Introduction
Climate change refers to the global shift in weather patterns occurring over long periods of time. These changes encompass temperature, rainfall, wind speeds and cloud cover. Climate change has accelerated rapidly in the past half century and while international focus has concentrated on the environmental and economic consequences, the effects on human diseases such as skin cancer have been relatively under-represented. The association between ultraviolet (UV) exposure from the sun and the development of malignant skin disease has long been recognized but is still not completely understood. The relationship between climate change and UV exposure will be explored in an attempt to qualify the impact of climate change on skin cancer.
Ultraviolet radiation
Ultraviolet radiation (UVR) occupies the space between visible light and X-rays on the electromagnetic spectrum. The colour violet corresponds to the shortest wavelength in visible light and UV actually means ‘beyond violet’ (from the Latin ultra, meaning ‘beyond’). UV light can be further subdivided in terms of wavelength; ‘long wave’ UV with a range of 320–400 nanometres (nm) is known as UV-A; ‘medium wave’ UV with a range of 290–320 nm is known as UV-B; and ‘short wave’ UV with a range of 100–290 nm is known as UV-C.1 The wavelength of light is inversely proportional to its frequency and higher frequencies of light possess more energy. Hence UV-C carries the most energy and is the most damaging to biological systems. While UVB causes considerable DNA damage in the skin, UVA has only recently been shown to induce pyrimidine dimerizations and generate reactive oxygen and nitrogen species which damage DNA, proteins and lipids.2 The immunosuppressive effect of UVR contributes to its carcinogenic activity. Any one of these effects of UVR may contribute to the induction of skin cancers by other agents such as viruses, X-rays or chemical carcinogens.
Skin cancer
The three most common types of skin cancer are basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and malignant melanoma (MM). Exposure to ultraviolet radiation is recognized as a risk factor in all three malignancies. Approximately 90% of skin cancers are non-melanocytic, the vast majority of these are BCCs.
BCCs are commonly known as rodent ulcers; they usually arise in sun-exposed areas of the body and have a propensity to cause extensive local tissue damage. Patients with these malignancies are usually fair-skinned and tend to burn rather than tan in sunlight. An Italian study has also highlighted a definite association between BCC development and recreational sun exposure during childhood and adolescence.3 The exact nature of the wavelengths and exposure patterns involved in BCC carcinogenesis is still equivocal to a large degree, however recent studies demonstrate a correlation between ultraviolet B radiation (UV-B, 290–320 nm) and BCC risk.4
SCCs account for a significant proportion of non-melanocytic skin cancer. SCCs are caused by sunlight-induced mutations in the p53 tumour suppressor gene.5 They are found almost exclusively on sun-exposed skin such as the neck, face and arms, and the incidence is linked with geographical location, being higher at latitudesreceiving more sun such as Australia.6
Malignant melanoma is the most serious form of skin cancer: it is responsible for around 80% of skin cancer deaths. Over the last 25 years the reported incidence of malignant melanoma has increased. This is likely to be due to increased UV exposure, however the number of skin biopsies now taking place has also risen. An American study revealed that an increase in skin biopsy rates corresponded to an increase in the incidence of local melanoma while mortality rates remained unchanged, the authors have attributed the rising incidence of melanoma to an increase in diagnostic scrutiny rather than an actual increase in the incidence of disease.7 Melanoma is also the third most common cancer among 15–39 year olds. Exposure to UVR, fair skin, dysplastic naevi syndrome and a family history of melanoma are major risk factors for melanoma development. UV-B appears more closely associated with the development of melanoma than UV-A (320–400 nm). This is supported by the higher incidence of melanoma in equatorial regions than in latitudes further from the equator, as UV-B radiation is most intense at the equator while UV-A intensity varies less across latitudes.8 Although UV-B appears to be more important than UV-A as a risk factor, a causal link to UV-A exposure is also supported by data from patients using tanning beds9 and or treated with psoralan UV-A (PUVA) for psoriasis.10
Ozone
The Earth receives UVR from the sun, all of the UV-C and the majority of UV-A and UV-B is filtered out by the ozone layer. Ozone is a triatomic oxygen molecule, O3, found mainly in the stratosphere, which is approximately 10–40 km above the Earth's surface. It is continually being regenerated from O2 through the UV dependent ozone-oxygen cycle. Free radicals such as chlorine and bromine atoms shift the cycle to produce more O2 than O3; this depletes the ozone layer. The emission of chloroflourocarbons (CFCs) massively increases the concentration of these free radicals which then leads to the depletion of the ozone layer.
CFCs are compounds made from carbon, fluorine and chlorine, which were invented in the 1920s. They were commonly found in solvents, aerosol sprays and coolants in refrigerators. Due to their long half-lives (lasting between 50–100 years), the long-term damage they can cause to ozone is quite extensive. It is estimated that a single CFC molecule typically degrades around 10,000 ozone molecules before its removal, this is a conservative estimate, it could potentially degrade many millions.
