Abstract
Dishevelled (Dsh) is a key signaling molecule in the canonical Wnt pathway. Although the mechanism by which Dsh transduces a Wnt signal remains elusive, the subcellular localization of Dsh may be critical for its function. In the early sea urchin embryo, Dsh is concentrated in punctate structures within the cytoplasm of vegetal blastomeres. In these cells, Dsh stabilizes β-catenin and causes it to accumulate in nuclei, resulting in the activation of transcriptional gene regulatory networks that drive mesoderm and endoderm formation. Here, we present a systematic mutational analysis of Lytechinus variegatus Dsh (LvDsh) that identifies motifs required for its vegetal cortical localization (VCL). In addition to a previously identified lipid-binding motif near the N-terminus of Dsh (Weitzel et al., 2004), we identify a short (21 amino acid) motif between the PDZ and DEP domains that is required for VCL. Phosphorylation of threonine residues in this region regulates both the targeting and stability of LvDsh. We also identify functional nuclear import and export signals within LvDsh. We provide additional evidence that LvDsh is activated locally in the vegetal region of the embryo but is inactive in animal blastomeres and show that the inability of LvDsh to function in animal cells is not a consequence of impaired nuclear import. The DIX domain of LvDsh functions as a potent dominant negative when overexpressed (Weitzel et al., 2004). Here, we show that the dominant negative effect of DIX is dependent on a highly conserved, lipid-binding motif that includes residues K57 and E58. The dominant negative effect of DIX is not a consequence of blocking VCL or the nuclear import of LvDsh. We provide evidence that isolated DIX domains interact with full-length LvDsh in vivo. In addition, we show that the K57/E58 lipid-binding motif of DIX is essential for this interaction. We propose that binding of the isolated DIX domain to full-length Dsh may be facilitated by interactions with lipids, and that this interaction may inhibit signaling by a) preventing endogenous Dsh from interacting with Axin, or b) blocking the ability of Dsh to recruit other proteins, such as GBP/Frat1, to the β-catenin degradation complex.
Introduction
Wnt proteins are secreted, glycoprotein ligands that regulate developmental events, including cell polarity, body axis specification, and cell fate (Cadigan and Nusse, 1997; Dickinson and McMahon, 1992; Logan and Nusse, 2004; Nusse and Varmus, 1992; Wodarz and Nusse, 1998). Wnt proteins signal through multiple pathways, the best understood of which is the highly conserved, canonical Wnt pathway (Huelsken and Behrens, 2002; Moon et al., 2002). The primary outcome of this pathway is the stabilization of cytosolic β-catenin.
A critical, early event in the patterning of the animal-vegetal (A-V) axis in the early sea urchin embryo is the maternally regulated entry of β-catenin into the nuclei of vegetal cells (Davidson, 1989; Ettensohn, 2006; Logan et al., 1999). Later differentiation of vegetal cell progeny requires augmentation of nuclear β-catenin through zygotic expression of Wnt and activation of the canonical Wnt pathway (Angerer and Angerer, 2000). Similar early embryonic events involving nuclear β-catenin have been observed in many deuterostome embryos (Imai et al., 2000; Larabell et al., 1997; Logan et al., 1999; Roeser et al., 1999; Schneider et al., 1996). Recent studies suggest that this mechanism of axis specification and polarity may have been established prior to the divergence of radially and bilaterally symmetrical metazoans (Wikramanayake et al., 2003).
According to current models, in the absence of a Wnt signal, β-catenin is part of a multiprotein complex with Axin, glycogen synthase kinase 3β (GSK3β), the tumor suppressor gene product adenomatous polyposis coli protein (APC), and other proteins (Hart et al., 1998; Kimelman and Xu, 2006; Kishida et al., 1999; Lee et al., 2003). Within this complex, β-catenin is phosphorylated at several N-terminal serine and threonine residues by casein kinase I (CKI) and GSK3β (Amit et al., 2002; Liu et al., 2002; Yanagawa et al., 2002; Yost et al., 1996). Phosphorylated β-catenin is recognized by the ubiquitin ligase subunit, β-TrCP, and targeted for degradation by the proteosome (Aberle et al., 1997; Latres et al., 1999; Liu et al., 1999). A Wnt signal is initiated by binding of secreted Wnt to its receptors, Frizzled and LPR5/6 which activates the protein Dishevelled (Dsh) by an unknown mechanism that may include phosphorylation (Rothbacher et al., 2000; Sun et al., 2001; Tomlinson et al., 1997; Willert et al., 1997; Wong et al., 2003). Activated Dsh antagonizes GSK3β-dependent phosphorylation of β-catenin, possibly by recruiting the GSK3β-binding protein FRAT1 to the degradation complex (Ferkey and Kimelman, 2002; Hino et al., 2003; Li et al., 1999). Decreased phosphorylation of β-catenin is associated with its dissociation from the degradation complex and stable accumulation in the cytoplasm. Increased cytoplasmic pools of β-catenin lead to translocation and accumulation of the protein in the nucleus where interaction with LEF/TCF transcription factors causes transcriptional activation of Wnt target genes (Guger and Gumbiner, 2000; Henderson and Fagotto, 2002).
Dsh was one of the first components of the Wnt signaling pathway to be defined; yet its biochemical functions remain poorly understood. Drosophila harboring the recessive viable allele, dsh1, display defects in the orientation of sensory bristles that cause an unkempt or “dishevelled” physical appearance (Krasnow et al., 1995). Dsh family members are 500 to 600 amino acids in length and have been found in animals ranging from Drosophila to humans (Wharton, 2003). In addition to playing a role in the canonical Wnt signaling pathway, Dsh proteins are also involved in the planar cell polarity (PCP) pathway. The primary outcome of this pathway is the regulation of cytoskeletal organization and cell polarity through c-Jun kinase (JNK) (Mlodzik, 1999; Shulman et al., 1998). Dsh is hypothesized to serve as the branch point at which the two pathways diverge, making its regulation of particular importance (Axelrod et al., 1998; Boutros and Mlodzik, 1999).
All Dsh family members contain three highly conserved domains: an N-terminal DIX domain, a central PDZ domain, and a DEP domain (Fig. 1) (Klingensmith et al., 1994; Sussman et al., 1994; Theisen et al., 1994). Genetic, biochemical and structure-function studies have been conducted in a variety of experimental systems to elucidate the roles of these domains. The PDZ domain mediates the interaction of Dsh with a variety of binding partners, including the Frizzled receptor, the kinases CKI and Par-1 and the cell signaling mediators Frodo and Dapper (Cheyette et al., 2002; Gloy et al., 2002; Lee, 2003; Peters et al., 1999; Sun et al., 2001). Through its association with such molecules, the PDZ domain is believed to function in both the canonical Wnt and PCP pathways. The DEP domain functions primarily in the PCP pathway by mediating Dsh association with the GTPase, RAC, a known JNK activator (Axelrod et al., 1998; Boutros et al., 1998; Habas et al., 2003). In contrast, the DIX domain functions principally in the canonical Wnt pathway and plays a role in Dsh multimerization and interaction with Axin, although other parts of the Dsh molecule may also be involved in these interactions (Itoh et al., 2000; Julius et al., 2000; Kishida et al., 1999; Moriguchi et al., 1999; Penton et al., 2002; Rothbacher et al., 2000; Smalley et al., 1999). When overexpressed independently, the DIX domain acts as a potent dominant negative with respect to canonical Wnt signaling in multiple organisms (Axelrod et al., 1998; Weitzel et al., 2004). In sea urchin embryos, overexpression of the DIX domain blocks the accumulation of β-catenin in vegetal blastomere nuclei and prevents development of vegetal tissues (Weitzel et al., 2004). The mechanism underlying the dominant negative phenotype remains elusive but evidence suggests that stray DIX domains might disrupt endogenous Dsh/Dsh or Dsh/Axin interactions (Julius et al., 2000; Kishida et al., 1999). Despite these findings, it remains unclear how Dsh functions to transduce a Wnt signal to downstream effectors.
Figure 1.
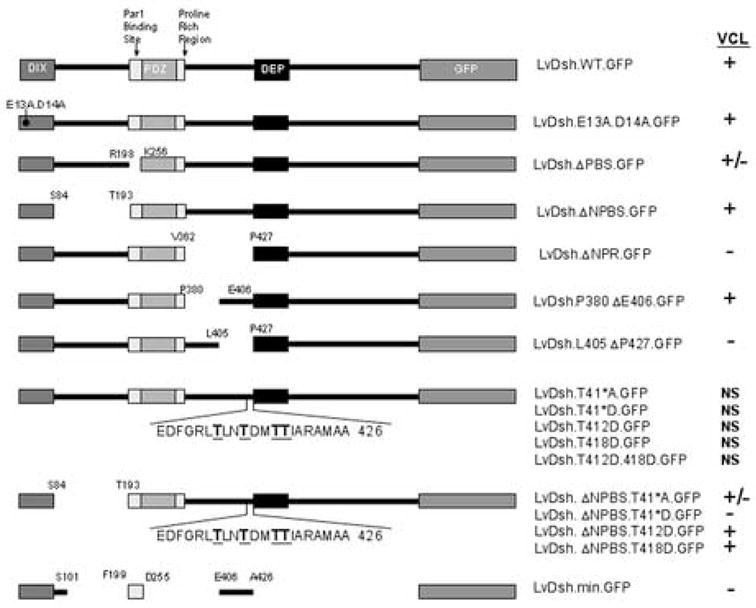
Summary of LvDsh constructs used to identify regions required for vegetal, cortical localization (VCL). VCL scoring for each construct is shown. “+” indicates that VCL was evident in most embryos oriented favorably; “+/−” indicates that some, but not all, favorably oriented embryos exhibited VCL. For the “+/−” scored embryos, the crescent of GFP tagged protein in the vegetal, cortical region was generally less pronounced than that observed following injection of mRNA encoding wild-type LvDsh.GFP. “−” indicates that none of the embryos showed VCL. “NS” indicates that the embryos were not scored due to low levels of GFP fluorescence.
