Abstract
HIV infection typically involves interaction of Env with CD4 and a chemokine coreceptor, either CCR5 or CXCR4. Other cellular factors supporting HIV replication have also been characterized. We previously demonstrated a role for CD63 in early HIV infection events in macrophages via inhibition by anti-CD63 antibody pretreatment. To confirm the requirement for CD63 in HIV replication, we decreased CD63 expression using CD63-specific short interfering RNAs (siRNA), and showed inhibition of HIV replication in macrophages. Surprisingly, pretreatment with CD63 siRNA not only silenced CD63 expression by 90%, but also inhibited HIV-1 replication in a cultured cell line (U373-MAGI) which had been previously shown to be insensitive to CD63 monoclonal antibody inhibition. Although the anti-CD63 antibody was previously shown to inhibit early HIV infection events only in macrophages, we now show a potential role for CD63 in later HIV replication events in macrophages and cell lines. Further delineation of the role of CD63 in HIV replication may lead to development of novel therapeutic compounds.
Keywords: Tetraspanin, CD63, HIV-1, siRNA, macrophages
Introduction
Human immunodeficiency virus type 1 (HIV-1) replication has been shown to extensively utilize cellular factors. A recent large-scale siRNA screen revealed over 250 candidate host factors which may aid successive steps in the viral replication cycle (Brass et al., 2008). Infection is initiated by high affinity Env binding to CD4 (Klatzmann et al., 1984) followed by interaction with chemokine receptors, either CCR5 or CXCR4 (Dragic et al., 1996; Feng et al., 1996). Reverse transcription is largely independent of cellular enzymes or cofactors, but HIV integration and proviral transcription require participation of host cell factors (Goodarzi et al., 1999; Greene 1991). Following viral gene expression, the cellular GTPase Rab9 facilitates trafficking of viral proteins from the late endosome to the trans-Golgi (Murray et al., 2005). Rab9 appears to play a role in the replication of several different viruses, including HIV. Finally, a set of protein complexes, the Endosomal Sorting Complexes Required for Transport (ESCRTs), are required to arrange viral proteins for assembly of several RNA viruses, perhaps best established for retroviruses (Morita et al., 2004; Pelchen-Matthews et al., 2004). Until last year, all antiviral treatments targeted viral proteins, but moving forward the first antiviral drug that targets a cellular gene (CCR5) has been approved by the FDA, maraviroc (Selzentry), which marks the beginning of a new era; like CCR5, CD63 is another cellular protein that may represent an important new therapeutic target for the development of antiretroviral drugs.
Tetraspanins cross the plasma membrane with four transmembrane domains and potentially serve critical roles in HIV-1 replication. These integral membrane proteins are ubiquitously expressed in human cells. We previously showed that anti-CD63 monoclonal antibody (mAb) treatment 30 minutes prior to and during infection markedly reduced HIV replication in macrophages, but not in primary T-cells or cell lines (von Lindern et al., 2003). Inhibition was shown to occur during early infection prior to initiation of reverse transcription, suggestive of involvement in virus entry or uncoating. CD63 closely associates with other integral membrane proteins, the β-2 integrins, as well as other tetraspanins (Hammond et al., 1998; Mannion et al., 1996; Rubinstein et al., 1996), and it is possible that CD63 antibody treatment may interfere with post-viral binding effects in macrophages, including signal transduction or viral uptake. CD63 and other tetraspanins are also known to form discrete membrane regions termed tetraspan enriched microdomains (TEMs), although only limited data are available to support a role for TEMs in viral replication. CD63 is particularly abundant in endosomes, which are involved in virion production (Ono et al., 2004; Kramer et al., 2005; Pelchen-Matthews et al., 2004; Murray et al., 2005). CD63 is also found in HIV virions, and may incorporate into nascent virions as HIV buds from endosomal structures or plasma membrane regions rich in CD63 and other tetraspanins (Pelchen-Matthews et al., 2003). Although CD63 appears to play a role in HIV entry of macrophages, but not T-cells, there may be an additional role for CD63 in later steps of virus replication. Here, we describe a potential role for CD63 in both primary macrophages and CD4+ cell lines, demonstrated by showing decreased HIV replication in cells following CD63 down regulation by RNA interference (RNAi) with short interfering RNA (siRNA).