In 1987, an international treaty called the Montreal Protocol was signed to phase out all CFC usage. Industry began substituting CFCs with hydrochlorofluorocarbons (HCFCs). HCFCs cause much less ozone depletion, but they are extremely powerful greenhouse gases being up to 10,000 times more potent than carbon dioxide. As a result of the Montreal Protocol CFC levels have now levelled off and have begun to decrease in some cases, this is in sharp contrast to HCFC levels which have shown a steep rise in their atmospheric concentrations since the 1980s. Amendments to the Montreal Treaty now call for a complete ban on all HCFCs by 2030.
Scientists have measured a steady decline in ozone of approximately 4% per decade since the 1970s with marked seasonal fluctuations occurring over the Earth's polar regions. The largest seasonal fluctuation in ozone was discovered in 1985 by British scientists from the British Antarctic Survey.11 They discovered a ‘hole’ in the ozone layer above Antarctica. The ozone ‘hole’ is really a reduction in concentrations of ozone high above the earth in the stratosphere. The ozone hole has steadily grown in size (up to 27 million km2) and its average annual duration has also increased (from August through early December) in the past two decades.
The short-term impact of the Montreal Protocol on ozone has been less than dramatic. Although the current Intergovernmental Panel on Climate Change models suggest that global ozone depletion has now stabilized, the long half-lives of these compounds mean that it will probably by another 50 or more years before the large Antarctic hole in the ozone layer recovers. The most current estimates predict that the first detectable change will only occur in 2024, assuming full compliance with the Montreal Protocol. Even with full compliance of the Montreal Protocol and assuming all other variables, such as cloud cover and human behaviour are kept constant, the depletion of ozone will drastically increase the incidence of all types of skin cancer. In 1998 the United Nations Environment Programme reported that in 2050 the incidence of skin cancer in north-west Europe will peak at an additional 90 per million per year, resulting in several thousand more cases of reported skin cancer in the UK. This time lag is due to the fact that skin cancer usually results from cumulative UV exposure over several decades.12
Climate change
In the past century the Earth has warmed up by 0.74°C. Over half of this increase has occurred since the 1970s.13 This global warming phenomenon is caused by greenhouse gases, such as carbon dioxide and nitrous oxide, trapping infrared radiation from the sun in the atmosphere. Deforestation combined with the burning of fossil fuels is thought to account for most of the anthropogenic increases in carbon dioxide levels.
Climate change will affect the UK climate in many ways. The two which will cause behaviour change, in terms of UV exposure, will be changes in temperature and rainfall. During the 20th century, the annual mean central England temperature warmed by about 1°C; the UK ministry for the environment, DEFRA, predicts that if high greenhouse gas emissions are maintained then the average annual temperature across the UK may rise by between 2°C and 3.5°C by the 2080s with the south of the UK experiencing the greatest change ( Figure 1). Very cold winters will become a rarity and high summer temperature will become the norm. The increase in temperature could make the carcinogenic effects of UVR even more lethal. Mouse experiments have shown that the carcinogenic effectiveness of UV radiation increases by 5% per °C, so a long-term elevation of temperatures by 3.5°C would increase the carcinogenicity by 7.5%. If modelled on the UK then this synergistic relationship between ozone depletion and a conservative 2°C increase would result in an extra 5000–6000 cases of skin cancer per year by 2050.14
Figure 1.
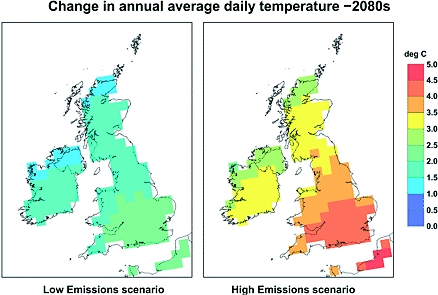
Daily temperature could increase by as much as 4°C by 2080 with the south of the UK experiencing the greatest amount of warming. Source: DEFRA
Precipitation patterns and frequencies over the last 100 years show that precipitation patterns have been quite erratic. There has however been a general trend showing decreased rainfall during summer and increasing rainfall during winter.13
Current simulations with high greenhouse emission scenarios suggest that winters will generally become wetter and that summers will become drier. The increased precipitation in winter could reduce snowfall by almost 90%.13 This could mean children living in the next century may never see snow during their childhoods. The reasons for the changing precipitation patterns remain equivocal.
With warmer, drier summers there is an increased tendency to spend more time outdoors; this would increase the population exposure to sunlight and the UV radiation associated with it. A study in 1996 examined the different causes of UV exposure in children from different parts of the UK. It demonstrated that children in the warmer south-east of England received a greater amount of UV radiation during recreational activities when compared to children in the colder north east. This study showed that ambient temperature and climate are more like to explain behaviour and hence UV exposure rather than ambient UV exposure.15 A Danish study recently compared UV exposure during winter versus summer in indoor workers by measuring the standard erthyma dose (SED) they received using personal dosimeters. The study found considerable differences in behaviour and UV exposure. The mean solar UV exposure was 3.1 SED per day in winter and 133 SED in summer. During winter the workers had not been outdoors during daylight hours on 77% of the days; this corresponded with 19% during the summer. They had on average 10 minutes per day with positive dosimeter readings in winter compared with 2 hours per day in summer.16 Fewer daylight hours along with colder temperatures may explain these differences. One can infer from this study that as winters get milder and the average temperature increases, the total time spent outdoors and hence the UV exposure is likely to increase.
