Abstract
Altered impulse control has been implicated in the shaping of habitual alcohol use and eventual alcohol dependence. We sought to identify the neural correlates of altered impulse control in 24 abstinent patients with alcohol dependence (PAD), as compared to 24 demographics matched healthy control subjects (HC). In particular, we examined risk taking and deficits in cognitive control as the neural endophenotypes of alcohol dependence. To this end, functional magnetic resonance imaging (fMRI) was conducted during a stop signal task (SST), in which a procedure was used to elicit errors in the participants. The paradigm allowed trial-by-trial evaluation of response inhibition, error processing, and post-error behavioral adjustment. Furthermore, by imposing on the subjects to be both fast and accurate, the SST also introduced a distinct element of risk, which participants may or may not avert during the task. Brain imaging data were analyzed with Statistical Parametric Mapping in covariance analyses accounting for group disparity in general performance. The results showed that, compared to HC, PAD demonstrated longer go trial reaction time (RT) and higher stop success rate (SS%). HC and PAD were indistinguishable in stop signal reaction time (SSRT) and post-error slowing (PES). In a covariance analysis accounting for go trial RT and SS%, HC showed greater activity in the left dorsolateral prefrontal cortex than PAD, when subjects with short and long SSRT were contrasted. By comparing PAD and HC directly during stop errors (SE), as contrasted with SS, we observed greater activity in PAD in bilateral visual and frontal cortices. Compared to HC, PAD showed less activation of the right dorsolateral prefrontal cortex during PES, an index of post-error behavioral adjustment. Furthermore, PAD who showed higher alcohol urge at the time of the fMRI were particularly impaired in dorsolateral prefrontal activation, as compared to those with lower alcohol urge. Finally, compared to HC subjects, PAD showed less activity in cortical and subcortical structures including putamen, insula and amygdala during risk-taking decisions in the SST. These preliminary results provided evidence for altered neural processing during impulse control in PAD. These findings may provide a useful neural signature in the evaluation of treatment outcomes and development of novel pharmacotherapy for alcohol dependence.
Keywords: alcohol abuse, impulsivity, response inhibition, error processing, go/nogo
Introduction
Alcohol dependence involves a wide range of serious medical and non-medical conditions such as alcohol-related liver diseases, violence and traffic accidents. Individuals as well as the society as a whole suffer a great deal from this serious mental illness. Understanding the psychological and neural processes leading to heavy, habitual and eventually uncontrollable use of alcohol is thus an important public health issue and poses great challenges to addiction neuroscience.
A number of investigators have hypothesized a critical association between drug and alcohol addiction and deficits in impulse control (Ernst and Paulus, 2005; Everitt and Robbins, 2005; Goldstein and Volkow, 2002; Kalivas and Volkow, 2005; Moeller et al., 2001; Volkow and Li, 2005). Broadly defined in the literature, impulse control could comprise two distinguishable psychological dimensions. On one hand, impulse control implies ability to avoid risk and to curb excessive desire to seek sensation (Finn, 2002; Kelley et al., 2004; Kreek et al., 2005; Verdejo-Garcíaet al., 2008). On the other hand, impulse control implies cognitive operations that allow individuals to change behaviors in a dynamic fashion on the basis of advance information or feedback derived from monitoring ongoing behavior (Botvinick et al., 2001; Carter et al., 1999; Kok et al., 2006; Ridderinkhof et al., 2004). This latter capability has specifically been referred to as cognitive control. By setting goals, inhibiting habitual acts, and monitoring performance, cognitive control allows behavioral flexibility for one to maneuver changing environment and optimize goal-directed actions (Dalley et al., 2004). Cognitive control thus serves to maintain homeostasis by a process that accommodates changing states of the decision maker (Paulus, 2007). It has been hypothesized that disrupted impulse control along with heightened salience attributed to alcohol could lead to a vicious cycle of withdrawal, craving, bingeing and intoxication (Goldstein and Volkow, 2002).