Results
CD63 down regulation by siRNA in macrophages
To confirm the role of CD63 in HIV replication in primary macrophages, we optimized siRNA transfections of these cells for RNAi studies. By Western dot-blot analysis, CD63 is barely detectable at 24h and was not observed at 48h (Fig. 1), whereas CD63 expression was unaffected in control siRNA-transfected or untransfected cells relative to β-actin controls. To gauge the effect of CD63 down regulation on HIV replication, macrophages were transfected with various specific siRNAs or scrambled control siRNA after 5 to 7 days of adherence. They were infected 48h later with the R5 strain HIV-SF162 and virus production was assessed in culture supernatant by p24 ELISA after 7 days. Virus production was significantly reduced following transfection with siRNA specific for CD4, CCR5 or CD63, but there was no effect on virus production following CXCR4-specific siRNA transfection or that of scrambled control siRNA (Fig. 2A).
Fig. 1.

Down regulation of CD63 in macrophages by RNAi. Following adherence for 6 days, macrophages (4×105 per well in 24-well plates) were transfected with 100 nM CD63-specific siRNA for 24 or 48 hrs using oligofectamine. Scrambled siRNA and untransfected cells were used as controls. Following transfection, cells were lysed in 1% TritonX-100 in PBS and subjected to Western dot-blot analysis with anti-CD63 antibody or anti-β-actin antibody internal control for cellular protein.
Fig. 2.
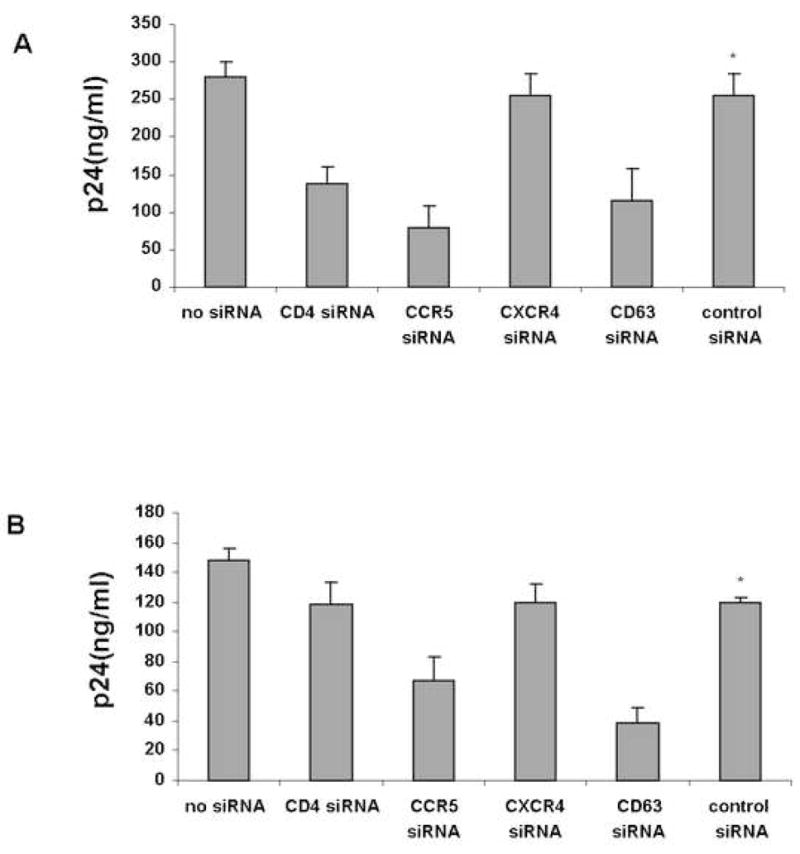
Inhibition of HIV-SF162 (R5) infection after CD63 down-regulation in macrophages. Macrophages were purified by adherence to plastic, and were treated with siRNA on day 5 (A) or on day 10 (B). In either case, cells (4×105) were infected with HIV-SF162 (R5) on day 7 using an MOI of 0.02 and extracellular virus was measured in the cultured supernatant 7d post-infection by p24 ELISA. *P<0.05, compared with CD63 siRNA group.