With domains capable of functioning in multiple ways, Dsh subcellular localization may play a pivotal role in its ability to signal in various pathways. A hallmark of PCP pathway signaling is the recruitment of Dsh to the cell membrane (Boutros and Mlodzik, 1999; Strutt, 2003). In Xenopus embryos, Dsh has been shown to translocate to the cell membrane in response to Frizzled1, a known receptor in the PCP pathway (Axelrod et al., 1998; Rothbacher et al., 2000). Signaling through the canonical Wnt pathway has also been reported to alter Dsh localization. Exposure of mouse mesenchymal cells to a Wnt stimulus results in perinuclear accumulation of Dsh (Torres and Nelson, 2000). Most consistently, Dsh has been observed to localize to punctate cytoplasmic structures, suggested to be critical for the propagation of Wnt signals (Axelrod et al., 1998; Choi and Han, 2005; Fagotto et al., 1999; Itoh et al., 2000; Rothbacher et al., 2000; Weitzel et al., 2004). In the sea urchin, Dsh cytoplasmic puncta are restricted specifically to the vegetal cortex of fertilized eggs and early vegetal blastomeres (Weitzel et al., 2004). The nature and molecular composition of the cytoplasmic puncta remains undetermined. It has been suggested that the puncta represent the association of Dsh through its DIX domain with cytoplasmic vesicles (Cliffe et al., 2003; Miller et al., 1999; Torres and Nelson, 2000). Supporting evidence comes from the finding that conserved motifs within the DIX domain are capable of binding micelles composed of dodecylphosphocholine (DPC), a phospholipid mimic (Capelluto et al., 2002; Capelluto and Overduin, 2005). Additionally, the puncta have been described as local accumulations of Dsh in association with actin filaments or microtubules (Ciani et al., 2004; Krylova et al., 2000; Torres and Nelson, 2000). Other evidence suggests that the puncta are protein aggregates or dynamic protein assemblies of Dsh in complex with Axin (Schwarz-Romond et al., 2005; Smalley et al., 2005).
While most reports have focused on the localization of Dsh within the cytoplasm, a role for Dsh in the nucleus has been described recently (Itoh et al., 2005). Previous studies in mouse embryonic kidney cells and Xenopus animal caps revealed nuclear localization of endogenous Dsh in the presence and absence of Wnt stimulation, respectively (Cheyette et al., 2002; Torres and Nelson, 2000). Using human cell lines, Itoh and colleagues (2005) demonstrated that endogenous Dsh translocates to and accumulates in nuclei in response to a Wnt stimulus. They identified both a traditional leucine-rich nuclear export signal (NES) and a unique nuclear import signal (NLS) in Dsh proteins from various organisms. Mutation of the NLS in X. laevis Dsh prevented its nuclear accumulation and abolished its ability to induce secondary axes in frog embryos. In addition, the localization-impaired mutant failed to stabilize β-catenin, despite retaining binding capacity for such key regulators of the canonical Wnt pathway as CKI and Axin. These data suggest that the nuclear localization of pathway components, in addition to β-catenin, may be pivotal for canonical Wnt signaling.
In the present study, we examine more closely the localization of Dsh in early sea urchin embryos to gain insight into its function within the canonical Wnt pathway and in early metazoan development. We report the identification of additional motifs within LvDsh that regulate its localization to the vegetal cortex. In addition, we identify functional nuclear import and export signals within LvDsh. We confirm that overexpression of LvDsh in animal blastomeres does not alter their fate and show that this is the case even when LvDsh is induced to accumulate at high levels in the nuclei of these cells. We have also explored the mechanisms underlying the dominant negative effect produced by the overexpression of the DIX domain of LvDsh (Weitzel et al., 2004). We report that the dominant negative phenotype is dependent on sequences within the DIX domain (K57/E58) that mediate micelle binding in vitro (Capelluto et al., 2002; Capelluto and Overduin, 2005). We determine that the dominant negative phenotype is not a consequence of blocking LvDsh nuclear or vegetal cortical localization. Finally, we report that the DIX domain alone is capable of forming a stable association with LvDsh in vivo and show that the K57/E58 lipid-binding motif of the isolated DIX domain is essential for this interaction.
Materials and Methods
Animals
Adult Lytechinus variegatus were obtained from the Duke University Marine Laboratory (Beaufort, NC), Carolina Biological Supply (Burlington, NC), and Sea Life Incorporated (Tavernier, FL). Spawning was induced by intracoelomic injection of 0.5M KCl. Embryos were cultured at 18–25°C in temperature-controlled water baths.
DNA Constructs
Deletion mutations were generated in L. variegatus Dishevelled (LvDsh) using a three-way ligation strategy. For all deletion mutants, the parent construct was full length, wild-type LvDsh cloned in the BamHI restriction site of the pCS2+GFP vector (pCS2+LvDsh.WT.GFP) (Weitzel et al., 2004). To delete the desired regions, PCR mutagenesis was employed using primers flanking the sequences to be removed. The primers generated silent mutations that introduced unique restriction sites. Point mutations were generated using the Quick-Change Site-Directed Mutagenesis Kit (Stratagene). To generate the minimal targeting construct (LvDsh.min.GFP), three LvDsh regions, M1-S101 (DIX domain), F199-D255 (Par1 binding site), and E406-A426, were PCR-amplified using primers containing compatible sticky-end restriction sites. The three PCR fragments were assembled into a single construct and subcloned into the pCS2+GFP vector. To specifically target LvDsh and its variants to the nucleus, cDNAs were subcloned into the pCS2+NLS vector, which places the SV40 large T-antigen nuclear localization signal upstream of the coding sequence.
RNA Injections
For mRNA injections, cDNAs were subcloned into variants of the pCS2+ vector. Plasmids were linearized with NotI and served as templates to generate 5′ capped mRNA using the SP6 mMessage mMachine High Yield Capped RNA Transcription Kit (Ambion). The average mRNA yield was 18 mg/ml from 2 μg of DNA template. Injection solutions consisted of 20% glycerol and 2–8 mg/ml mRNA in RNAse-free, sterile water. Fertilized L. variegatus eggs were injected as described by (Cheers and Ettensohn, 2004).
Light Microscopy
Living embryos expressing GFP-tagged forms of Dsh were examined using a Bio-Rad MRC-600 confocal laser scanning microscope equipped with 20x and 40x water-immersion objectives. Z-stacks (6–20 images/stack) of 512×512 pixel images were collected with Kalman filtering and a slow scan rate. Two-dimensional projections were generated from z-stacks using NIH Image software. Additional image processing was performed using Adobe Photoshop. For observation of dominant-negative phenotypes, living embryos were immobilized on polylysine-coated coverslips and photographed using differential interference contrast optics.
Immunoblot Analysis
Late gastrula stage embryos were collected by mouth pipette and placed in a 1.5 ml centrifuge tube. Embryos were held on ice for 5 minutes and spun briefly (5–10 sec) in a clinical centrifuge. After removing as much seawater as possible, the embryos were dissolved in 30 μl of hot Laemmli sample buffer and boiled for 5 minutes. Lysed embryo samples were subjected to SDS-PAGE analysis and blotted onto Trans-Blot nitrocellulose membranes (Bio-Rad). Immunoblots were probed with a mouse monoclonal antibody that recognizes the decapeptide Myc epitope tag (Invitrogen) followed by incubation with a goat anti-mouse alkaline phosphatase conjugate (Jackson ImmunoResearch Laboratories). Blots were developed by colorimetric methods using NBT/BCIP (Roche).
Results
Vegetal cortical localization of LvDsh
Previous studies showed that expression of a GFP-tagged form of LvDsh resulted in targeting of the tagged protein to the vegetal cortex during early cleavage (Fig. 3A) (Weitzel et al., 2004). These studies showed that the proline-rich SH3-binding motif (Penton et al., 2002) and the region C-terminal to the DEP domain are not required for vegetal cortical localization (VCL). In contrast, the DIX domain, the region between the DIX and PDZ domains, and the region between the proline-rich and DEP domains are essential. To define more precisely targeting sequences required for VCL, we generated new deletions and point mutations within LvDsh (Fig. 1). All constructs were injected over a wide (>10-fold) range of concentrations, and mRNA levels had no effect on subsequent patterns of VCL. All constructs were expressed at levels sufficient to be detected by fluorescence microscopy unless otherwise noted (Fig. 1).
Figure 3.
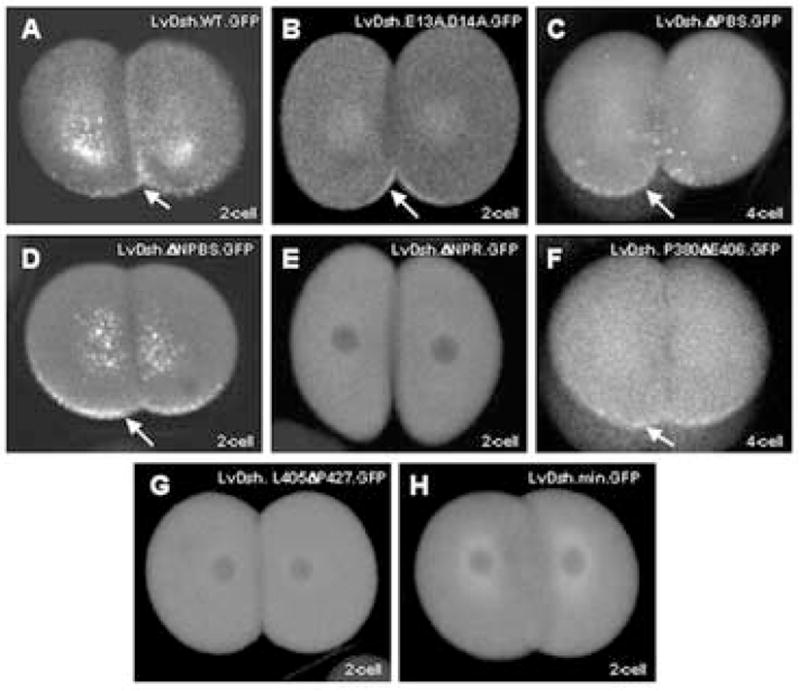
Analysis of residues in LvDsh required for VCL. (A–H) mRNAs encoding wild-type LvDsh or one of its variants were injected at a concentration of 8 mg/ml into fertilized L. variegatus eggs. Embryos were scored for GFP fluorescence during early cleavage stages. Specific embryological stages are noted. (A) Wild-type LvDsh injected embryos display typical vegetal, cortical localization (arrow). (B) Residues within the DIX domain shown to mediate lipid interaction were changed to alanine. Mutation of these residues does not prevent VCL (arrow). (C) Deletion of the Par1 binding site only partially attenuates protein targeting (arrow). (D) Deletion of the segment between the DIX domain and the Par1 binding site does not affect VCL (arrow). (E) VCL is completely abolished by deletion of the segment between the proline-rich region and the DEP domain. (F) Within this segment, deletion of the residues between P380 and E406 has no effect on VCL (arrow). (G) In contrast, deletion of the residues between L405 and P427 completely abolishes VCL. (H) A minimal construct containing the regions shown to be necessary for VCL is not sufficient to produce VCL.