In this study we employed the stop signal task (SST) as a behavioral proxy to explore whether neural process associated with impulse control are altered in patients in alcohol dependence (PAD). The SST is widely used in the cognitive and imaging neuroscience literature (Logan, 1994; Logan and Cowan, 1984). In a “tracking” SST in which the difficulty of the stop trials were adjusted according to participants’ performance, we delineated the neural correlates of response inhibition, error processing, and post-error behavioral adjustment, which are key component processes of cognitive control (Li et al., 2006a; Li et al., 2008a; Li et al., 2008b; Li et al., 2008c). Furthermore, by imposing on the participants to be both fast and accurate, the SST introduced a component of risk, which participants may avert by slowing down, or ignore by responding “as usual,” during go trials. We observed greater activity in a number of cortical and subcortical structures including the amygdala when participants take risk as compared to when they avoid risk (Li et al., In Press). Thus, with the SST that allowed us to examine the neural processes of cognitive control and risk taking, we sought to establish a neural signature of impaired impulse control in PAD.
Materials and Methods
Subjects, informed consent and assessment of alcohol urge
Twenty-four abstinent patients with alcohol dependence (PAD, 6 women) and 24 age- and education-matched healthy control subjects (HC, 6 women) participated in the study (Table 1). PAD met criteria for current alcohol dependence, as diagnosed by the Structured Clinical Interview for DSM-IV (First et al., 1995). PAD did not meet current DSM-IV criteria for dependence on other psychoactive substances, other than nicotine, and were also excluded if they met current criteria for any DSM IV Axis I psychiatric disorder. Recent use of other illicit substances was ruled out by urine toxicology screens upon admission. Women were excluded from the study if there were using any form of birth control or were either peri or post menopausal. In addition, individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. They were drug-free while staying in an inpatient treatment unit prior to the current fMRI study. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None of them reported having a history of head injury or neurological illness. The Human Investigation committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent prior to study participation.
Table 1.
Demographics of the subjects
| subject characteristic | PAD (n=24) | HC (n=24) | p value | |
|---|---|---|---|---|
| men/women | 18/6 | 18/6 | ||
| age (years) | 38.7 ± 8.3 | 35.5 ± 5.9 | 0.13a | |
| ethnicity | African American | 7 (29.2%) | 5 (20.8%) | 0.22b |
| Caucasian | 17 (70.8%) | 19 (79.2%) | ||
| education (years) | 12.5 ± 1.7 | 13.1 ± 1.6 | 0.23a | |
| average number of days of alcohol use / month prior to admission | 24.2 ± 8.3 | 4.3 ± 3.1* | <0.0001a | |
| average number of years of alcohol use | 10.2 ± 7.3 | 5.7 ± 4.5* | <0.0001a | |
Note: values are mean ± s.d.
data not available in two HC
2-sample t test
binomial test
PAD were assessed for their alcohol urge with the Alcohol Urge Questionnaire (AUQ, Bohn et al., 1995; Fox et al., 2008). AUQ was used to measure current alcohol urge on a Likert scale ranging from 1 (strongly disagree) to 7 (strongly agree), with a total of 8 items addressing desire for drink, expectation of positive affect from drinking, and inability to avoid drinking if alcohol was available. PAD were assessed with AUQ every 3−4 days during their inpatient stay. PAD participated in the fMRI study between 11 to 17 days (average = 2 weeks) after admission.
Behavioral task and experimental procedures
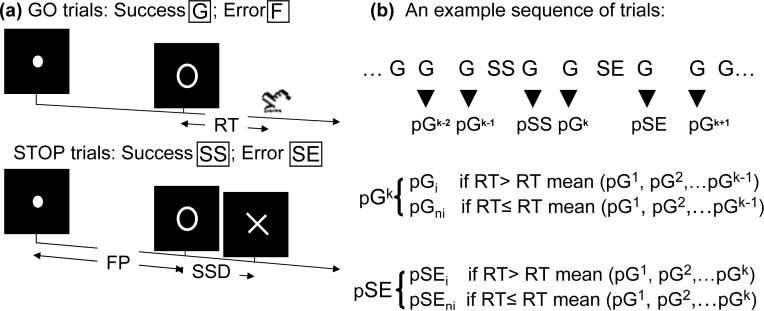
We employed a simple reaction time (RT) task in this stop-signal paradigm (Fig. 1). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention and eye fixation at the beginning of a go trial. After a randomized time interval (fore-period) anywhere between 1 and 5 s, the dot turned into a circle, prompting the subjects to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. In a stop trial, an additional “X,” the “stop” signal, appeared after the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop trials constituted the remaining one quarter of the trials. There was an inter-trial-interval of 2 s.
Figure 1.
