Abstract
An agglutination assay was used to examine the binding of purified human S protein (vitronectin, serum spreading factor) to 201 clinical isolates of Neisseria gonorrhoeae. Strains belonging to the protein IA serovars were significantly (P less than 0.001) more reactive in agglutination tests with human S protein and were more serum resistant than strains belonging to the protein IB serovars. The strains from patients with disseminated infections belonged predominantly to the IA serovar (19 of 23) and, with the exception of IA-4 and certain IB serovars, avidly agglutinated with S protein. The serovar IA-4 and IB strains isolated from joint or cerebrospinal fluid failed to agglutinate with S protein and appeared to be less serum resistant than most other IA isolates. Cysteine hydrochloride or 2-mercaptoethanol inhibited agglutination of S protein and a more than twofold increase in resistance to killing by fresh human serum following preincubation with S protein; the serum-sensitive parent strain did not agglutinate S protein, and serum resistance was not increased following preincubation with this protein. Binding of S protein by gonococci may represent a novel pathogenic mechanism that can contribute to serum resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Mandrell R. E., Shero M., Wilson M. E., Griffiss J. M., Brooks G. F., Lammel C., Breen J. F., Rice P. A. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990 Aug;162(2):506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Preissner K. T., Müller-Berghaus G., Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987 Aug;55(8):1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Lee T. J., Sparling P. F. Penicillin sensitivity and serum resistance are independent attributes of strains of Neisseria gonorrhoeae causing disseminated gonococcal infection. Infect Immun. 1977 Mar;15(3):834–841. doi: 10.1128/iai.15.3.834-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Counts G. W., Beaty H. N. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCutchan J. A., Katzenstein D., Norquist D., Chikami G., Wunderlich A., Braude A. I. Role of blocking antibody in disseminated gonococcal infection. J Immunol. 1978 Nov;121(5):1884–1888. [PubMed] [Google Scholar]