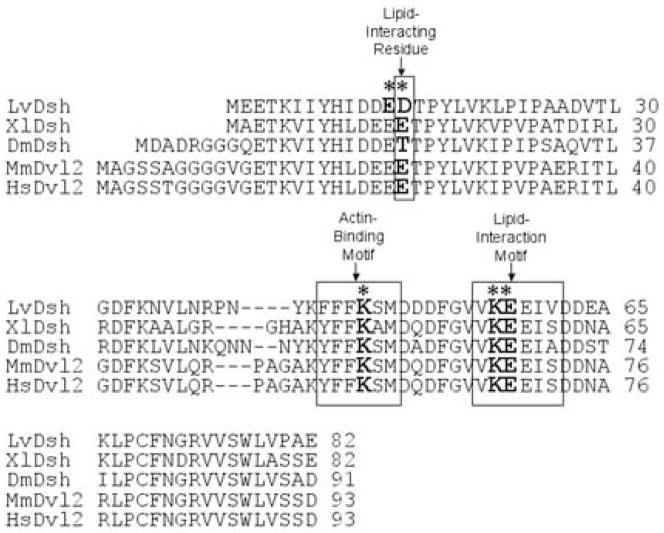
Two conserved motifs within the DIX domain have been identified that are capable of binding liposomes (Fig. 2) (Capelluto et al., 2002; Capelluto and Overduin, 2005). A double mutation (K57A/E58A) in one conserved, lipid-interaction motif was shown previously to abolish VCL (Weitzel et al., 2004). When introduced into mouse Dishevelled-2 (mDvl2), this point mutation abolishes lipid-binding activity without altering the structure of the DIX domain (Capelluto et al., 2002). A second, lipid-interacting site containing several charged residues has been identified in the DIX domain (Capelluto and Overduin, 2005). To determine whether this second site is critical for VCL, we replaced charged residues E13 and D14 with alanine (LvDsh.E13A.D14A.GFP, Fig. 1). Similar mutations have been shown to abrogate the lipid-binding properties of the DIX domain (Capelluto et al., 2002; Capelluto and Overduin, 2005). We found that these mutations had no effect on VCL, however (Fig. 3B).
Figure 2.
ClustalW alignment of the DIX domain of Dishevelled (Dsh) from L. variegatus (Weitzel et al., 2004), X. laevis (Sokol et al., 1995), D. melanogaster (Klingensmith et al., 1994), M. musculus (Sussman et al., 1994), and H. sapiens (Pizzuti et al., 1996a). Defined motifs for lipid interaction and actin binding are boxed. An asterisk indicates the specific residues within each motif targeted for mutation.
The region between the DIX and PDZ domains is also required for VCL (Weitzel et al., 2004). Further dissection of this region, however, did not reveal shorter motifs that play an essential role in LvDsh targeting. Removal of the Par1 binding site (PBS) (Sun et al., 2001) mildly attenuated VCL (LvDsh.ΔPBS.GFP, Fig. 3C). The remaining sequence between the DIX and PDZ domains, which we referred to as the non-Par1 binding site (NPBS), was completely dispensable for VCL (LvDsh.ΔNPBS.GFP, Fig. 3D). We consistently observed more robust expression of LvDsh.ΔNPBS.GFP than the wild-type protein (Figs. 3A and D). The region removed from the ΔNPBS mutant encompasses part of the binding site for inversin, a known Dsh antagonist (Simons et al., 2005). Our observations support the recent hypothesis that inversin targets cytosolic Dsh for degradation, as removal of the inversin binding region consistently resulted in higher levels of fluorescence in several different mutant backgrounds (data not shown). Taken together, our findings and those of Weitzel et al. (2004) suggest that PBS may make a modest contribution to VCL, but the major role of the region between the DIX and PDZ domains may be to serve as a spacer between other critical motifs.
Analysis of the region between the PDZ and DEP domains revealed a small (21 amino acid) motif that was essential for targeting. Our initial experiments showed that deletion of the region between the proline-rich and DEP domains, termed the non-proline-rich region (NPR), completely abolished targeting (LvDsh.ΔNPR.GFP, Fig. 3E). We divided the NPR domain into two sub-regions. Deletion of amino acids M381 to L405 had no effect on the targeting of LvDsh to the vegetal cortex (LvDsh.P380ΔE406.GFP, Fig 3F). Deletion of residues E406 to A426, however, resulted in a complete loss of VCL (LvDsh.L405ΔP427.GFP, Fig. 3G).
Four threonine residues (T412, T415, T418, and T419) are located within the 21-amino acid region E406-A426 (Fig. 1). The effects of the potential phosphorylation states of these threonine residues were investigated by introducing point mutations (Fig. 1). In order to abolish potential phosphorylation and mimic the de-phosphorylated state, all four threonine residues were replaced by alanines (LvDsh.T41*A.GFP). To mimic the phosphorylated state, all four threonines were replaced by aspartic acids (LvDsh.T41*D.GFP) (Cong et al., 2004; Huang and Erikson, 1994). In the wild-type background, both constructs were expressed at levels too low to be scored unambiguously, despite multiple trials using different preparations of mRNA and a wide range of concentrations. A double mutation of T412 and T418 (the two residues most likely to be targets of phosphorylation based on several algorithms) to alanines also resulted in levels of expression too low to be scored (data not shown).
To overcome the low levels of expression and to allow us to test the effects of various phosphorylation mutants on VCL, we transferred the alanine and aspartic acid mutants into the more robustly-expressing LvDshΔNPBS background (LvDshΔNPBS.T41*A.GFP and LvDshΔNPBS.T41*D.GFP). Levels of expression of the phosphorylation mutants were again consistently lower than the parent construct (LvDshΔNPBS.GFP) but were sufficiently high to identify embryos expressing the GFP-tagged proteins and to score VCL with confidence. Mutation of all four conserved threonine residues to alanines resulted in a partial attenuation of VCL (38% of embryos injected with LvDshΔNPBS.T41*A.GFP showed VCL, compared with 93% of sibling embryos injected with LvDshΔNPBS.GFP). Mutating either T412 or T418 individually to alanines (LvDshΔNPBS.T412A.GFP and LvDshΔNPBS.T418A.GFP) had no effect on VCL. Mutation of the four threonines to aspartic acids in the ΔNPBS background resulted in very low levels of expression, and in only one experiment (from a total of five trials) was expression sufficiently high to unambiguously score the embryos. In this experiment, the LvDshΔNPBS.T41*D.GFP mutant failed to localize to the vegetal cortex (7% of the injected embryos showed VCL). Taken together, our findings strongly suggest that the phosphorylation states of T412, T415, T418, and T419 affect both the stability and targeting of Dsh.
We allowed embryos expressing GFP-tagged, mutant forms of Dsh to develop for at least 24 hr at 23°C to assess possible effects on later development. None of the constructs had the robust dominant negative properties of the DIX domain, which when overexpressed results in a high proportion of embryos (>75%) that completely lack mesoderm and endoderm (Weitzel et al., 2004). Overexpression of LvDsh.ΔPBS.GFP or LvDsh.ΔNPBS.GFP resulted in small numbers of embryos (<10%) with mild dominant negative phenotypes, characterized by a reduction in the size of the archenteron and reduced numbers of mesenchyme cells. In later experiments (below), we found that a form of Dsh lacking only the DIX domain (LvDsh.ΔDIX.GFP) had a similar effect. It is important to note that the level of expression of different mutant forms of Dsh is likely to be variable. In addition, we have not tested untagged forms of the various mutants to determine whether these have more potent dominant effect effects than the GFP-tagged forms.
The observations reported here, in combination with those of Weitzel et al. (2004) have revealed three regions that influence the vegetal, cortical localization of LvDsh: the DIX domain, the Par1 binding site, and amino acids E406 to A426 between the proline-rich region and the DEP domain. To test whether these motifs are sufficient for VCL, we constructed a minimal form of LvDsh that consisted solely of these three defined regions (LvDsh.min.GFP, Fig 1). This mutant protein did not target to the vegetal cortex, although it was expressed robustly (Fig. 3H). Normal spatial relationships between the various domains were drastically altered in the mutant protein, however.
Nuclear import/export of LvDsh
Nuclear import and export of Dsh has been reported recently to play an important role in canonical Wnt signaling (Itoh et al., 2005). A leucine-rich nuclear export signal (NES) with the consensus sequence M/LxxLxL has been described in the carboxyl tail of Xenopus laevis Dishevelled (Itoh et al., 2005). Specific amino acid sequences that direct the transport of X. laevis Dsh to the nucleus (NLS) have also been identified and are conserved among homologs from Drosophila to humans (Itoh et al., 2005). We identified similar sequences in LvDsh (Fig 4). To determine whether the putative localization signals within LvDsh are functional, we designed a series of constructs that lacked either the NES alone or both the NES and NLS (Fig. 5A). Wild-type LvDsh showed little or no nuclear accumulation during early development (Fig. 5B). To investigate the potential function of the NES of LvDsh, we deleted the C-terminus, which harbors the NES (LvDsh.ΔC.GFP; Figs. 4 and 5A). Early cleavage stage embryos had no detectable LvDsh.ΔC within their nuclei (Fig. 5C). By the 28-cell stage, however, nuclear accumulation was visible in interphase micromere nuclei. The early accumulation of LvDsh in micromere nuclei may reflect the prolonged interphase of these cells (Dan et al., 1980; Summers et al., 1993). By the 56-cell stage, nuclear accumulation was robust throughout the embryo and persisted at least to the blastula stage, the latest stage examined by confocal microscopy (Fig. 5D). Nuclear accumulation of LvDsh.ΔC.GFP during early development had no apparent effect on later embryonic development (see also the discussion of SV40.NLS.LvDsh.GFP, below). To verify that the NLS we identified in LvDsh was responsible for driving LvDsh.ΔC into the nuclear compartment, we altered key residues within the putative nuclear localization signal in the LvDsh.ΔC background (LvDsh.NLSm.ΔC.GFP, Fig. 5A) (Itoh et al., 2005). This import-impaired mutant did not accumulate in nuclei (Fig. 5E). These observations show that the conserved NLS and NES sequences are functional in LvDsh.