(a) Stop signal paradigm. In “go” trials (75%) observers responded to the go signal (a circle) and in “stop” trials (25%) they had to withhold the response when they saw the stop signal (an X). In both trials the go signal appeared after a randomized time interval between 1 to 5 s (the fore-period or FP) following the appearance of the fixation point. The stop signal followed the go signal by a time delay – the stop signal delay (SSD). The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success (SS) and stop error (SE) trial, respectively. (b) An example sequence of trials to illustrate the definition of post-go slowing vs. post-go speeding in go trial reaction time; and post-error slowing vs. post-error speeding in go trial reaction time.
The time interval between the stop and the go signals (or the stop-signal delay, SSD) started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 64 ms, making it more difficult for them to succeed again in the next stop trial; conversely, if they failed, SSD decreased by 64 ms, making it easier for the next stop trial. With the staircase procedure, a “critical” SSD could be computed that represents the time delay required for the subject to succeed in withholding a response half of the time in the stop trials (Levitt, 1970). One way to understand the stop signal task is in terms of a horse race model with a go process and a stop process racing toward a finishing line (Logan, 1994). The go process prepares and generates the movement while the stop process inhibits movement initiation: whichever process finishes first determines whether a response will be initiated or not. Importantly, the go and stop processes race toward the activation threshold independently. Thus, the time required for the stop signal to be processed so a response is withheld (i.e., stop signal reaction time or SSRT) can be computed on the basis of the go trial RT distribution and the odds of successful inhibits for different time delays between go and stop signals. This is done by estimating the critical SSD at which a response can be correctly stopped in approximately 50% of the stop trials. With the assumptions of this “horse-race” model, the SSRT could then be computed for each individual subject by subtracting the critical SSD from the median go trial RT. Generally speaking, the SSRT is the time required for a subject to cancel the movement after seeing the stop signal. A long SSRT indicates poor response inhibition.
Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Prior to the fMRI study each subject had a practice session outside the scanner. Each subject completed four 10-min runs of the task with the SSD updated manually across runs. Depending on the actual stimulus timing (e.g., trials varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure we anticipated that the subjects would succeed in withholding their response in approximately 50% of the stop trials. This was thus an event-related fMRI study, with the go and stop trials randomly jittered to improve the efficiency of the study design.
We computed the fore-period effect as an index of motor preparedness during the SST (Li et al., 2005; Li et al., 2006a; Tseng and Li, 2008). Briefly, longer fore-period is associated with faster response time (Bertelson and Tisseyre, 1968; Woodrow, 1914). Thus, RT of go trials with a fore-period between 3 and 5 s were compared to those with one between 1 and 3 s, and the effect size of RT difference was defined as fore-period effect. It is also known that in a reaction time (RT) task the RT of a correct response is prolonged following an error, compared to other correct responses, and this prolonged RT is thought to reflect cognitive processes involved in error monitoring (Rabbit, 1966). We thus computed the RT difference between the go trials that followed a stop error and those that followed another go trial, and termed this RT difference “post-error slowing” (Hajcak et al., 2003; Li et al., 2008a).
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio, Erlangen, Germany). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4mm and no gap. Three hundred images were acquired in each run for a total of 4 runs.
Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping version 2 (SPM2, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999; Friston et al., 1995a). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 10 mm at Full Width at Half Maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
Four main types of trial outcome were distinguished: go success (G), go error (F), stop success (SS), and stop error (SE) trial (Fig. 1). A statistical analytical design was constructed for each individual subject, using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston et al., 1995b). Realignment parameters in all 6 dimensions were also entered in the model. Serial autocorrelation was corrected by a first-degree autoregressive or AR(1) model. The GLM estimated the component of variance that could be explained by each of the regressors. We constructed for each individual subject statistical contrasts: SS > SE and SE > SS.
In a second GLM, G, F, SS, and SE trials were first distinguished. G trials were divided into those that followed a G (pG), SS (pSS) and SE (pSE) trial. Furthermore, pSE trials were divided into those that increased in RT (pSEi) and those that did not increase in RT (pSEni), to allow the isolation of neural processes involved in post-error behavioral adjustment (Li et al., 2008a). To determine whether a pSE trial increased or did not increase in RT, it was compared to the pG trials that preceded it in time during each session. The pG trials that followed the pSE trial were not included for comparison because the neural/cognitive processes associated with these pG trials occurred subsequent to and thus could not have a causal effect on the pSE trial (Li et al., 2008a). We constructed for each individual subject two contrasts: SS > SE, to compare with the first GLM and verify the model; and pSEi vs. pSEni, to identify activations associated with post-error slowing.