CD63 down regulation after HIV infection in macrophages
In order to determine if CD63 plays a role in later events in HIV replication, macrophages were transfected with control, CD63, or CD4, or HIV coreceptor-specific siRNAs 3 days post-infection (Fig. 2B). Treatment with CD4-specific siRNA 3 days after infection did not significantly affect virus production, but virus production was still inhibited when CD63-specific siRNA treatment was initiated 3 days after infection, suggesting an important role for CD63 in macrophages during HIV replication events occurring after proviral integration.
To further assess the role of CD63 in the late stage of HIV replication, CD63-specific siRNA was transfected into U1/HIV-1 cells, which are chronically infected monocytoid cells harboring 2 integrated copies of provirus per cell. Early steps of HIV replication are not required for virus production in these cells, and virus production can be induced with 3 phorbol 12-myristate 13-acetate (PMA). HIV viral production was reduced in CD63-depleted U1 cells to the same level as in cells treated with Rab 9-specific siRNA (Fig. 3), which is involved in post-integration trafficking of HIV proteins from the late endosome to the trans-Golgi (Murray et al., 2005). We also observed a decrease in virus production when we used JC53 CD4+ cells transfected with both CD63-specific siRNA followed by HIV-SX plasmid (data not shown); these findings further support the role of CD63 in later stages of HIV replication, specifically occurring after proviral integration.
Fig. 3.
Inhibition of late stage of HIV replication in an HIV-producing cell line (U1/HIV) depleted of CD63. U1/HIV-1 cells were plated (1×106) on day 1. Cells were transfected with CD63- or Rab 9-specific siRNA, and on day 3 phorbol 12-myristate 13-acetate (PMA) was added to induce virus expression. Extracellular virus was measured in the culture supernatant on day 4 by p24 ELISA. *P<0.05, with CD63 siRNA compared to virus (PMA) alone and control scrambled siRNA.
RNA interference in U373-MAGI-CCR5 cells
Down regulation of CD63 by RNAi was assessed in the U373-MAGI-CCR5 cell line. CD63 protein levels were reduced at 24h and 48h following transfection of CD63-specific siRNA, as shown by Western dot-blot analysis (Fig. 4). To assess surface expression of these molecules, effects of siRNA treatment were also determined by flow cytometry. CD4-specific siRNA resulted in modest reduction of CD4 expression (Fig. 5A), but specific expression was markedly reduced by both CCR5- and CD63-specific siRNA treatment (Figs. 5B and 5C). Notably, CD63 siRNA treatment did not affect expression of either CD4 or CCR5 (Fig. 5D).
Fig. 4.
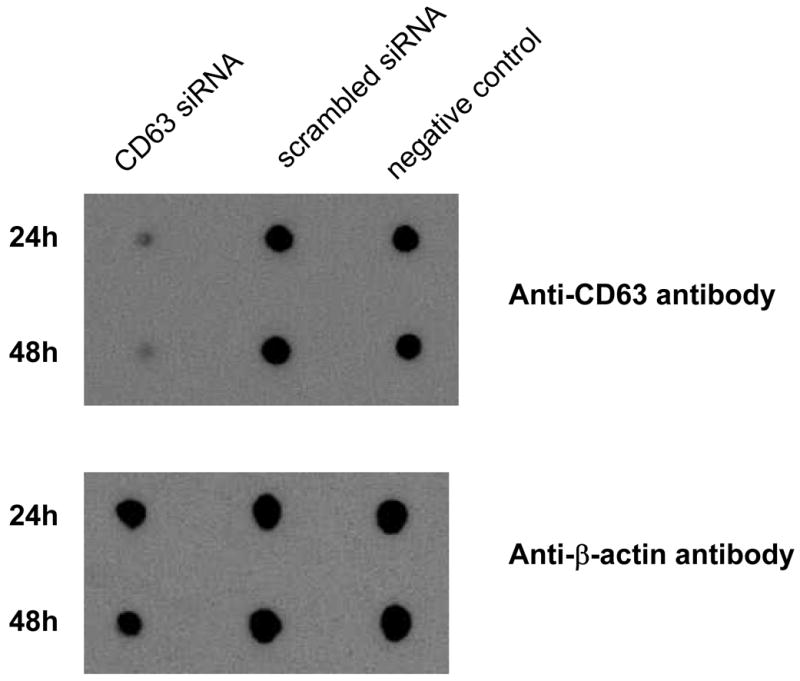
Down regulation of CD63 in U373-MAGI-CCR5 by siRNA. U373-MAGI-CCR5 cells were transfected 24h or 48h with 100 nM CD63-specific siRNA. Scrambled control siRNA and untransfected cells were also included. Cells were lysed in 1% TritonX-100 in PBS and subjected to Western dot-blot analysis with anti-CD63 antibody or anti-β-actin antibody internal control for cellular protein.