Figure 4.
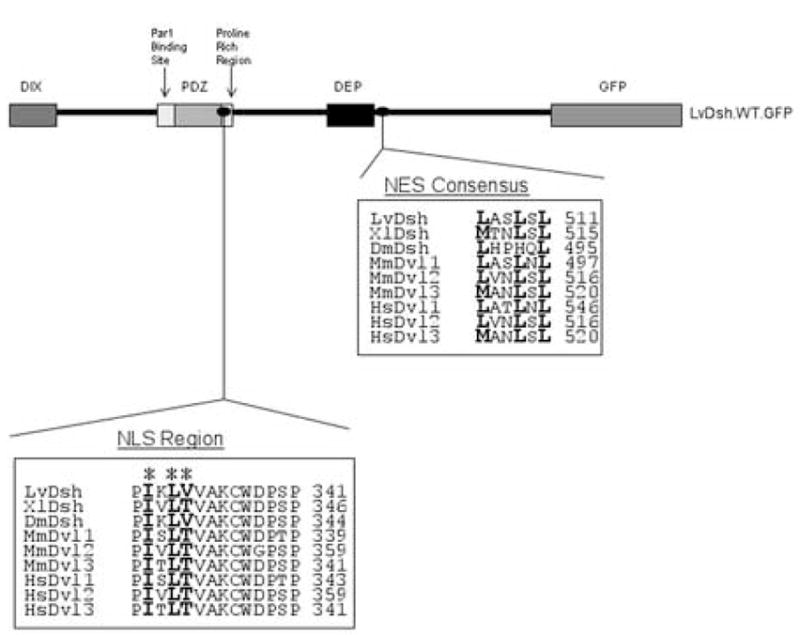
ClustalW alignment of predicted nuclear import (NLS) and export (NES) signals within Dsh proteins from L. variegatus (Weitzel et al., 2004), X. laevis (Sokol et al., 1995), D. melanogaster (Klingensmith et al., 1994), M. musculus (Klingensmith et al., 1996; Sussman et al., 1994; Tsang et al., 1996), and H. sapiens (Greco et al., 1996; Pizzuti et al., 1996a; Pizzuti et al., 1996b). Asterices indicate the NLS residues targeted for mutation.
Figure 5.
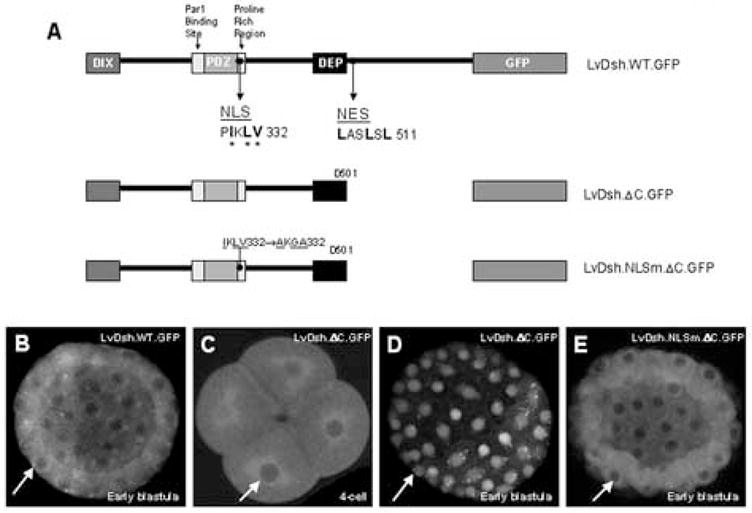
Predicated nuclear import (NLS) and export signals (NES) in LvDsh are functional. (A) LvDsh constructs used in nuclear localization studies. Asterices indicate NLS residues targeted for mutation. (B–E) mRNAs encoding wild-type LvDsh or one of its truncation mutants were injected into fertilized L. variegatus eggs. Embryos were scored for GFP fluorescence at both early and late stages. (B) Wild type LvDsh shows diffuse cytoplasmic staining at all stages. (C) Embryos injected with LvDsh.ΔC also show diffuse cytoplasmic staining up through the 16-cell stage. (D) Robust nuclear accumulation of LvDsh.ΔC is observed beginning at the 56-cell stage and persists through blastula stage. (E) Nuclear accumulation is driven by the nuclear localization signal within LvDsh. Mutation of the NLS in the LvDsh.ΔC background completely abolishes nuclear accumulation of the protein.
LvDsh associates with actin and liposomes through conserved motifs in the DIX domain (Capelluto et al., 2002; Capelluto and Overduin, 2005) (Fig. 2). To determine whether actin or lipid binding is required for the translocation of LvDsh to the nucleus, we generated mutations in the actin-binding motif (K47A) and individually mutated the two lipid-binding motifs (K57A/E58A and E13A/D14A) of LvDsh.ΔC (Fig. 6A). Mutation of the actin-binding site or the original lipid-binding motif (K57A/E58A) did not alter nuclear accumulation (Fig. 6B and C). Alteration of the charged, lipid-associating residues (E13A/D14A), however, resulted in accumulation of fluorescence in distinct cytoplasmic puncta (Fig. 6D). In a variety of cell types, Dsh has been observed in cytoplasmic puncta that may represent vesicles or large protein assemblies (Miller et al., 1999; Schwarz-Romond et al., 2005; Smalley et al., 2005; Torres and Nelson, 2000). Further investigation is needed to ascertain the structure of the puncta generated by the mutation of the charged, lipid-associating residues of LvDsh.
Figure 6.
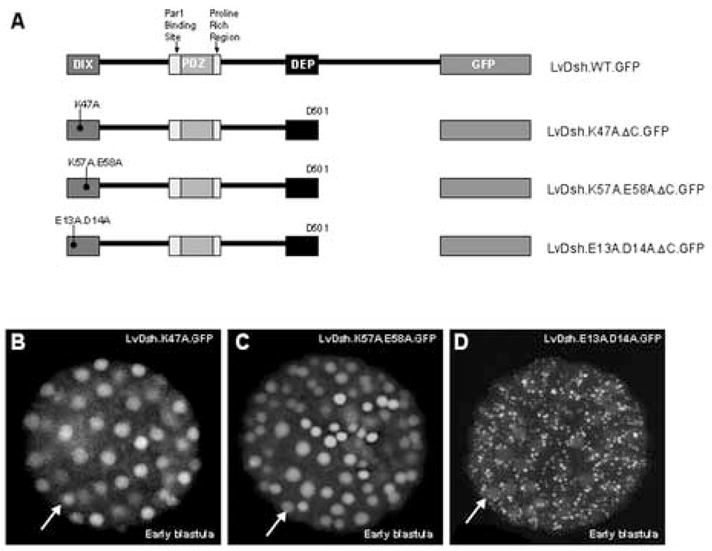
Lipid interaction at charged residues E13 and D14 may be important for nuclear localization of LvDsh. (A) Graphic representation of LvDsh constructs used in determining amino acid residue requirements for nuclear localization. (B–D) mRNAs encoding point mutants of LvDsh truncates were injected at a concentration of 8 mg/ml into fertilized L. variegatus eggs. All embryos were scored for GFP fluorescence at the blastula stage. (B) The DIX domain contains a well-defined actin-binding motif. The most highly conserved residue of the motif, K47, was changed to alanine. Mutation of this residue does not alter nuclear accumulation of LvDsh.ΔC (arrow). (C) In addition to the actin-binding motif, the DIX domain contains two regions shown to be involved in lipid association. One region is a conserved motif across species. Residues K57 and E58 within this motif were changed to alanine. Mutation of these residues has no effect on the nuclear accumulation of LvDsh.ΔC (arrow). (D) The second lipid association region consists of charged residues. Amino acids E13 and D14 of this region were changed to alanine. Mutation of these residues results in little nuclear accumulation (arrow). However, bright cytoplasmic puncta are observed.
Overexpression of wild-type LvDsh (GFP-tagged or untagged) had no effect on the phenotype of the developing embryo (Fig. 7D). We hypothesized that a missing regulator in the animal cells of the embryo might be preventing transduction of a signal. Because nuclear import of Dsh is required for its function in the canonical pathway (Itoh et al., 2005), we reasoned that the absence of a regulatory factor might be circumvented by forcing LvDsh into the nucleus. To test this hypothesis, we placed the SV40 large T- antigen nuclear localization signal upstream of the wild-type LvDsh coding sequence (SV40.NLS.LvDsh.GFP, Fig 7A). Overexpression of nuclear-targeted LvDsh resulted in a striking accumulation in nuclei, where the protein was concentrated in multiple puncta (Fig. 7B). The localization of LvDsh to the nuclear compartment had no visible effect on the development of the embryo, however, over a wide range of mRNA concentrations (Fig. 7C). These findings argue that Dsh is not functional in animal blastomeres even when targeted specifically to the nucleus and suggest that other aspects of Dsh function are compromised in animal cells.
Figure 7.
