection of the affected portion of bone is shown in figures 4 through 7. This case illustrates the excellent suitability of iliac crest flaps for further reconstructive treatment with the implantation of prosthetic teeth.
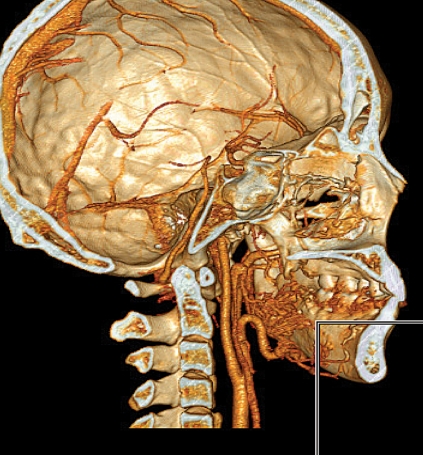
Figure 4.
This cranial CT angiogram (lateral view) of a 15-year-old boy shows an arteriovenous malformation located mainly in the body of the left mandible and extending from the angle of the jaw into the premolar region. This lesion had come to attention through bleeding from the alveola during attempted extraction of a loose tooth.
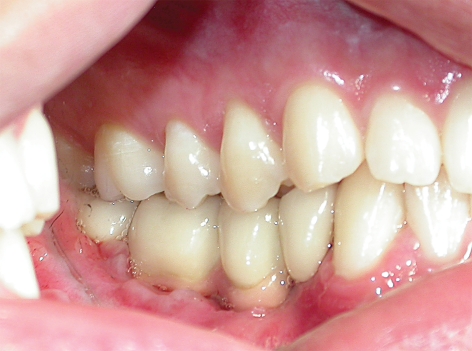
Figure 7.
The left side of the patient’s bite at age 17, after total repair with implanted prostheses. The occlusion is very good.
The most common complications are postoperative pain at the donor site, the formation of hernias, and sensory disturbances of the lateral portion of the thigh. Osteomyocutaneous flaps are generally very large and are therefore rarely suitable for the coverage of intraoral defects of small or intermediate size.
Fibular flaps
Hidalgo was the first to reconstruct the mandible with the fibula and also reported quite early on that the entire mandible could be replaced with this type of flap: One can resect practically the entire fibula without causing any notable functional deficit in the lower limb (20). The flap derives its blood supply from the peroneal artery, which gives off periosteal and medullary branches to the bone as well as further branches to the skin. The incorporation of the latter vessels in the flap enables the surgeon to harvest a skin flap that, like a radial flap, can be used to replace the oral mucosa.
The osteocutaneous fibular flap offers the possibility of adequately treating not just extensive bony defects, but also combined bony and soft-tissue defects, using two skin islands if necessary.
Scapular flaps
The scapula was introduced as a source of bone for mandibular reconstruction (14) only after the shoulder had been described as a donor site for soft-tissue flaps. The lateral margin and inferior angle of the scapula can provide enough bone to repair defects up to approximately the size of a hemimandible. Although scapular bone flaps, like fibular bone flaps, are of limited height, dental prostheses can nearly always be implanted in them afterward.
Current developments
Perforator-based flaps
The applicability of microvascular tissue transfer has been further extended recently by the introduction of perforator-based flaps (21), i.e., thin, flexible skin flaps based exclusively on their terminal cutaneous branches, which are simultaneously used as a vascular pedicle. In contrast to the conventional flap types that were described above, the manner in which perforator-based flaps are harvested depends on the variable individual localization of the terminal cutaneous branch ("perforator"). This vessel is first exposed and then followed and dissected free, in retrograde fashion, through the fatty tissue, fascia, and musculature up to the main arterial trunk. Because this method leaves the superfluous fatty and muscular tissue at the donor site rather than taking it along with the flap, a very thin flap results that is highly suitable for coverage of the usually flat defects of the oral cavity or facial skin.
Unlike standard flaps, a perforator-based flap has a short vascular pedicle, if the surgeon opts not to take along the supplying arterial trunk(s). This feature may seem to be a disadvantage at first; yet, by enabling the microsurgical anastomosis of the flap to smaller vessels in the immediate vicinity of the defect, it obviates the need for dissection of the cervical vessels, which can be quite difficult after prior surgery or radiotherapy.
The anterolateral thigh (ALT) and the proximal lateral calf have been described as particularly suitable donor sites for reconstruction in oral and maxillofacial surgery (22, 23). The widest experience to date has been with ALT perforator-based flaps up to 8 × 25 cm in size, which are currently used routinely by some authors as an alternative to radial flaps because of the very low morbidity at the donor site, and which have a wide spectrum of indications. In contrast, soleus perforator-based flaps are usually used only for smaller defects, because the donor site in the proximal lateral thigh can only be primarily closed if the flap is 5 cm wide or less. Preservation of the cutaneous vessels of the calf keeps the morbidity of flap harvesting to a minimum.