Fig. 5.

CD4, CCR5 and CD63 levels on U373-MAGI-CCR5 24h after siRNA transfection. U373-MAGI-CCR5 cells (5×104) were seeded in 24-well plates and transfected with CD4, CCR5, CD63 or scrambled control siRNAs. At 24h post-transfection, cells were detached using 1X trypsin and stained with (A) anti-CD4-PE antibody, (B) anti-CCR5-PE antibody, or (C) anti-CD63-PE antibody and measured by flow cytometry. Levels of CD4, CCR5 and CD63 in control or CD63 siRNA transfectants are compared in panel (D). An IgG1 isotype antibody control was used to determine the rate of down regulation.
Effect of CD63 down regulation on HIV replication in U373-MAGI cells
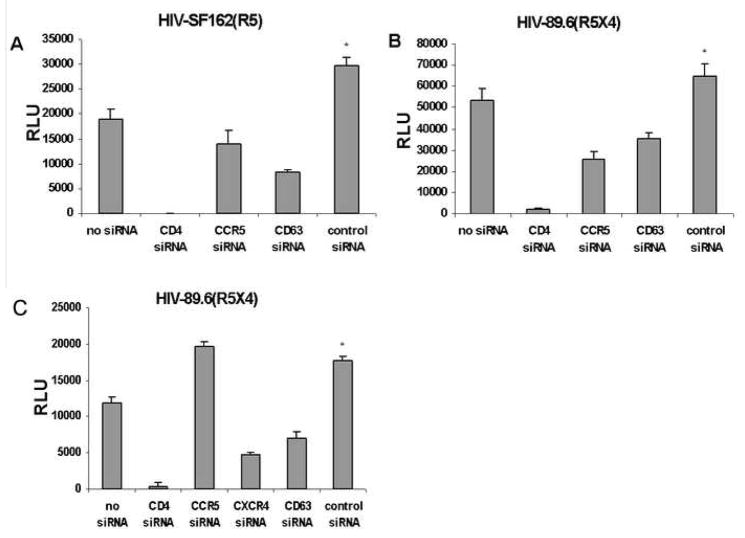
Replication of HIV- SF162 (R5; Fig. 6A) or HIV 89.6 (R5X4; Fig. 6B), as determined by β-gal activity three days after infection, was reduced following CD63 or CCR5 down regulation in U373-MAGI-CCR5 cells; reduction was greater with CD4 down regulation (Figs. 6A & B). Using a related indicator cell line, U373-MAGI-CXCR4 (Fig. 6C) expressing the chemokine coreceptor CXCR4 and not CCR5, down-regulation of CD4, CD63 or CXCR4 significantly reduced virus replication, compared to transfection with scrambled or negative control siRNAs (Fig. 6C); P<0.05, compared with CD63 siRNA. As expected, CCR5-specific siRNA treatment did not affect virus replication in these cells, since U373-MAGI-CXCR4 cells do not express CCR5 (Fig. 6C). Although the RLU of control siRNA-transfected cells was higher than that of the untransfected cells in each case (Fig. 6A–6C), statistically there is no difference.
Fig. 6.
Infection of U373-MAGI-CCR5 or U373-MAGI-CXCR4 30h post-transfection of siRNA. (A & B) U373-MAGI-CCR5 or (C) U373-MAGI-CXCR4 cells (104) were seeded in 96-well plates 24h prior to transfection. Cells were transfected with CD4 siRNA, CCR5 siRNA, CD63 siRNA or scrambled control siRNA in quadruplicate. Transfection reagent was included in the no siRNA transfection control. After 30h, cells were infected with an m.o.i. of approximately 0.02 with (A) HIV-SF162 (R5), or with (B) HIV-89.6 (R5X4). (C) U373-MAGI-CXCR4 cells were infected with HIV-89.6 (R5X4). β-galactosidase activity was measured by Beta-Glo™ Assay System (Promega) by luminometry performed 48h post-infection, with subtraction of signal from uninfected U373-MAGI cells. *P<0.05, compared with CD63 siRNA group.