In this second GLM, pG trials were also divided into those that increased in RT (pGi) and those that did not increase in RT (pGni, Li et al., In Press). Similarly, to determine whether a pG trial increased or did not increase in RT, it was compared to the pG trials that preceded it in time during each session. We contrasted pGni>pGi (i.e., post-go speeding > post-go slowing) for each individual subject to identify activations associated with risk taking decisions in the SST.
Random effect analyses of brain imaging data
RESPONSE INHIBITION
The SS and SE trials were identical in stimulus condition, with SS trials involving inhibition success and SE trials involving inhibition failure. The contrast SS > SE thus engaged processes related to response inhibition and was used in the random effect analysis (Li et al., 2006a). With the SSRT to index response inhibition, PAD and HC were each grouped into those with short (n=12) and long (n=12) SSRT on the basis of a median split, following the rationale of the race model (Li et al., 2006a; Logan, 1994). In a 2×2 analysis of variance (ANOVA), the contrasts HC > PAD (short > long SSRT) allowed us to identify structures showing greater activation in HC as compared to PAD during response inhibition and vice versa.
ERROR PROCESSING
We compared PAD and HC using the contrast SE > SS in a covariance analysis accounting for go trial RT and SS%.
POST-ERROR SLOWING
We compared PAD and HC using the contrast pSEi > pSEni in a covariance analysis accounting for go trial RT and SS%.
RISK TAKING
We compared PAD and HC using the contrast pGni > pGi in a covariance analysis accounting for go trial RT and SS%.
In addition to voxelwise whole brain exploration, we also performed region of interest (ROI) analysis based on our previous findings (Li et al., 2006; Li et al., 2008a; Li et al., 2008b; Li et al., In Press). We used MarsBaR (Brett et al., 2002; http://marsbar.sourceforge.net/) to compute for each individual subject the effect size (t-statistic) of activity change for functional ROIs derived from our published studies. The effect size rather than mean difference in brain activity was derived in order to account for individual differences in the variance of the mean. These ROIs included two cortical regions related to response inhibition (Li et al., 2006a): the dorsal medial frontal cortex (dmFC, x=−4, y=32, z=51) and the other focused on the rostral anterior cingulate cortex (rACC, x=−8, y=35, z=19); and the left caudate head (Li et al., 2008c). Seven regions related to error processing were also designated as ROIs (Li et al., 2008b): dorsal anterior cingulate cortex (dACC, x=−4, y=16, z=44) extending to include supplementary motor area (SMA); cuneus including retrosplenial cortex (x=16, y=−64, z=8); thalamus (x=−12, y=−16, z=8); left insula probably including inferior frontal cortex (x=−48, y=8, z=−8); superior frontal and precentral gyrus (x=−36, y=−8, z=52); superior temporal gyrus (x=−48, y=−28, z=24); and right insula (x=44, y=16, z=0). One region related to post-error slowing was identified as an ROI (Li et al., 2008a): ventrolateral prefrontal cortex (VLPFC, x=44, y=24, z=−4; BA 47). Finally, two regions related to risk taking were identified as ROIs (Li et al., In press): amygdala (x=−16, y=−4, z=−16) and the posterior cingulate cortex (PCC, x=−4, y=−40, z=44).
Results
Behavioral performance
Table 2 summarizes the stop signal performance for PAD and HC. Compared to HC, PAD were significantly slower in median go trial RT, suggesting that they adopted a more conservative response strategy. PAD also showed a higher stop success rate, compared to HC. The results suggested that, compared to HC, PAD exercised greater attention in monitoring for the stop signal. This discrepancy in attentional monitoring thus needed to be accounted for when regional brain activities were compared for response inhibition and post-error slowing. The two groups otherwise did not differ in stop signal performance. Furthermore, PAD demonstrated an average of −78 ± 20 ms in post-go speeding in RT and 93 ± 18 ms in post-go slowing in RT, not differently from HC, who demonstrated an average of −82 ± 26 ms in post-go speeding in RT and 99 ± 25 ms in post-go slowing in RT.
Table 2.