In an Australian behavioural study, it was reported that when the ambient temperature was 19–27°C the chances of sunburn doubled, compared to temperatures of 18°C or lower (currently typical average maximum summer temperatures in the UK). Interestingly, at temperatures above 27°C, the likelihood of sunburn fell again presumably because people sought shade from the more intense heat.12
Climate change will also affect the recreational activities of adults, the exact nature of the changes is not known but a warmer climate may encourage the greater participation of outdoor sports. The multicentre southern European study, Helios II, reported that UVR is associated with a risk of BCC even for relatively short periods of exposure, such as playing sports.17 The same study established that water sports and beach holidays emerged as separate risk factors for BCCs. In addition, a case-control study by the same group showed that sun exposure during leisure time activities such as beach sports and outdoor sports showed only a slightly increased, statistically insignificant, odds ratio for cutaneous melanoma.18 Sun exposure during childhood outdoor activities was associated with a significant risk increase for MM and BCC.
Conclusion
In conclusion, ozone depletion and climate change are separate entities which are intricately linked. They both have the potential to increase the incidence of skin cancer through different means. Over the last 30 years ozone depletion has received much of the attention, leading to the Montreal Protocol; heralded by Kofi Annan as ‘perhaps the single most successful international agreement to date’. Ozone depletion has lead to an increase in skin cancers and worryingly this is still rising. The depletion will however peak and then the ozone layer will begin to repair itself. Focus must now shift towards analysing the social and behavioural changes that will come about through climate change. Warmer, drier weather in the UK is likely to encourage people to spend more time outdoors and increase their exposure to UVR. The consequence will be an increase in the incidence of skin cancer brought about by behavioural change rather than environmental change. The world has had 30 years of public health initiatives and awareness campaigns. These must be heeded and acted upon now to protect the public from this preventable threat.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantor AKB
Contributorship AKB decided to write the article and undertook the literature search, design, collection, analysis of data and wrote the paper. RJT provided a clinical overview
Acknowledgements
The authors would like to thank PH Tan, A Sud and N Cox for their suggestions on a previous version of the paper
References
- 1.Tyrrell RM. Ultraviolet radiation and free radical damage to skin. Biochem Soc Symp 1995;61:47–53 [DOI] [PubMed] [Google Scholar]
- 2.Runger TM, Kappes UP. Mechanisms of mutation formation with long-wave ultraviolet light (UVA). Photodermatol Photoimmunol Photomed 2008;24:2–10 [DOI] [PubMed] [Google Scholar]
- 3.Corona R, Dogliotti E, D'Errico M, et al. Risk factors for basal cell carcinoma in a Mediterranean population: role of recreational sun exposure early in life. Arch Dermatol 2001;137:1162–8 [DOI] [PubMed] [Google Scholar]
- 4.Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol 2005;152:1108–24 [DOI] [PubMed] [Google Scholar]
- 5.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature 1994;372:773–6 [DOI] [PubMed] [Google Scholar]
- 6.Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Austr 2006;184:6–10 [DOI] [PubMed] [Google Scholar]
- 7.Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ 2005;331:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JA, Scotto J. Melanoma: linked temporal and latitude changes in the United States. Canc Causes Contr 1993;4:413–18 [DOI] [PubMed] [Google Scholar]
- 9.Autier P. Perspectives in melanoma prevention: the case of sunbeds. Eur J Cancer 2004;40:2367–76 [DOI] [PubMed] [Google Scholar]
- 10.Whitmore SE, Morison WL. Melanoma after PUVA therapy for psoriasis. N Engl J Med 1997;337:502–3 [DOI] [PubMed] [Google Scholar]
- 11.Farman JC, Gardiner BG, Shanklin JD. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 1985;315:207–10 [Google Scholar]
- 12.Diffey B. Climate change, ozone depletion and the impact on ultraviolet exposure of human skin. Phys Med Biol 2004;49:R1–11 [DOI] [PubMed] [Google Scholar]
- 13.DEFRA Climate change: what is climate change? London: DEFRA; 2008. See http://www.defra.gov.uk/environment/climatechange/about/index.htm [Google Scholar]
- 14.van der Leun JC, de Gruijl FR. Climate change and skin cancer. Photochem Photobiol Sci 2002;1:324–6 [DOI] [PubMed] [Google Scholar]
- 15.Diffey BL, Gibson CJ, Haylock R, McKinlay AF. Outdoor ultraviolet exposure of children and adolescents. Br J Dermatol 1996;134:1030–4 [PubMed] [Google Scholar]
- 16.Thieden E, Philipsen PA, Wulf HC. Ultraviolet radiation exposure pattern in winter compared with summer based on time-stamped personal dosimeter readings. Br J Dermatol 2006;154:133–8 [DOI] [PubMed] [Google Scholar]
- 17.Rosso S, Zanetti R, Martinez C, et al. The multicentre south European study ‘Helios’. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer 1996;73:1447–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer 2006;94:743–51 [DOI] [PMC free article] [PubMed] [Google Scholar]