Discussion
CD63, a tetraspanin membrane glycoprotein found in nearly all human cells on the plasma membrane and especially abundant in endosomes, was previously shown to be involved in early events of HIV replication in macrophages (von Lindern et al., 2003). In this study, we present evidence for an expanded role of CD63 in HIV replication, not only limited to early events, but also likely involving later HIV replication events, in cell lines as well as in macrophages. This effect was shown for infection of R5 and R5X4 viruses following down regulation of CD63 by RNA interference with siRNA. Although PBL and cell lines were resistant to inhibition of HIV infection by a CD63 antibody, HIV replication in cell lines was inhibited following down regulation of CD63.
There are important cell-specific differences between the entry process in macrophages and cell lines or T cells that could explain the lack of anti-CD63 antibody inhibition of HIV replication in U373-MAGI cells or PBL. It is possible that this mode of entry may be more important in phagocytic cells such as macrophages. CD63, which is present but less abundant in lysosomal membranes, may be involved in preventing degradation of the virus through lysosomal pathways, instead routing the virus core through late endosomes, which can give rise to productive infection. For example, studies by Marechal et al showed that macrophages take up viral particles bound to the cell surface through macropinocytosis (Marechal et al., 2001). Shortly after exposure of macrophages to HIV (independent of viral envelope-receptor interactions), viral particles were visualized in intracellular vesicles. While most X4 and all Env-deleted virions were subsequently degraded, virions with R5-tropic envelopes achieve virus fusion, leading to capsular release into the cytoplasm and productive infection (Marechal et al., 2001). Although early studies indicated that HIV infection is pH-independent and does not require endocytosis of the CD4 receptor (Maddon et al., 1988; McClure et al., 1988; Stein et al., 1987), HIV can enter through clathrin-coated vesicles which fuse with endosomal membranes (Bourinbaiar et al., 1991; Grewe et al., 1990; Pauza et al., 1988). CD4+ HeLa cell line endocytosis was shown to contribute to HIV entry using trans dominant-negative mutants of dynamin and Eps15, which are required for endocytosis. Inhibition was shown by analysis of reverse transcription products by real-time PCR and by entry by delivery of virion-associated Vpr-β-galactosidase fusion protein, (Daecke et al., 2005), whereas dynamin is essential for both clathrin - and caveolar- dependent transport. The trans dominant-negative mutants decreased HIV entry up to 95%, confirming a role for endocytosis in productive infection. Expression of dominant-negative variants Eps15 is specific for inhibition of clathrin-dependent endocytosis.
Another important difference between macrophages or trophoblasts and T-cells or cell lines is the density of CD4 expression. As calculated by quantitative flow cytometry (QFACS), CD4 binding sites were estimated to number only about 200/cell on monocytes, as compared with 5000/cell on primary T cells and T cell lines (Lee et al., 1999). The relatively low density of CD4 may limit infectibility of primary macrophages by some virus strains, as well as the effects of CD63 antibody. CD63 may fulfill requirements for HIV entry into cells with relatively low CD4 expression.
Inhibition of infection of U373-MAGI cells by CD63 siRNA treatment (Fig. 6A) suggests a more fundamental role for CD63 in HIV replication in addition to the previously described role at the level of virus entry into macrophages. In the cell line U373-MAGI-CCR5 or U373-MAGI-CXCR4, cells are stably transfected with an LTR-β-galactosidase construct that allows quantitative assessment of HIV replication based on Tat production. Tat is an early protein, expressed prior to expression of structural genes, and therefore, decreased β-galactosidase production in siRNA-treated cells indicates a block to HIV replication in a step prior to initial HIV translation events. Using a similar detection system, in this case using cells stably transfected with LTR-luciferase, down regulation of Rab9, known to be necessary for transport of cargo proteins from the late endosome to the trans-Golgi, no effects are seen since this intracellular protein trafficking occurs after expression of Tat (Murray et al., 2005). Therefore, the effects of CD63 appear to occur during very early viral translation, prior to production of the structural genes.