General performance in the stop signal task
| group | Median go RT (ms) | %go | %stop | SSRT (ms) | FP effect^ (effect size) | PES (effect size) | RT difference between pG speeding and slowing (ms)^ |
|---|---|---|---|---|---|---|---|
| PAD | 687 ± 114 | 96.2 ± 1.63 | 53.3 ± 3.6 | 190 ± 30 | 1.78 ± 1.68 | 1.56 ± 2.06 | −171 ± 33 |
| HC | 595 ± 150 | 96.5 ± 1.81 | 50.9 ± 2.6 | 195 ± 36 | 2.27 ± 1.65 | 1.42 ± 1.52 | −181 ± 49 |
| P value | 0.020* | 0.850 | 0.010* | 0.645 | 0.310 | 0.791 | 0.386 |
Note: PAD: patients with alcohol dependence; HC: healthy controls; %go and %stop: percentage of successful go and stop trials; SSRT: stop-signal reaction time; FP (fore-period) effect: 42 ± 42 ms (AD) vs. 54 ± 34 ms (HC); PES (post-error slowing): 38 ± 51 ms (AD) vs. 36 ± 37 ms (HC); all numbers are mean ± standard deviation. P value based on 2-talied two sample t test.
<0.05
pG: post-go; =extent of post-go speeding – extent of post-go slowing. See text for further explanation.
Table 3 summarizes the stop signal performance separately for short and long SSRT group each for PAD and HC. PAD and HC showed near-trend and trend difference in median go trial RT and stop success rate.
Table 3.
General performance of PAD and HC in the stop signal task, grouped by SSRT
| group | SSRT (ms) | Median go RT (ms) | %go | %stop | FP effect (effect size) | PES (effect size) |
|---|---|---|---|---|---|---|
| PAD, short | 170 ± 18 | 743 ± 77 | 95.5 ± 1.1 | 54.6 ± 4.1 | 1.77 ± 1.63 | 0.89 ± 2.11 |
| PAD, long | 211 ± 25 | 632 ± 120 | 96.8 ± 1.8 | 51.9 ± 2.4 | 1.78 ± 1.81 | 2.23 ± 1.85 |
| HC, short | 168 ± 25 | 593 ± 166 | 96.1 ± 1.7 | 50.7 ± 3.2 | 2.08 ± 1.11 | 1.26 ± 1.85 |
| HC, long | 222 ± 22 | 597 ± 138 | 96.9 ± 1.9 | 51.1 ± 1.8 | 2.46 ± 2.09 | 1.58 ± 1.17 |
| P value, Interaction effect | 0.327 | 0.131 | 0.623 | 0.076 | 0.703 | 0.322 |
Note: PAD: patients with alcohol dependence; HC: healthy controls; %go and %stop: percentage of successful go and stop trials; SSRT: stop-signal reaction time; PES: post-error slowing; all numbers are mean ± standard deviation. P values of the interaction effect are all based on 2-way ANOVA: subject by SSRT group.
Other analyses indicated that performance of both PAD and HC was well tracked by the staircase procedure. These findings included that both PAD and HC subjects succeeded in approximately half of the stop trials; and both showed a significant linear correlation between the RT of stop error trials and the stop signal delay (p's<0.005, 0.446<R's<0.941, Pearson regression; Logan and Cowan, 1984).
Whole brain and region of interest (ROI) analyses
We applied the same threshold of p<0.001, uncorrected and 5 voxels in the extent of activation to all second-level whole brain analyses of imaging data. In ROI analyses, a threshold of p<0.05, uncorrected was first set up for the whole brain exploration, and the results were reported with small volume correction using p<0.001, uncorrected.
RESPONSE INHIBITION
PAD and HC were compared for the contrast SS>SE, in a group (PAD vs. HC) by SSRT (short vs. long) ANOVA. The results showed greater activation in the short as contrasted to long SSRT group in HC compared to PAD in the left dorsolateral prefrontal cortex (DLPFC, x=−48, y=24, z=36, Z=3.70, 8 voxels, Fig. 2). Since the two SSRT groups showed near-trend or trend differences in go trial RT and SS%, we performed another ANOVA covaried for these two variables. The results were essentially identical: HC showed greater left DLPFC activity (x=−48, y=24, z=36, Z=3.57, 6 voxels). PAD did not show greater regional brain activity than HC for the same contrast. In ROI analyses, PAD and HC did not differ in the dmFC, rACC, left caudate head masks in the same ANOVA.
Figure 2.