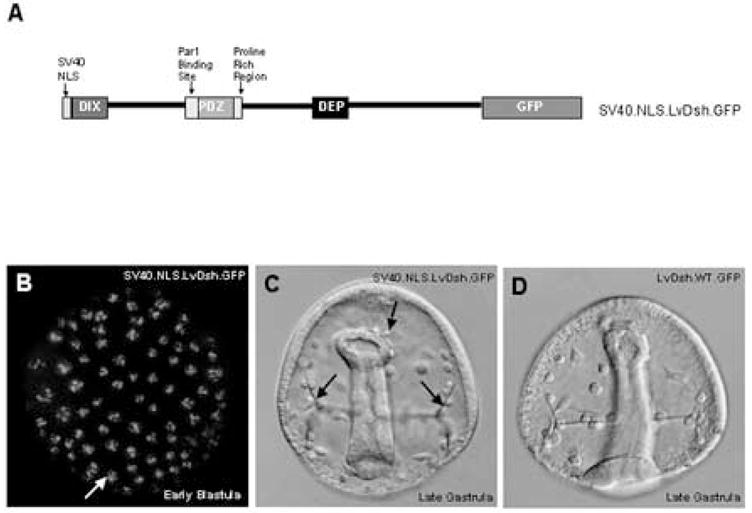
Targeted nuclear localization of LvDsh does not alter cell fates. (A) Schematic of the LvDsh construct used to target LvDsh to the nucleus. (B–D) mRNA encoding nuclear-targeted LvDsh was injected at a concentration of 8 mg/ml into fertilized L. variegatus eggs. (B) Fluorescent image of nuclear-targeted LvDsh.GFP in an early blastula stage embryo. SV40 NLS LvDsh.GFP localizes to the nucleus. Embryos are normal and display no aberrant phenotype. The nuclear-targeted LvDsh.GFP accumulates in distinct puncta within the nuclei. (C) Brightfield image of late gastrula stage embryo expressing nuclear-targeted LvDsh.GFP (SV40.NLS.LvDsh.GFP). Secondary mesenchyme cells are budding from the tip of the developing archenteron (upper arrow). Primary mesenchyme cell (PMC) cynicism and normal triradiate spicules (lower arrows) are visible. Gross morphology is intact. (D) Brightfield image of late gastrula stage embryo expressing wild-type LvDsh.GFP. The archenteron, PMC cynicism and triradiate spicules are visible.
The dominant negative effect of the DIX domain
The DIX (Dishevelled and Axin proteins) domain of Dsh is required for canonical β-catenin signaling and is believed to mediate Dsh multimerization and/or interaction with Axin (Fagotto et al., 1999; Itoh et al., 2000; Kishida et al., 1999; Penton et al., 2002; Rothbacher et al., 2000). In Drosophila, overexpression of the DIX domain alone phenocopies the Dsh genetic null mutation, supporting the view that this fragment of the protein acts as a dominant negative (Axelrod et al., 1998). In the sea urchin, overexpression of the DIX domain produces the same animalized phenotype as overexpression of cadherin, GSK3β, or a dominant negative form of TCF/LEF (Emily-Fenouil et al., 1998; Logan et al., 1999; Vonica et al., 2000; Wikramanayake et al., 1998) and blocks vegetal accumulation of β-catenin (Weitzel et al., 2004). The specific molecular mechanisms underlying the dominant negative effect remain elusive, however.
We sought to identify elements within the DIX domain that are required for the dominant negative effect (Fig. 8A). Injection of the original dominant negative construct, LvDsh.DIX ([mRNA] = 8 mg/ml), resulted in the anticipated animalized phenotype in >90% of the injected embryos (n = 200) (Fig. 8B). Similarly, DIX constructs harboring mutations in either the actin-binding domain (K47) or the charged, lipid-interacting motif (E13/D14) ([mRNA] = 8 mg/ml) had potent, dominant negative effects (>90% of injected embryos, n = 200) (Figs. 8C–D). When residues K57/E58 of DIX were mutated to alanines, however, the dominant negative phenotype was completely abolished ([mRNA] = 8 mg/ml, <2% of embryos with phenotype, n = 200). Western blot analysis demonstrated that the K57/E58 mutant was expressed at least as robustly as the other constructs (Fig. 10). These data demonstrate that the dominant negative phenotype generated by LvDsh.DIX is dependent on the lipid-interacting motif that contains residues K57 and E58 (Capelluto et al., 2002).
Figure 8.
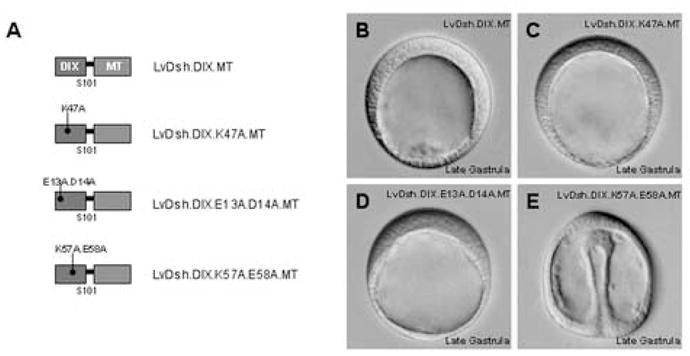
A conserved lipid interaction motif is necessary for the dominant negative function of LvDsh.DIX. (A) Graphic representation of LvDsh.DIX constructs used to determine the amino acids required for dominant negative function. (B–E) mRNA encoding Myc-tagged LvDsh.DIX or one of its point mutants was injected at a concentration of 8 mg/ml into fertilized L. variegatus eggs. Injected embryos were allowed to develop to late gastrula stage and scored for presence of the dominant negative phenotype. Bright field images were taken of representative embryos. (B) Injection of native LvDsh.DIX mRNA results in the previously described dominant negative phenotype characterized by the suppression of endoderm and mesoderm formation (Weitzel et al., 2004). This phenotype is indistinguishable from that produced by overexpression of cadherins or GSK3β (Emily-Fenouil et al., 1998; Logan et al., 1999; Wikramanayake et al., 1998). (C–D) Overexpression of LvDsh.DIX harboring alanine mutations within either the actin-binding motif (K47A) or charged residue, lipid association region (E13A/D14A) does not alter the dominant negative phenotype. (E) Overexpression of LvDsh.DIX containing alanine mutations within the conserved lipid-interaction motif (K57A/E58A) produces a normal embryo with no apparent dominant negative phenotype.
Figure 10.
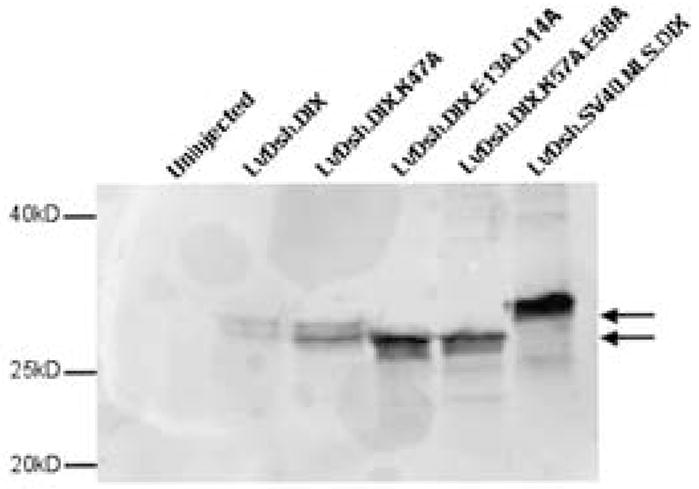
The dominant negative construct, DIX, and its mutants are expressed. mRNA encoding DIX or one of its mutants tagged with a Myc epitope was injected at a concentration of 8 mg/ml. Forty late gastrula stage embryos from each injection were harvested, lysed in Laemmli sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose. All proteins were detecting using a primary antibody against the Myc epitope tag. Forty, gastrula stage, uninjected embryos are shown as a control. Arrows indicate the doublet bands of LvDsh.DIX and LvDsh.DIX.K47A. Molecular weight standards are shown.
Myc-tagged forms of the original dominant negative protein, LvDsh.DIX, and the actin-binding mutant protein, LvDsh.DIX.K47A, appeared as doublets on immunoblots (Fig. 10, lanes 2 and 3). In contrast, the two forms of Dsh with mutations in lipid-interaction motifs (LvDsh.DIX.K57A.E58A and LvDsh.DIX.E13A.D14A) each appeared as a single, fast-migrating polypeptide (Fig. 10, lanes 4 and 5). No bands were seen in control homogenates prepared from uninjected embryos (Fig. 10, lane 1). Based on similar observations by Capelluto and colleagues (2002) with full-length mDvl2, we hypothesize that the higher molecular weight species might represent a phosphorylation variant of the DIX domain. Further investigation is needed, however, to determine the difference between the lower molecular weight species and the higher one.
The DIX domain lacks nuclear localization signals (Fig. 4), and LvDsh.DIX.GFP is expressed uniformly throughout the cytoplasm of early blastomeres (Weitzel et al., 2004). To determine whether the DIX domain can still exert a dominant negative phenotype when targeted to the nucleus, we fused the SV40 large T-antigen nuclear localization signal upstream of the DIX domain coding sequence (Figs. 9A and C). The nuclear-targeted DIX domain was expressed robustly and resulted in a strong, dominant negative phenotype indistinguishable from LvDsh.DIX ([mRNA] = 8 mg/ml, >90% of embryos with phenotype, n = 200) (Figs. 9B and 10, lane 6).
Figure 9.
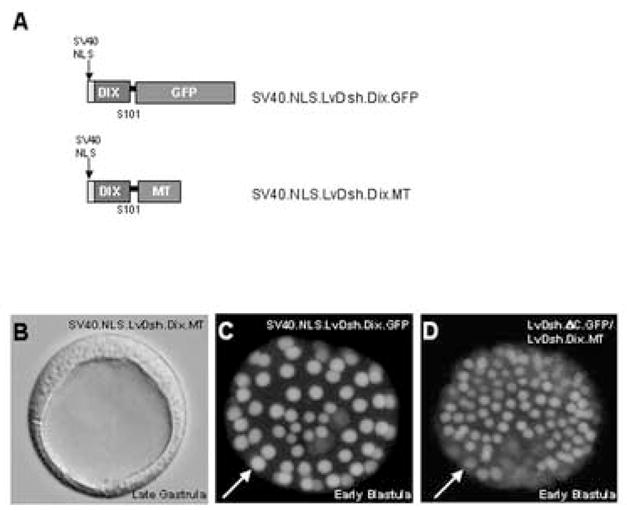
The dominant negative phenotype produced by the DIX domain of LvDsh is not caused by the specific localization of LvDsh.DIX or inhibition of the nuclear accumulation of LvDsh. (A) Summary of constructs used to target LvDsh.DIX to the nucleus. (B) Brightfield image of a representative embryo overexpressing a nuclear-targeted form of LvDsh.DIX. Nuclearizing LvDsh.DIX has no effect on its ability to generate a dominant negative phenotype. (C) Confocal image of GFP fluorescence confirms the presence of the protein within the nuclei of cells of a blastula stage embryo. (D) Confocal image of GFP fluorescence confirms the presence of LvDsh.ΔC within the nuclei of cells of a blastula stage embryo. This embryo, co-injected with LvDsh.DIX, develops a typical dominant negative phenotype.