The role of CD63 in post-entry HIV replication events is reinforced by data in primary macrophages, in which extracellular virus production is inhibited in cells in which RNAi via CD63-specific siRNA transfection 3 days after infection still inhibits HIV replication (Fig. 2B). Although this was not tested in a single cycle infection system, reverse transcription proceeds over 48 hours in macrophages (O’Brien et al., 1994), and the effects were tested 7 days later. This measurement of virus production would be just one day after maximal CD63 down regulation, and demonstrating effects in later stages of HIV replication in macrophages. The lack of effect with CD4 down regulation in parallel helps define the specificity of the effect, and supports a role in late replication events, at least after integration. This was shown by viral inhibition in a CD63-depleted promonocyte cell line that expresses HIV-1 from stably integrated provirus (Fig. 3). There are important differences in late replication events in macrophages, as compared with other HIV target cells. Although virus production in T cells and cultured cell lines is thought to occur as result of budding through the plasma cell membrane in T cells (Garrus et al., 2001), virus assembly in macrophages appears to occur on a subset of endocytic organelles that carry markers found in late endosomes of multivesicular bodies (MVBs), most notably CD63 (Pelchen-Matthews et al., 2003). Electron microscopy and immunoprecipitation experiments suggest that virus release from macrophages involves initial budding into endosomal organelles, which are then are released by fusion of these organelles with the plasma membrane (Raposo et al., 2002; Pelchen-Matthews et al., 2003; Kramer et al., 2005). Nonetheless, despite different modes of egress between macrophages and T cells, events prior to virus assembly and budding into MVBs require CD63, and are affected by CD63 down regulation.
Although virus maturation and extracellular release mechanisms appear to differ between macrophages and T cells or cell lines, CD63 appears to be involved in late replication events in all cell types tested. It is likely that CD63 is involved with virus protein processing and possibly in the process by which virus is released. Further studies are required to delineate the precise role of CD63 in HIV infection of phagocytic and non-phagocytic cells, especially in virus protein transportation both after entry and before virus production.
Materials and Methods
Cells and viruses
Primary macrophages were purified from peripheral blood mononuclear cells by adherence to plastic tissue culture dishes. Briefly, primary monocyte-derived macrophages were isolated from healthy HIV-1-negative blood donors by Ficoll-Hypaque centrifugation followed by adherence for 7 days to plastic petri dishes initially coated with human AB serum. Previously this was shown to yield cells with >98% esterase positivity (Rich et al., 1992). During differentiation, macrophages were cultured in Iscove’s modified Dulbecco’s medium supplemented with 20% fetal calf serum. U373 cells, obtained through the NIH AIDS Research and Reference Reagent Program (contributed by Dr. Michael Emerman and Dr. Adam Geballe), are a cell line derived from a glioblastoma that has been modified by stable transfection of LTR-β-galactosidase (U373-MAGI) (Vodicka et al., 1997; Harrington et al., 1993). U373-MAGI cells were stably transfected with either CCR5 or CXCR4 to enable infection by HIV (Vodicka et al., 1997). Cells were propagated in DMEM and 10% fetal calf serum.
U1/HIV-1 cells (1×106) obtained from the NIH AIDS Research and Reference Reagent Program (Folks, et al., 1988) were plated in 24-well microtiter plates on day 1. Cells were transfected with siRNA (200 nM final concentration) using Oligofectamine (Invitrogen) following the manufacturer’s instructions. Complete cell media (RPMI 1640 containing 2.0 mM L-glutamine; 10% heat-inactivated fetal bovine serum) was changed after 24h. Forty-eight hours post-transfection (day 3), complete RPMI media plus phorbol 12-myristate 13-acetate (PMA) was added to cells. Extracellular virus was measured in the culture supernatant on day 5 by p24 ELISA.
Viruses and antibodies
The following HIV-1 strains were obtained from the NIH AIDS Research and Reference Reagent Program: R5 HIV strains SF162 (Shioda et al., 1991), contributed by Cecilia Cheng-Mayer and ADA (Westervelt et al., 1992) contributed by Howard Gendelman, R5X4 HIV strain 89.6 (Collman et al., 1992) contributed by Ron Collman, and X4 HIV strain 92HT599, contributed by Neal Halsey. All HIV-1 stocks containing HIV p24 200 ng/ml, were further expanded in 40 ×106 phytohemagglutin antigen (PHA)-stimulated peripheral blood lymphocytes (PBLs). Supernatants were harvested 6, 7, and 8 days after infection, and virus production was determined by p24 antigen capture assay (Immunodiagnostics). PHA-stimulated PBLs were propagated in 80% RPMI 1640 (Cellgro), 20% fetal bovine serum, 1% L-glutamine, 1% penicillin/streptomycin, and 20 U/ml IL-2. All antibodies used in infection assays were dialyzed before use to remove sodium azide. Anti-CD4 (Leu3a) antibody was obtained from BD Biosciences and anti-CD63 antibody (CLB-gran12) was obtained from Caltag. For flow cytometry studies, fluorescein isothiocyanate (FITC/phycoerythrin (PE)-labeled anti-CD4 antibody, anti-CD63 antibody, and matching mouse immunoglobulin G1 (IgG1) isotype (BD Immunocytometry Systems mA6 were used.