Carrier technique
A further, technically demanding method of reconstruction has been developed for patients whose vascular status is so poor because of prior radical surgery, often combined with radiotherapy or preexistent arteriosclerosis, that no suitable vessels for anastomosis can be found in the head and neck area. Typically, these mutilated patients have already undergone multiple failed reconstructive procedures and may well be in an emotional crisis with the danger of suicide.
There is almost always a total or subtotal loss of the mandible, usually in combination with extensive intra- and extraoral soft-tissue defects, making it impossible for the patient to eat, drink, or speak understandably.
If a vascular anastomosis to the thoracoacromial vessels is no longer possible in such a patient, or if interposition flaps or vascular loops have already been tried without success, then one can use the "wrist-carrier" technique that was originally described for limb reconstruction. In this technique, the flap needed for reconstruction in the face is anastomosed to forearm vessels (the radial artery and cephalic vein), so that its blood supply is fully independent of the vascular supply of the head and neck region (24). After suitable fixation of the arm to the head, this flap, temporarily perfused by the forearm vessels, can be reliably transplanted into the defect in the usual manner. One can even perform serial grafting of multiple flaps for complex reconstructions. The flap must become autonomous as a precondition for division of its vascular pedicle; this can be accelerated by the early application of ischemic conditioning.
Summary
The treatment of tumors of the head and neck requires contributions not only from surgical disciplines centered on this region, i.e., ophthalmology, otorhinolaryngology, neurosurgery, plastic surgery, and oral and maxillofacial surgery, but also from radiotherapists and oncologists. The cases that we have presented here also exemplify the importance of concurrent care by psychologists and/or psychiatrists as well as family physicians. Microvascular tissue transfer has been an established component of oral and maxillofacial surgery for nearly three decades, and its spectrum of applications has been further widened recently by the introduction of perforator-based flaps and the carrier technique. Thus, the possibilities for reconstructive procedures in the head and neck are now quite extensive, not only in tumor surgery but also in traumatology and in the repair of congenital malformations. Despite the success rates of over 90% that are reported in the literature, the major surgical project of a microvascular tissue transfer must always be carefully weighed against other reconstructive options on the basis of the patient’s individual wishes.
Prospective, randomized, controlled studies must still be done in reconstructive microsurgery in order to raise the level of the evidence for the procedures that are performed. For the present, at least, knowing what reconstructive options are available will enable the involved physicians to counsel the affected patients appropriately and to refer them onward for treatment.
Figure 5.
Right, the resected piece of bone, including the vascular malformation; left, the simultaneously harvested microsurgical iliac crest flap with its vessels, which are about to be used for vascular anastomosis and reconstruction of the mandibular defect.
Figure 6.
Orthopantogram 6 months after mandibular reconstruction. The osteosynthesis plates have already been removed and four intraosseous implants have been inserted as preparation for the provision of teeth. Also visible at the lower edge of the mandible are residual intravascular radio-opaque particles from the embolization that was performed one day before the resection.
Key messages.
Alongside local flap plasties, microsurgical tissue transfer is a main pillar of reconstructive oral and maxillofacial surgery, with a greater than 90% success rate.
The full benefit of tumor surgery in the oral and maxillofacial region cannot be realized without microsurgical tissue transfer.
As soon as it becomes evident that the available types of simple, local plastic surgical reconstruction will be inadequate, the patient should be referred to an experienced center for a functional reconstruction.
The therapeutic spectrum of microsurgical reconstructions has been extended into the oral and maxillofacial area by the introduction of perforator-based flaps, carrier flaps, and flow-through flaps.