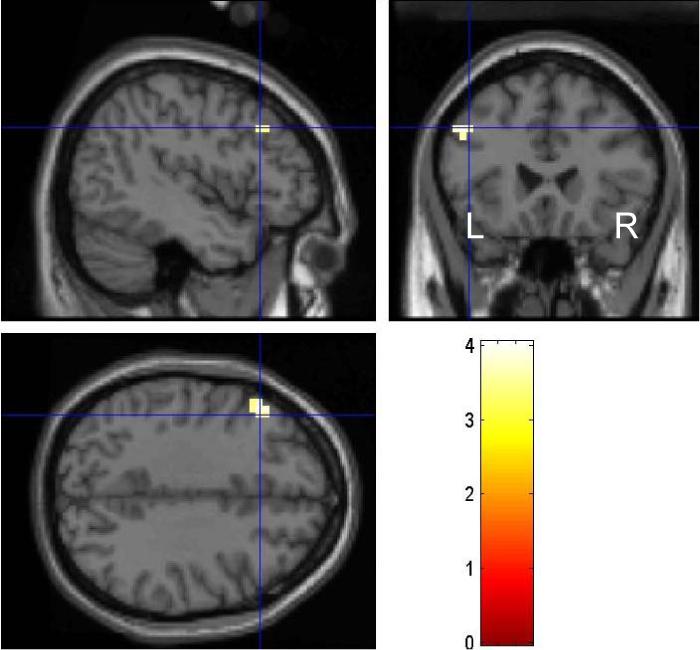
Compared to healthy control subjects, patients with alcohol dependence showed less activation of the lateral prefrontal cortex during response inhibition. Color bar represents voxel T value. See text for details.
ERROR PROCESSING
Contrasting stop error (SE) with stop success (SS) trials, PAD showed increased activity compared to HC in a number of brain regions including bilateral visual and frontal cortices in a 2-sample t test (Fig. 3; Table 4). Conversely, no brain regions showed greater activity in the same covariance analysis in HC, as compared to PAD. In ROI analysis for error processing, PAD and HC did not show differential activity in any of the seven masks identified from our earlier studies.
Figure 3.

Compared to healthy control subjects, patients with alcohol dependence showed greater activation in a number of cortical structures during error processing. Contrast in BOLD signal was overlaid on a T1 structural image in axial sections. Orientation is neurological: R=R. These brain regions are summarized in Table 4.
Table 4.
Brain regions showing greater activity in PAD, compared to HC, during stop error > stop success
| cluster size (voxels) | voxel Z value | MNI coordinate (mm) | Identified brain region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 49 | 4.39 | −56 | −72 | 12 | Middle temporal G |
| 24 | 4.30 | 20 | −64 | 36 | Superior parietal G |
| 56 | 4.18 | 12 | 28 | 56 | Superior frontal G |
| 3.90 | −4 | 28 | 60 | Superior frontal G | |
| 115 | 4.13 | 36 | −84 | 20 | Intraoccipital sulcus/superior occipital G |
| 39 | 4.10 | −36 | 12 | 44 | Inferior precentral S/middle frontal G |
| 77 | 3.96 | 40 | 12 | 44 | Middle frontal G |
| 3.69 | 44 | 4 | 20 | Inferior precentral S | |
| 19 | 3.72 | −12 | 36 | 20 | Anterior cingulate G |
| 16 | 3.58 | −28 | 44 | 32 | Superior frontal S/middle frontal G |
| 11 | 3.58 | 28 | −84 | −12 | Middle occipital G |
| 12 | 3.52 | 52 | −52 | 4 | Middle temporal G |
| 8 | 3.47 | −20 | −76 | 28 | Superior parietal G |
| 5 | 3.44 | −8 | −28 | 32 | Cingulate G |
| 7 | 3.39 | 12 | −40 | 72 | Paracentral lobule |
Note: PAD: patients of alcohol dependence; HC: healthy controls; G=gyrus; S=sulcus
POST-ERROR SLOWING
Compared to HC, PAD showed less activation of the right dorsolateral prefrontal cortex (x=44, y=32, z=40; Z=3.62, 14 voxels; Fig. 4) during post-SE go trials with RT increase (pSEi) contrasted with post-SE go trials without RT increase (pSEni); i.e., post-error slowing (PES). No brain regions showed greater activity during PES in PAD as compared to HC. In ROI analysis, we compared PAD and HC for pSEi>pSEni on the basis of small volume correction for the right VLPFC mask. The results showed that PAD and HC did not differ in activation for this contrast in right VLPFC.
Figure 4.

Compared to healthy control subjects, patients with alcohol dependence showed less activation of the right dorsolateral prefrontal cortex during post-error slowing. Color bar represents voxel T value. See text for details.