HIV infection assays
HIV-1 stocks were titered, and for most experiments, the inoculum was 2 ng per 5×104 cells (MOI 0.02). U373-MAGI cells (1×104) were plated in 96-well microtiter plates the day before infection. After 2h incubation of virus supernatant (50 μl/well) at 37°C, fresh medium (150μl) was added to each well. β-galactosidase activity was measured 48h after infection using standard protocol (Invitrogen). Primary macrophages adherent for 7 days were infected 1 day after plating at 5×104 cells per well in 96-well microtiter plates, with an approximate MOI of 0.02 in 50 μl/well, with fresh medium (150 μl) added to each well after 2h incubation. Cells were fed with fresh medium 4 days later, and supernatant was assessed for p24 by ELISA on day 7 postinfection.
Cell surface protein expression and HIV replication in siRNA-transfected cells
The CD63-specific target siRNA was designed and synthesized by Dharmacon. siRNA was delivered intracellularly using Oligofectamine (Invitrogen) following the manufacturer’s instructions. Primary macrophages adherent for 5 days were transfected with 100 nM siRNA using oligofectamine (siRNA:oligofectamine = 1:1) in serum free Iscove’s medium after plating at 4×105 cells per well in 24-well plates. Cells were fed with Iscove’s medium and 20% FCS after 4h to terminate transfection. To measure protein down regulation, cells were subjected post-infection to flow cytometry for surface protein analysis or were lysed after 2 days by 1% Triton X-100 in PBS for Western blot analysis. Primary macrophages were infected with HIV two days after siRNA transfection in Iscove’s medium at an MOI of approximately 0.02. Cells were fed with Iscove’s medium and 20% FCS 4 days later, and supernatants were harvested and diluted for p24 ELISA on day 7 postinfection.
U373-MAGI cells were transfected with 100nM siRNA one day after plating at 1×104 cells per well in 96-well microtiter plates. Protein down regulation was assessed 24h and 48h after siRNA transfection by lysis with 1% Triton X-100 in PBS for Western dot-blot analysis, or subjected to flow cytometry for surface protein analysis. β-galactosidase activity was measured 48h after infection using standard protocol (Invitrogen).
Flow cytometry
U373-MAGI cells were washed twice with PBS and scraped into Falcon 2054 tubes, 5×105 cells per tube in 100μl cold PBS (with 5% serum). Fluorescent antibodies (anti-CD4-PE antibody; anti-CCR5-PE antibody, and antiCD63-PE antibody) were added to each tube according to the manufacturer’s protocol, and were incubated for 20 min on ice. After staining, cells were washed twice with 4ml cold PBS (with 5% serum), and resuspended in 150 μl cold PBS. Cells were fixed with 150 μl 4% paraformaldehyde and analyzed by flow cytometry. An anti-IgG1 isotype control antibody was used in all flow cytometry experiments.
Statistical method
Statistical comparisons among differently treated groups were made using one-way Anova with post hoc Newman-Keuls multiple comparison test.
Acknowledgments
We thank Penny Munoz for manuscript preparation and Eric Freed for helpful discussions. Also, we thank Mark Griffin for assistance with flow-cytometry services.
This work was supported by Public Health Service grant AI52041 from the National Institute of Allergy and Infectious Diseases and National Heart, Lung, and Blood Institute grant HL088999. J.V.L. was supported by institutional funds from the McLaughlin Endowment.