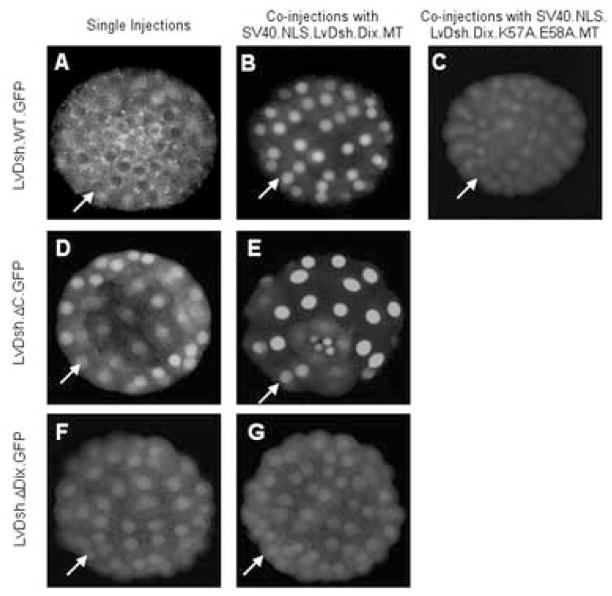
We hypothesized that the DIX domain might exert its dominant negative effect by interfering with nuclear import of endogenous LvDsh. To test this hypothesis, we co-injected LvDsh.DIX.MT in combination with LvDsh.ΔC.GFP. We confirmed that >90% of the co-injected embryos exhibited a dominant negative phenotype at the late gastrula stage, demonstrating that LvDsh.DIX.MT was expressed at sufficiently high levels (data not shown). In these embryos, LvDsh.ΔC.GFP accumulated in the nucleus at levels similar to those seen when this construct was injected alone (Fig. 9D). We also noted that co-injection did not prevent LvDsh.ΔC.GFP from localizing to the vegetal cortex at early cleavage stages, confirming the findings of Weitzel et al. (2004). These findings argue strongly against the possibility that DIX exerts its dominant negative effects by blocking accumulation of LvDsh in the nucleus or by preventing VCL.
In the course of these studies on the potential effects of DIX on the nuclear localization of Dsh, we co-injected nuclear-targeted DIX (SV40.NLS.LvDsh.DIX.MT) and LvDsh.ΔC.GFP. This dominant negative form of DIX also had no effect on the vegetal, cortical localization of LvDsh.ΔC.GFP, consistent with the findings reported above (data not shown). Unexpectedly, beginning at the 16-cell stage and persisting through the blastula stage, the normal nuclear accumulation of LvDsh.ΔC.GFP (Fig. 11D) was greatly enhanced and much more tightly restricted to nuclei (Fig. 11E), indistinguishable from the pattern of localization of nuclear-targeted DIX (SV40.NLS.LvDsh.DIX.GFP) when expressed alone (Fig. 9C). We also found that nuclear-targeted DIX dramatically altered the subcellular distribution of full-length, wild-type LvDsh. Expression of LvDsh.GFP alone resulted in punctate, cytoplasmic localization of the protein with little or no expression in the nucleus (Fig. 11A), while co-injection with SV40.NLS.LvDsh.DIX.MT resulted in striking nuclear accumulation of LvDsh.GFP (Fig. 11B).
Figure 11.
LvDsh.DIX interacts via residues K57 and E58 with the DIX domain of full length LvDsh. LvDsh.GFP constructs were injected alone (A, D, and F) or co-injected with either nuclear-targeted LvDsh.DIX (B, E, and G) or nuclear-targeted mutant (K57A/E58A) LvDsh.DIX (C). Confocal images of representative embryos were taken at the blastula stage. Arrows indicate nuclei. When injected alone, wild-type LvDsh.GFP shows diffuse cytoplasmic staining with no nuclear accumulation (A). Co-injection with nuclear-targeted LvDsh.DIX results in a marked decline in cytoplasmic staining and a corresponding increase in nuclear LvDsh.GFP (B). The previously observed nuclear accumulation of LvDsh.ΔC.GFP is replicated when injected alone (D). Co-injection of LvDsh.ΔC.GFP with nuclear-targeted LvDsh.DIX greatly enhances the nuclear accumulation of the protein (E). The interaction between DIX and LvDsh is mediated by the DIX domain of the full-length protein, because LvDsh lacking the DIX domain (LvDsh.ΔDIX.GFP) shows minimal nuclear fluorescence in the absence (F) or presence (G) of nuclear-targeted LvDsh.DIX. Conserved, lipid-associating residues K57 and E58 within the DIX domain are required for its interaction with full length LvDsh.GFP (C).
We hypothesized that nuclear-targeted DIX might alter the localization of both LvDsh.ΔC.GFP and LvDsh.GFP by associating directly or indirectly with these proteins and concentrating them in the nucleus. In vitro, isolated DIX domains can bind directly to the DIX domain of full-length Dsh (Kishida et al., 1999). We therefore asked whether such an interaction might be required for the redistribution of LvDsh.GFP by nuclear-targeted DIX. We injected a LvDsh construct lacking the DIX domain (LvDsh.ΔDIX.GFP) alone and in combination with nuclear-targeted DIX (SV40.NLS.LvDsh.DIX.MT). Overexpression of the ΔDIX construct alone resulted in low levels of both cytoplasmic and nuclear staining (Fig. 11F). When co-injected with SV40.NLS.LvDsh.DIX.MT, the expression and localization of LvDsh.ΔDIX.GFP remained completely unchanged (Fig. 11G). This observation shows that redistribution of LvDsh to the nucleus by SV40.NLS.DIX is dependent on the DIX domain of LvDsh.
Because a double mutation (K57A/E58A) in a lipid-binding motif of DIX blocked the dominant negative effect of this protein, we asked whether the same mutation might also inhibit the ability of DIX to interact with full-length Dsh. We therefore co-injected a nuclear-targeted form of the mutant DIX domain (SV40.NLS.LvDsh.DIX.K57A.E58A.MT) and full-length, GFP-tagged Dsh (LvDsh.GFP). The K57A/E58A mutation dramatically reduced the nuclear accumulation of LvDsh-GFP, although it did not eliminate it completely (Fig. 11C). Control experiments with a GFP-tagged form of the same nuclear-targeted, DIX mutant (SV40.NLS.LvDsh.DIX.K57A.E58A.GFP) confirmed that this form of the DIX domain was efficiently targeted to nuclei by the SV40 NLS, to an extent indistinguishable from wild-type DIX (Fig. 9C and data not shown). These findings demonstrate that the K57/E58 lipid-binding motif of DIX is critical in mediating the interaction between DIX and full-length LvDsh in vivo.
Discussion
Dsh, Wnts, and early axis specification in metazoan embryos
Components of the canonical Wnt signaling pathway play a critical role in early axis specification in many metazoans (Ettensohn, 2006; Heasman et al., 1994; Imai et al., 2000; Kelly et al., 2000; Nasevicius et al., 1998; Pelegri and Maischein, 1998; Wikramanayake et al., 2003; Zeng et al., 1997). In Xenopus, the role of Wnt signaling in axis formation has been studied intensively, and a maternal Wnt (Wnt11) has been shown to be required for activation of the canonical pathway in dorsal blastomeres (Tao et al., 2005). The role of Dsh in axis specification in Xenopus remains controversial. Miller et al. (1999) found that Dsh is localized to vesicle-like organelles in the vegetal cortex of unfertilized eggs and showed that the DIX domain is required for Dsh to associate with these structures. During cortical rotation, the Dsh-containing organelles translocate to the dorsal side of the egg, where they may function to stabilize β-catenin. Despite these intriguing findings, questions remain concerning the significance of the polarized distribution of Dsh and the function of Dsh in endogenous axis formation in Xenopus. Dominant negative forms of Dsh do not suppress the formation of the endogenous dorsal-ventral axis (Sokol, 1996). It is possible, however, that dominant negative constructs are not expressed at high enough levels in the early embryo to be effective, or that maternal Dsh is already tightly associated with other proteins in the unfertilized Xenopus egg and therefore refractory to a dominant negative approach.
In the sea urchin embryo, Dsh plays an essential role in axis formation (Weitzel et al., 2004). Overexpression of the DIX domain, a dominant negative form of Dsh that interferes with canonical signaling (see below), prevents the accumulation of β-catenin in the nuclei of vegetal blastomeres and suppresses endoderm and mesoderm formation. The functional significance of the polarized distribution of Dsh in sea urchins, however, as in Xenopus, remains unclear. The inability of ectopically expressed Dsh to alter the fates of animal blastomeres suggests that an essential positive regulator of Dsh is missing or inactive in these cells (alternatively, a negative regulator may be concentrated or activated in animal cells). Because recent findings have identified a role for Dsh in the nucleus (see below), we hypothesized that a critical regulator of nuclear import might be missing in animal cells and that we might circumvent the need for such a regulator by driving high levels of Dsh into the nuclei of animal cells. Nuclear targeting of Dsh using the SV40 NLS did not perturb embryo patterning, however, strongly suggesting that a feature of Dsh function other than nuclear transport is compromised in animal cells. In addition, our observation that LvDsh.ΔC.GFP, which lacks nuclear export sequences, accumulated both in animal and vegetal nuclei, suggests that all factors required for nuclear import are present in animal cells.
One hypothesis is that in sea urchins, as in Xenopus, a localized Wnt signal activates Dsh only on one side of the early embryo. Although mRNAs encoding several Wnt proteins have been identified in the unfertilized sea urchin egg, none are localized preferentially in the vegetal region (D. McClay, personal communication). Sea urchins lack a homologue of Wnt-11 (Weinstock et al., 2006), and over-expression of Xenopus Wnt-11 in sea urchins does not result in axial defects (Leonard and Ettensohn, unpublished observations). Thus, it is too early to know whether a localized Wnt signal is present in the early sea urchin embryo. Further work will be needed to identify the upstream regulators of Dsh in sea urchins and other metazoans. These studies will reveal the extent to which mechanisms that control β-catenin nuclearization in early embryogenesis have been conserved during animal evolution.