- McShan W. M., Williams R. P., Hull R. A. A recombinant molecule from a disseminating strain of Neisseria gonorrhoeae that confers serum bactericidal resistance. Infect Immun. 1987 Dec;55(12):3017–3022. doi: 10.1128/iai.55.12.3017-3022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello J. A., Bohnhoff M. Serovars and serum resistance of Neisseria gonorrhoeae from disseminated and uncomplicated infections. J Infect Dis. 1989 Dec;160(6):1012–1017. doi: 10.1093/infdis/160.6.1012. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Nairn C. A., Cole J. A., Patel P. V., Parsons N. J., Fox J. E., Smith H. Cytidine 5'-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988 Dec;134(12):3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- Pettit R. K., Szuba J. C., Judd R. C. Comparison of two serum bactericidal assays for Neisseria gonorrhoeae. J Immunol Methods. 1990 May 8;129(1):15–22. doi: 10.1016/0022-1759(90)90415-r. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Preissner K. T., Müller-Eberhard H. J. Inhibition of C9 polymerization within the SC5b-9 complex of complement by S-protein. Acta Pathol Microbiol Immunol Scand Suppl. 1984;284:89–96. [PubMed] [Google Scholar]
- Preissner K. P., Podack E. R., Müller-Eberhard H. J. SC5b-7, SC5b-8 and SC5b-9 complexes of complement: ultrastructure and localization of the S-protein (vitronectin) within the macromolecules. Eur J Immunol. 1989 Jan;19(1):69–75. doi: 10.1002/eji.1830190112. [DOI] [PubMed] [Google Scholar]
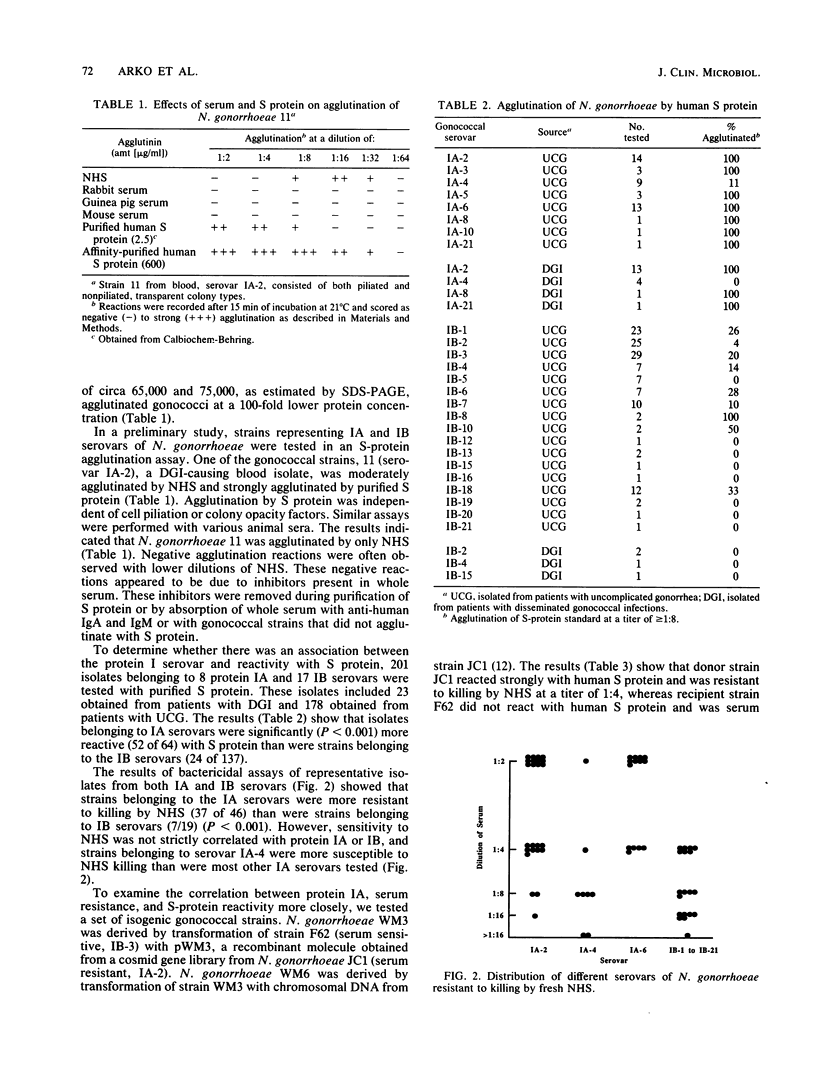
- Rice P. A., Goldenberg D. L. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981 Aug;95(2):175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
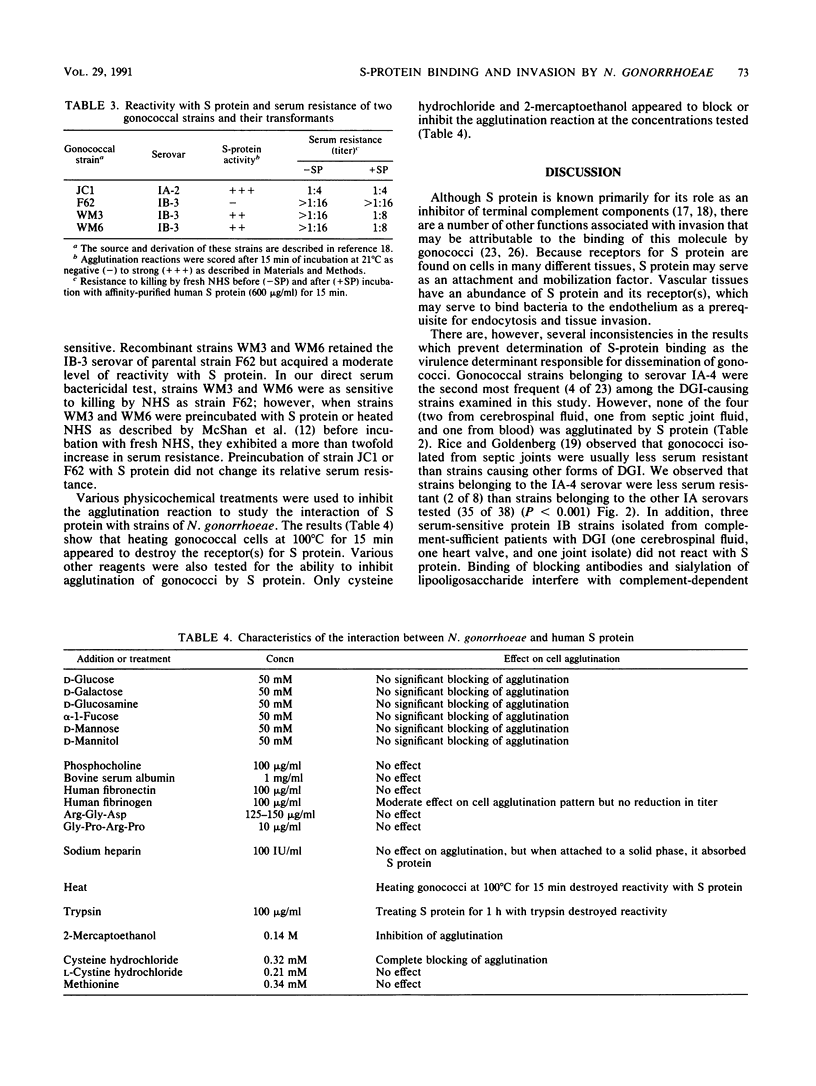
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Masson D., Schäfer S., Peitsch M., Preissner K. T. The heparin binding domain of S-protein/vitronectin binds to complement components C7, C8, and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Biochemistry. 1988 May 31;27(11):4103–4109. doi: 10.1021/bi00411a029. [DOI] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]