Footnotes
We confirm that we have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourinbaiar AS, Phillips DM. Transmission of human immunodeficiency virus from monocytes to epithelia. J Acquir Immune Defic Syndr. 1991;4:56–63. [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genome screen. Science. 2008;319:921–6. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Collman R, Balliet JW, Gregory SA, Friedman H, Kolson DL, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–21. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daecke J, Fackler OT, Dittmar MT, Krausslich HG. Involvement of clathrin- mediated endocytosis in human immunodeficiency virus type 1 entry. J Virol. 2005;79:1581–94. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Folks TM, Justement J, Kinter A, Schnittman S, Orenstein J, Poli G, Fauci AS. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13- phorbol-12-myristate acetate. J Immunol. 1988;140:1117–22. [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, Myszka DG, Sundquist WI. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Goodarzi G, Pursley M, Felock P, Witmer M, Hazuda D, Brackmann K, Grandgenett D. Efficiency and fidelity of full-site integration reactions using recombinant simian immunodeficiency virus integrase. J Virol. 1999;73:8104–11. doi: 10.1128/jvi.73.10.8104-8111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WC. The molecular biology of human immunodeficiency virus type 1 infection. New England Journal of Medicine. 1991;324:308–17. doi: 10.1056/NEJM199101313240506. [DOI] [PubMed] [Google Scholar]
- Grewe C, Beck A, Gelderblom HR. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3:965–74. [PubMed] [Google Scholar]
- Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J Immunol. 1998;161:3282–91. [PubMed] [Google Scholar]
- Harrington RD, Geballe AP. Cofactor requirement for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–47. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann K, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kramer B, Pelchen-Matthews A, Deneka M, Garcia E, Piguet V, Marsh M. HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol Dis. 2005;35:136–42. doi: 10.1016/j.bcmd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte- derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon PJ, McDougal JS, Clapham PR, Dalgleish AG, Jamal S, Weiss RA, Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988;54:865–74. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29) J Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–77. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. Embo J. 1988;7:513–8. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Murray JL, Mavrakis M, McDonald NJ, Yilla M, Sheng J, Bellini WJ, Zhao L, Le Doux JM, Shaw MW, Luo CC, Lippincott-Schwartz J, Sanchez A, Rubin DH, Hodge TW. Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. J Virol. 2005;79:11742–51. doi: 10.1128/JVI.79.18.11742-11751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WA, Namazi A, Mao SH, Kalhor H, Zack JA, Chen ISY. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J Virol. 1994;68:1258–1263. doi: 10.1128/jvi.68.2.1258-1263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A, Freed EO. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J Virol. 2004;78:1552–63. doi: 10.1128/JVI.78.3.1552-1563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza CD, Price TM. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988;107:959–68. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol. 2003;162:443–55. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Raposo G, Marsh M. Endosomes, exosomes and Trojan viruses. Trends Microbiol. 2004;12:310–6. doi: 10.1016/j.tim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Raposo G, Moore M, Innes D, Leijendekker R, Leigh-Brown A, Benaroch P, Geuze H. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic. 2002;3:718– 29. doi: 10.1034/j.1600-0854.2002.31004.x. [DOI] [PubMed] [Google Scholar]
- Rich EA, I, Chen S, Zack JA, Leonard ML, O’Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) Journal of Clinical Investigation. 1992;89:176–83. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein E, Le Naour F, Lagaudriere-Gesbert C, Billard M, Conjeaud H, Boucheix C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. European J Immunol. 1996;26:2657–2665. doi: 10.1002/eji.1830261117. [DOI] [PubMed] [Google Scholar]
- Shioda T, Levy JA, Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature (London) 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, Engleman EG. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–68. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, Goh WC, Wu LI, Rogel ME, Bartz SR, Schweickart VL, Raport CJ, Emerman M. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology. 1997;233:193–8. doi: 10.1006/viro.1997.8606. [DOI] [PubMed] [Google Scholar]
- von Lindern JJ, Rojo D, Grovit-Ferbas K, Yeramian C, Deng C, Herbein G, Ferguson MR, Pappas TC, Decker JM, Singh A, Collman RG, O’Brien WA. Potential role for CD63 in CCR5-mediated human immunodeficiency virus type 1 infection of macrophages. J Virol. 2003;77:3624–33. doi: 10.1128/JVI.77.6.3624-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P, Henkel T, Trowbridge DB, Orenstein J, Heuser J, Gendelman HE, Ratner L. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J Virol. 1992;66:3925–31. doi: 10.1128/jvi.66.6.3925-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]