Intracellular targeting of LvDsh
In the present study, we sought to more precisely define domains within Dsh that are required for the targeting of this protein to the vegetal cortex. Previous work showed that VCL is dependent on a conserved lipid-binding motif in the DIX domain (Capelluto et al., 2002; Weitzel et al., 2004). In this study, we identified two additional regions that play a role in VCL. Deletion of the Par1 binding/phosphorylation site (F199-D255) (Sun et al., 2001) partially attenuated VCL. Par-1 is capable of inducing β-catenin-mediated transcription coincident with Dsh phosphorylation (Ossipova et al., 2005; Sun et al., 2001). Although Par-1 can phosphorylate Dsh, it is unknown whether the transcriptional activity induced by Par-1 is mediated directly or indirectly through Dsh. Par-1 phosphorylation of Dsh has been shown to have a direct impact on its localization in Xenopus animal caps. Ossipova and colleagues have shown that Xenopus Dsh is recruited from punctate structures in the cytoplasm to the cell cortex in response to Par-1 (Ossipova et al., 2005). We observed that removal of the Par-1 responsive element from Dsh reduced its ability to accumulate in the vegetal cortex of the embryo, supporting the notion that Par-1 may play a limited role in Dsh localization in the early embryo. We were not able to identify deletions within the DIX-PDZ intervening region, however (including the deletion of the Par-1 responsive element), that had as dramatic an effect on VCL as elimination of this entire domain. One possible explanation for this finding is that the region has an important spacer function and serves to maintain proper spatial relationships between other motifs.
We have also identified a short (21-amino acid) motif located N-terminal to the DEP domain (E406-A426) that is essential for VCL. Although this sequence does not lie within a well-defined domain, it corresponds to a region of mouse Dishevelled-1 (mDvl1) that can be phosphorylated by casein kinase Iε (CKIε) (Cong et al., 2004). When aspartic acid residues that have been shown to mimic constitutive phosphorylation are substituted for the serine/threonine residues in this region, the ability of mDvl1 to mediate β-catenin signaling increases. Aspartic acid-substituted mDvl1 exhibits a diffuse distribution in the cytoplasm rather than the punctate pattern typical of wild-type mDvl1. Conversely, a mutant form of mDvl1 in which the serine/threonine residues are replaced by the neutral amino acid alanine localizes in a pattern similar to the wild-type protein (Cong et al., 2004).
The E406-A426 region of LvDsh contains four threonine residues. Mutational analysis of these residues points to their role in regulating both the subcellular localization of Dsh and the stability of the protein. Manipulation of the four residues, either by transformation to aspartic acids or alanines, compromised the ability of GFP-tagged Dsh to target to the vegetal cortex. In a variety of contexts, mutations in these residues also significantly reduced levels of protein expression based on GFP fluorescence. This effect is unlikely to be an indirect consequence of the inability of these mutant proteins to target properly, as several other constructs that fail to show VCL (e.g., LvDsh.K57A.E58A.GFP and LvDsh.L405ΔP427.GFP) are expressed very robustly. We have not tested all possible combinations of phosphorylation states of the four threonine residues in the E406-A426 region, and additional mutational analysis may clarify the connection between specific phosphorylated residues in this region, VCL, and Dsh stability.
In the vegetal cortex, LvDsh is concentrated in puncta (Weitzel et al. 2004). The function of Dsh-containing puncta is unknown, but several lines of evidence suggest they play a physiological role in the development of the early embryo. Using antibodies against endogenous Dsh protein, puncta have been observed in developing embryos from C. elegans to mouse (Chang et al., 2005; Miller et al., 1999; Torres and Nelson, 2000; Yanagawa et al., 1995). The ability of Dsh to form puncta correlates with its phosphorylation state and with activation of the canonical Wnt pathway (Capelluto et al., 2002). Overexpression of Frizzled-1 is associated with the recruitment of Dsh from cytoplasmic punctate structures to the plasma membrane (Choi and Han, 2005; Yang-Snyder et al., 1996).
The molecular composition of Dsh-containing puncta may vary in different cell types and under different physiological conditions. It has been proposed that the puncta represent an association between Dsh and cytoplasmic vesicles. Evidence that sequences within the DIX domain are capable of binding dodecylphosphocholine (DPC) micelles supports this hypothesis (Capelluto et al., 2002). Recent data show, however, that Dsh puncta do not co-localize with known vesicle, membrane, or endocytic markers (Schwarz-Romond et al., 2005; Smalley et al., 2005). Dsh puncta have also been found in association with the actin cytoskeletal network (Torres and Nelson, 2000). In addition, local accumulations of Dsh puncta have been observed in association with microtubules and may play a role in their stability (Ciani et al., 2004; Krylova et al., 2000). It has been proposed that punctate accumulations of DIX domain-containing proteins such as Dsh and Axin are large protein aggregates in dynamic equilibrium with cytosolic protein pools (Schwarz-Romond et al., 2005). Further studies concerning the nature of the cytoplasmic puncta are needed and should provide insight into their function in early embryonic development.
Recent findings have revealed an unexpected role for Dsh in the nucleus. Itoh and colleagues have shown that Drosophila Dsh (DmDsh) shuttles between the nuclear and cytoplasmic compartments in early Xenopus embryos (Itoh et al., 2005). A form of DmDsh impaired in its ability to localize to the nucleus fails to induce secondary axis formation in Xenopus and cannot stimulate canonical Wnt signaling. In the present study, we identified similar, functional nuclear import and export signals within LvDsh. We determined that nuclear import of LvDsh was not affected by mutation of highly conserved actin (K47) or liposome-binding (K57/E58) residues (Figs. 6B and C). Mutation of charged, lipid-association residues (E13/D14) in the background of the nuclear export-compromised form of Dsh (LvDsh.ΔC.GFP) resulted in accumulation of LvDsh in cytoplasmic puncta unlike those seen with the wild-type protein, a phenomenon that we have not explored further. Because ectopic expression of Dsh in the sea urchin, unlike Xenopus, does not alter cell fates, other assays will be required to test whether nuclear shuttling of Dsh has an essential function in this organism.
The dominant negative effect of the DIX domain
When overexpressed, the isolated DIX domain of Dsh has a potent dominant negative effect (Axelrod et al., 1998; Weitzel et al., 2004). In the context of the intact Dsh molecule, the DIX domain is required for canonical β-catenin signaling (Itoh et al., 2000; Penton et al., 2002; Rothbacher et al., 2000). In the present study, we explored the mechanisms underlying the dominant negative effect of DIX overexpression. We found that over-expression of the DIX domain of LvDsh inhibited neither VCL nor the nuclear accumulation of LvDsh, ruling out the possibility that the dominant negative effects of DIX are mediated by either of these two mechanisms. Targeting the DIX domain to the nuclear compartment did not impair its ability to act as a dominant negative, although under these conditions the protein was still present transiently in the cytoplasm and may have interacted with partners en route to the nucleus.
We have shown that the dominant negative effect of the DIX domain is dependent on the micelle-binding motif that includes residues K57 and E58. Surprisingly, a double mutation in a second, putative, lipid-binding motif (E13A/D14A) did not affect the dominant negative function of the DIX domain. We note, however, that this double mutation did not affect the ability of full-length Dsh to target to the vegetal cortex, suggesting that its effects are distinct from those of the K57A/E58A mutation. It is important to note that the lipid-binding functions of these motifs have studied in vitro and it remains possible that the sequences mediate other kinds of molecular interactions in living cells.
Our studies show that the isolated DIX domain interacts with full-length Dsh in vivo. This finding is consistent with reports that Dsh forms multimers and that various C-terminally-truncated forms of Dsh associate with one another in living cells (Kishida et al., 1999; Rothbacher et al., 2000). A direct interaction between the isolated DIX domain and Dsh has been demonstrated in vitro (Kishida et al., 1999). The DIX domain of full-length Dsh is required for this interaction in vitro, as we have observed in living cells. In vivo, Dsh molecules are present in larger complexes that contain additional proteins, and there are likely to be other, indirect interactions among Dsh molecules mediated by regions other than the DIX domain (Kishida et al., 1999: Rothbacher et al., 2000). We report for the first time that the K57 and E58 residues of the isolated DIX domain are essential for the interaction with Dsh. These residues may have a direct function in mediating homotypic binding between DIX domains, independent of their putative lipid-binding properties. An intriguing, alternative possibility is that the binding between DIX and Dsh, and perhaps Dsh oligomerization in normal cells, is mediated by interactions with lipids.
Based on the findings reported here and by others, we propose that the isolated DIX domain acts as a dominant negative by binding to the DIX domain of full-length Dsh. Axin is another DIX domain-containing protein in the β-catenin degradation complex, but biochemical studies have shown that the isolated DIX domain of Dsh does not bind to full-length Axin (Kishida et al., 1999). Dsh and Axin are present in a complex in vivo and direct binding between the purified molecules has been demonstrated, although reports differ concerning the domains within Axin and Dsh that mediate this interaction (Cliffe et al., 2003; Fagotto et al., 1999; Itoh et al., 2000; Julius et al., 2000; Kishida et al., 1999; Li et al., 1999; Salic et al., 2000; Smalley et al., 1999). The interaction between Dsh and Axin is essential for canonical signaling, possibly by recruiting GBP/FRAT1 to the degradation complex (Salic et al., 2000; Yost et al., 1998). We therefore propose that binding of DIX to the N-terminal DIX domain of Dsh interferes with the ability of the full-length Dsh molecule to associate with Axin or otherwise prevents Dsh from recruiting accessory proteins such as GBP/FRAT1 to the β-catenin degradation complex. Further analysis of the mechanisms underlying the dominant negative effect of the DIX domain may provide important insights concerning the normal mechanisms of Wnt signaling.