Further solutions need to be devised especially for patients who have undergone surgery and radiotherapy and have no available neck vessels for anastomosis.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Bootz F, Preyer S. Microvascular tissue transplantation in plastic reconstruction of the external head-neck area. Laryngorhinootologie. 1994;73:538–542. doi: 10.1055/s-2007-997190. [DOI] [PubMed] [Google Scholar]
- 2.Urken ML, Weinberg H, Buchbinder D, et al. Microvascular free flaps in head and neck reconstruction. Report of 200 cases and review of complications. Arch Otolaryngol Head Neck Surg. 1994;120:633–640. doi: 10.1001/archotol.1994.01880300047007. [DOI] [PubMed] [Google Scholar]
- 3.Nahabedian MY, Singh N, Deune EG, Silverman R, Tufaro AP. Recipient vessel analysis for microvascular reconstruction of the head and neck. Ann Plast Surg. 2004;52:148–155. doi: 10.1097/01.sap.0000095409.32437.d4. [DOI] [PubMed] [Google Scholar]
- 4.Harris JR, Lueg E, Genden E, Urken ML. The thoracoacromial/ cephalic vascular system for microvascular anastomoses in the vessel-depleted neck. Arch Otolaryngol Head Neck Surg. 2002;128:319–323. doi: 10.1001/archotol.128.3.319. [DOI] [PubMed] [Google Scholar]
- 5.Hölzle F, Kesting MR, Hölzle G, et al. Clinical outcome and patients’ satisfaction after mandibular reconstruction with free fibula flaps. Int J Oral Maxillofac Surg. 2007;36:802–806. doi: 10.1016/j.ijom.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Riediger D, Schwenzer N. Die Transplantation eines Haut-Fett-Lappens aus der Leiste mit mikrochirurgischem Gefäßanschluss im vorbestrahlten Wangenbereich. Dtsch Z Mund-Kiefer-Gesichts-Chir. 1980;4:233–237. [Google Scholar]
- 7.Thoma A, Sprague S. Methodologics issues in the comparison of microsurgical flaps/ techniques in head and neck reconstruction. Clin Plastic Surg. 2005;32:347–359. doi: 10.1016/j.cps.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Urshel J, Goldsmith C, Tandan V, Miller J. The evicence-based surgery working group. User`s guide to evidence-based surgery: how to use an article evaluating surgical interventions. Can J Surg. 2001;44:95–100. [PMC free article] [PubMed] [Google Scholar]
- 9.Seidenberg B, Rosenak SS, Hurwitt ES, Som ML. Immediate reconstruction of the cervical esophagus by a revascularized isolated jejunal segment. Ann Surg. 1959;149:162–171. doi: 10.1097/00000658-195902000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrel A. The surgery of blood vessels. Johns Hopkins Hosp Bull. 1906;18:18–20. [Google Scholar]
- 11.Yang G, Chen B, Gao Y, Liu X, Li J, Jiang S, He S. Forearm free skin transplantation. Nat Med J China. 1981;61:139–145. [Google Scholar]
- 12.Bardsley AF, Soutar DS, Elliot D, Batchelor AG. Reducing morbidity in the radial forearm flap donor site. Plast Reconstr Surg. 1990;86:287–292. [PubMed] [Google Scholar]
- 13.Dos Santos LF. Retalho circular: un novo retalho livre microcirurgio. Rev Bras Cir. 1980;70:133–138. [Google Scholar]
- 14.Swartz WM, Banis JC, Newton EJ, Ramasastry SS, Jones NF, Acland R. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg. 1986;77:530–545. doi: 10.1097/00006534-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Song RS, Song Y, Yu Y, Song Y. The upper arm free flap. Clin Plast Surg. 1982;9:27–31. [PubMed] [Google Scholar]
- 16.Tansini I. Nuovo processo par l’amputazione della mamella. Riforma Medica. 1896;12:3–7. [Google Scholar]
- 17.Yamada A, Harii K, Ueda K, Asato H. Free rectus abdominis muscle reconstruction of the anterior skull base. Br J Plast Surg. 1992;45:302–306. doi: 10.1016/0007-1226(92)90057-5. [DOI] [PubMed] [Google Scholar]
- 18.Hölzle F, Watola A, Kesting MR, Wolff KD. Atrophy of free fibular graft after mandibular reconstruction. Plast Reconstr Surg. 2007;119:151–156. doi: 10.1097/01.prs.0000240703.02620.24. [DOI] [PubMed] [Google Scholar]
- 19.Taylor GI, Watson N. One stage repair of compound leg defects with free, revascularized flaps of groin skin and iliac bone. Plast Reconstr Surg. 1978;61:494–506. doi: 10.1097/00006534-197804000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hidalgo DA. Fibula free flap: A new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71–79. [PubMed] [Google Scholar]
- 21.Koshima I, Moriguchi T, Fukuda H, Yoshikawa Y, Soeda S. Free, thinned, paraumbilical perforator-based flaps. J Reconstr Microsurg. 1991;7:313–317. [PubMed] [Google Scholar]
- 22.Wei FC, Jain V, Celik N, Chen HC, Chuang DC, Lin CH. Have we found the ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109:2219–2226. doi: 10.1097/00006534-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Wolff KD, Hölzle F, Nolte D. Perforator flaps from the lateral lower leg for intraoral reconstruction. Plast Reconstr Surg. 2004;113:107–113. doi: 10.1097/01.PRS.0000095936.56036.CD. [DOI] [PubMed] [Google Scholar]
- 24.Wolff KD, Hölzle F, Eufinger H. The radial forearm flap as a carrier for the osteocutaneous fibula graft in mandibular reconstruction. Int J Oral Maxillofac Surg. 2003;32:614–618. doi: 10.1054/ijom.2002.0395. [DOI] [PubMed] [Google Scholar]