RISK TAKING
PAD and HC were compared for the contrast: post-go go trials without RT increase (pGni) > post-go go trials with RT increase (pGi). The results showed decreased activation in a number of cortical and subcortical structures including the putamen and insula in PAD, compared to HC (Fig. 5; Table 5). In ROI analyses, we compared PAD and HC for pGni>pGi for a mask of the amygdala and the posterior cingulate cortex (PCC). The results showed that, compared to HC, PAD demonstrated decreased activation in the amygdala (p<0.025, corrected for FWE, Z=2.64, x=−20, y=−4, z=−16) and in the PCC (p<0.030, corrected for FWE, Z=2.62, x=−8, y=−36, z=44).
Figure 5.

Compared to healthy control subjects, patients with alcohol dependence showed less activation in several cortical and subcortical structures during risk taking decision in the stop signal task. Contrast in BOLD signal was overlaid on a T1 structural image in axial sections. Orientation is neurological: R=R. These brain regions are summarized in Table 5.
Table 5.
Brain regions showing greater activity in HC, compared to PAD, during risk taking decisions
| cluster size (voxels) | voxel Z value | MNI coordinate (mm) | Identified brain region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 43 | 4.01 | 40 | −60 | 0 | middle occipital/temporal G |
| 23 | 3.84 | 32 | 40 | 24 | middle frontal G |
| 53 | 3.81 | 24 | 12 | −12 | putamen |
| 3.42 | 40 | −8 | −16 | insula | |
| 9 | 3.68 | 28 | 36 | −12 | medial orbital G |
| 19 | 3.62 | 44 | −12 | 64 | superior precentral sulcus/precentral G |
| 27 | 3.55 | 4 | −88 | 4 | cuneus/superior occipital G |
| 9 | 3.51 | −52 | −52 | −20 | middle temporal G |
| 10 | 3.49 | −52 | −24 | 44 | supramarginal G |
| 22 | 3.48 | 68 | −20 | 12 | lateral fissure/postcentral G |
| 5 | 3.38 | −28 | 24 | −12 | medial orbital G |
| 11 | 3.36 | −44 | −16 | 60 | superior precentral sulcus/precentral G |
| 6 | 3.34 | 32 | −68 | 36 | intraparietal sulcus |
| 10 | 3.33 | −4 | 16 | 40 | cingulate G/sulcus |
Note: PAD: patients of alcohol dependence; HC: healthy controls; G=gyrus
Correlation of neural measures with alcohol use in PAD
Linear regression analyses indicated that left DLPFC activity during response inhibition, right DLPFC activity during post-error slowing, amygdala or PCC/precuneus activity during risk taking, or any of the regional activities during error processing did not correlate with years of alcohol use or the total amount of alcohol use in the 90 days prior to admission in the PAD (−0.122<r's<0.025; 0.122<p's<0.571).
Comparing PAD with high and low alcohol urge rating
PAD showed a rating of 20.3 ± 15.0 (mean ± standard deviation; range: 8−50) on alcohol use urge based on the Alcohol Urge Questionnaire on the first day of admission. Alcohol urge decreased over a course of 4−5 weeks of inpatient stay. Because of varying alcohol urge rating across individuals, we normalized the ratings to individual means. Functional MR scans were performed between day 11 and 17 after admission, a period when the average relative urge was under 1.0. However, individual PAD varied in alcohol urge, with 5 PAD showing a relative urge greater than 1.0 (high urge group) and 19 PAD showing a relative urge less than 1.0 (low urge group) at the time when fMRI was conducted (1.18 ± 0.16 versus 0.86 ± 0.14, p<0.001, 2-sample t test).
We compared these two groups of PAD for the effect size of activity changes for the left DLPFC (response inhibition), right DLPFC (post-error slowing), amygdala and PCC (risk taking) that showed differential activity between PAD and HC. Because of multiple comparisons, we guarded against false positive results at an alpha of 0.012. The results showed that, compared to the low urge group, the high urge group showed significantly less activity in the right DLPFC during post-error slowing (effect size: −1.54 ± 0.91 vs. 0.45 ± 1.37, p<0.006; 2-sample t test).