Acknowledgments
The authors thank Kathryn R. Caperna, Michele R. Illies and Heather E. Weitzel for their contributions to the construction of LvDsh mutant clones and Dr. David L. Turner (University of Michigan) for generous providing pCS2+ vectors. This work was supported by NSF Grant IBN-0128140. and NIH Grant HD042251.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer LM, Angerer RC. Animal-vegetal axis patterning mechanisms in the early sea urchin embryo. Dev Biol. 2000;218:1–12. doi: 10.1006/dbio.1999.9553. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–22. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–18. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–9. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- Capelluto DG, Overduin M. Secondary structure, 1H, 13C and 15N resonance assignments and molecular interactions of the dishevelled DIX domain. J Biochem Mol Biol. 2005;38:243–7. doi: 10.5483/bmbrep.2005.38.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Lloyd CE, Zarkower D. DSH-2 regulates asymmetric cell division in the early C. elegans somatic gonad. Mech Dev. 2005;122:781–9. doi: 10.1016/j.mod.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Cheers MS, Ettensohn CA. Rapid microinjection of fertilized eggs. Methods Cell Biol. 2004;74:287–310. doi: 10.1016/s0091-679x(04)74013-3. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–61. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK. Rap2 is required for Wnt/beta-catenin signaling pathway in Xenopus early development. Embo J. 2005;24:985–96. doi: 10.1038/sj.emboj.7600571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol. 2004;164:243–53. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–6. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24:2000–11. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan K, Tanaka S, Yamazaki K, Kato Y. Cell cycle study up to time of hatching. Dev Growth Differ. 1980;22:589–598. doi: 10.1111/j.1440-169X.1980.00589.x. [DOI] [PubMed] [Google Scholar]
- Davidson EH. Lineage-specific gene expression and the regulative capacities of the sea urchin embryo: a proposed mechanism. Development. 1989;105:421–45. doi: 10.1242/dev.105.3.421. [DOI] [PubMed] [Google Scholar]
- Dickinson ME, McMahon AP. The role of Wnt genes in vertebrate development. Curr Opin Genet Dev. 1992;2:562–6. doi: 10.1016/s0959-437x(05)80172-8. [DOI] [PubMed] [Google Scholar]
- Emily-Fenouil F, Ghiglione C, Lhomond G, Lepage T, Gache C. GSK3beta/shaggy mediates patterning along the animal-vegetal axis of the sea urchin embryo. Development. 1998;125:2489–98. doi: 10.1242/dev.125.13.2489. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA. The emergence of pattern in embryogenesis: Regulation of beta-catenin localization during early sea urchin development. Sci STKE. 2006;(361):pe48. doi: 10.1126/stke.3612006pe48. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–56. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey DM, Kimelman D. Glycogen Synthase Kinase-3beta Mutagenesis Identifies a Common Binding Domain for GBP and Axin. J Biol Chem. 2002;277:16147–16152. doi: 10.1074/jbc.M112363200. [DOI] [PubMed] [Google Scholar]
- Gloy J, Hikasa H, Sokol SY. Frodo interacts with Dishevelled to transduce Wnt signals. Nat Cell Biol. 2002;4:351–7. doi: 10.1038/ncb784. [DOI] [PubMed] [Google Scholar]
- Greco TL, Sussman DJ, Camper SA. Dishevelled-2 maps to human chromosome 17 and distal to Wnt3a and vestigial tail (vt) on mouse chromosome 11. Mamm Genome. 1996;7:475–6. doi: 10.1007/s003359900144. [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM. A mode of regulation of beta-catenin signaling activity in Xenopus embryos independent of its levels. Dev Biol. 2000;223:441–8. doi: 10.1006/dbio.2000.9770. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–81. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep. 2002;3:834–9. doi: 10.1093/embo-reports/kvf181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S-i, Michiue T, Asashima M, Kikuchi A. Casein Kinase Iepsilon Enhances the Binding of Dvl-1 to Frat-1 and Is Essential for Wnt-3a-induced Accumulation of beta -Catenin. J Biol Chem. 2003;278:14066–14073. doi: 10.1074/jbc.M213265200. [DOI] [PubMed] [Google Scholar]
- Huang W, Erikson RL. Constitutive Activation of Mek1 by Mutation of Serine Phosphorylation Sites. PNAS. 1994;91:8960–8963. doi: 10.1073/pnas.91.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–8. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- Imai K, Takada N, Satoh N, Satou Y. (beta)-catenin mediates the specification of endoderm cells in ascidian embryos. Development. 2000;127:3009–20. doi: 10.1242/dev.127.14.3009. [DOI] [PubMed] [Google Scholar]
- Itoh K, Antipova A, Ratcliffe MJ, Sokol S. Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol Cell Biol. 2000;20:2228–38. doi: 10.1128/mcb.20.6.2228-2238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius MA, Schelbert B, Hsu W, Fitzpatrick E, Jho E, Fagotto F, Costantini F, Kitajewski J. Domains of axin and disheveled required for interaction and function in wnt signaling. Biochem Biophys Res Commun. 2000;276:1162–9. doi: 10.1006/bbrc.2000.3607. [DOI] [PubMed] [Google Scholar]
- Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Xu W. β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–22. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–30. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- Krasnow RE, Wong LL, Adler PN. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development. 1995;121:4095–102. doi: 10.1242/dev.121.12.4095. [DOI] [PubMed] [Google Scholar]
- Krylova O, Messenger MJ, Salinas PC. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3beta. J Cell Biol. 2000;151:83–94. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–36. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E, Chiaur DS, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18:849–54. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT. Molecular links between X-inactivation and autosomal imprinting: X-inactivation as a driving force for the evolution of imprinting? Curr Biol. 2003;13:R242–54. doi: 10.1016/s0960-9822(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. Embo J. 1999;18:4233–40. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A. 1999;96:6273–8. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Logan CY, Miller JR, Ferkowicz MJ, McClay DR. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development. 1999;126:345–57. doi: 10.1242/dev.126.2.345. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–37. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. Embo J. 1999;18:6873–9. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kawachi K, Kamakura S, Masuyama N, Yamanaka H, Matsumoto K, Kikuchi A, Nishida E. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J Biol Chem. 1999;274:30957–62. doi: 10.1074/jbc.274.43.30957. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Hyatt T, Kim H, Guttman J, Walsh E, Sumanas S, Wang Y, Ekker SC. Evidence for a frizzled-mediated wnt pathway required for zebrafish dorsal mesoderm formation. Development. 1998;125:4283–92. doi: 10.1242/dev.125.21.4283. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–87. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell. 2005;8:829–41. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Pelegri F, Maischein HM. Function of zebrafish beta-catenin and TCF-3 in dorsoventral patterning. Mech Dev. 1998;77:63–74. doi: 10.1016/s0925-4773(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Penton A, Wodarz A, Nusse R. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics. 2002;161:747–62. doi: 10.1093/genetics/161.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–50. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Amati F, Calabrese G, Mari A, Colosimo A, Silani V, Giardino L, Ratti A, Penso D, Calza L, Palka G, Scarlato G, Novelli G, Dallapiccola B. cDNA characterization and chromosomal mapping of two human homologues of the Drosophila dishevelled polarity gene. Hum Mol Genet. 1996a;5:953–8. doi: 10.1093/hmg/5.7.953. [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Novelli G, Mari A, Ratti A, Colosimo A, Amati F, Penso D, Sangiuolo F, Calabrese G, Palka G, Silani V, Gennarelli M, Mingarelli R, Scarlato G, Scambler P, Dallapiccola B. Human homologue sequences to the Drosophila dishevelled segment-polarity gene are deleted in the DiGeorge syndrome. Am J Hum Genet. 1996b;58:722–9. [PMC free article] [PubMed] [Google Scholar]
- Roeser T, Stein S, Kessel M. Nuclear beta-catenin and the development of bilateral symmetry in normal and LiCl-exposed chick embryos. Development. 1999;126:2955–65. doi: 10.1242/dev.126.13.2955. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. Embo J. 2000;19:1010–22. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–32. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–8. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–77. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- Shulman JM, Perrimon N, Axelrod JD. Frizzled signaling and the developmental control of cell polarity. Trends Genet. 1998;14:452–8. doi: 10.1016/s0168-9525(98)01584-4. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. Embo J. 1999;18:2823–35. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley MJ, Signoret N, Robertson D, Tilley A, Hann A, Ewan K, Ding Y, Paterson H, Dale TC. Dishevelled (Dvl-2) activates canonical Wnt signalling in the absence of cytoplasmic puncta. J Cell Sci. 2005;118:5279–5289. doi: 10.1242/jcs.02647. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–67. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:1637–47. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–13. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Summers RG, Morrill JB, Leith A, Marko M, Piston DW, Stonebraker AT. A stereometric analysis of karyokinesis, cytokinesis, and cell arrangements during and following fourth cleavage in the sea urchin, L. variegatus. Dev Growth Differ. 1993;35:41–57. doi: 10.1111/j.1440-169X.1993.00041.x. [DOI] [PubMed] [Google Scholar]
- Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3:628–36. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–71. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–60. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Strapps WR, Heemskerk J. Linking Frizzled and Wnt signaling in Drosophila development. Development. 1997;124:4515–21. doi: 10.1242/dev.124.22.4515. [DOI] [PubMed] [Google Scholar]
- Torres MA, Nelson WJ. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol. 2000;149:1433–42. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Dev Dyn. 1996;207:253–62. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vonica A, Weng W, Gumbiner BM, Venuti JM. TCF Is the Nuclear Effector of the β-catenin Signal That Patterns the Sea Urchin Animal-Vegetal Axis. Developmental Biology. 2000;217:230–243. doi: 10.1006/dbio.1999.9551. [DOI] [PubMed] [Google Scholar]
- Weinstock GM, Gibbs RA, Sodergren E, Davidson EH, Cameron RA Consortium TSUGS. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006 in press. [Google Scholar]
- Weitzel HE, Illies MR, Byrum CA, Xu R, Wikramanayake AH, Ettensohn CA. Differential stability of beta-catenin along the animal-vegetal axis of the sea urchin embryo mediated by dishevelled. Development. 2004;131:2947–56. doi: 10.1242/dev.01152. [DOI] [PubMed] [Google Scholar]
- Wharton KA., Jr Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, Xu R, Martindale MQ. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–50. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- Wikramanayake AH, Huang L, Klein WH. beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc Natl Acad Sci U S A. 1998;95:9343–8. doi: 10.1073/pnas.95.16.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. Embo J. 1997;16:3089–96. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–60. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. Embo J. 2002;21:1733–42. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 1995;9:1087–97. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–6. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–41. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]