Discussion
Prefrontal deficits, cognitive control and alcohol dependence
Chronic and heavy alcohol use is known to be associated with a wide range of altered cognitive and affective states (Sullivan and Pfefferbaum, 2005; Sher, 2006). A number of functional imaging studies have examined the neural processes underlying motor dysfunction (Parks et al., 2003), working memory dysfunction (Akine et al., 2007; Caldwell et al., 2005; Schweinsburg et al., 2005; Tapert et al., 2004), cue-elicited craving (Filbey et al., In Press; Grüsser et al., 2004; Heinz et al., 2004; Myrick et al., 2004; Park et al., 2007; Tapert et al., 2004a; Tapert et al., 2003; Tapert et al., 2004b; Wrase et al., 2002; Wrase et al., 2007), altered perceptual detection (Hermann et al., 2007) and affective processing (Heinz et al., 2007; Salloum et al., 2007), in alcohol dependent patients. Some have specifically addressed altered inhibitory control in these patients (Anderson et al., 2005; Karch et al., In Press; Karch et al., 2008; Schweinsburg et al., 2004). For instance, Anderson and colleagues showed that greater blood oxygenation level-dependent (BOLD) response to inhibition during a go/nogo task predicted more expectancies of cognitive and motor impairment from alcohol in adolescents who were assessed with alcohol expectancies (Anderson et al., 2005). These results suggested that decreased inhibitory control may contribute to more positive and less negative expectancies, which could eventually lead to problem drinking (Anderson et al., 2005).
The current study assessed impulse control in patients with alcohol dependence (PAD) using the stop signal task. In particular, we attempted to examine cognitive control independent of general task performance. On the basis of our previous findings from the same behavioral paradigm in healthy individuals, we sought to identify altered cerebral processes in PAD during component processes of cognitive control (Li and Sinha, 2008). Overall, our results suggested that PAD showed altered activity in a number of cortical structures during response inhibition, error processing, and post-error behavioral adjustment. In particular, the findings of decreased dorsolateral prefrontal cortical activation during response inhibition and post-error slowing are broadly in accord with previous studies demonstrating altered prefrontal cortical activity in patients with alcohol misuse (Akine et al., 2007; Bowden-Jones et al., 2005; Chanraud et al., 2007; Clark et al., 2007; Dao-Castellana et al., 1998; De Bellis et al., 2005; de Greck et al., In press; Fein et al., 2006; Goldstein et al., 2004; Heinz et al., 2007; Pfefferbaum et al., 2001; Rupp et al., 2006; Schecklmann et al., 2007; Verdejo-García et al., 2006; see also Kopelman, 2008; Sinha and Li, 2007; Scheurich, 2005; Uekermann et al., 2008 for a review). For instance, the finding of decreased left DLPFC activation during response inhibition in PAD as compared to healthy controls (HC) is consistent with an earlier report showing diminished activity in the same brain region in chronic alcoholics during performance of the Stroop test (Dao-Castellana et al., 1998). Our finding of decreased right DLPFC activity during post-error slowing is also consistent with an earlier study of alcoholic patients showing decreased bilateral DLPFC activation during a working memory task (Pfefferbaum et al., 2001).
Altered prefrontal activity has been reported in alcoholic patients in response to alcohol cues and craving (George et al., 2001; Olbrich et al., 2006; Wilson et al., 2004). For instance, PAD showed increased activity in the DLPFC and anterior thalamus in response to alcohol cues as compared to control visual cues (George et al., 2001). Furthermore, transcranial electrical stimulation of the prefrontal cortices appeared to ameliorate alcohol craving in these patients (Boggio et al., 2008). Thus, the current findings of greater changes in prefrontal cortical activity in PAD with higher alcohol urge are broadly consistent with the idea of prefrontal regulation of alcohol craving (Sinha and Li, 2007). On the other hand, craving and cognitive control represent opposing constructs in theorizing the regulation of alcohol use behavior. One possibility is that cue induced prefrontal activation represents an inflow of subcortical activity form the reward and emotion circuits rather than a signal of top-down executive control during the cue induction paradigms (Boggio et al., 2008). Thus, although the current finding of prefrontal functional changes associated with alcohol urge highlighted a crucial aspect of prefrontal functions, further studies are required to clarify the specific roles of prefrontal cortices in (the regulation of) alcohol craving.
Previous evoked potential and fMRI studies have reported disrupted error-related brain activity and connectivity associated with alcohol consumption (Holroyd and Yeung, 2003; Meda et al., In Press; Ridderinkhof et al., 2002). For instance, moderate alcohol intake is associated with diminished error-related anterior cingulate activity and failure to initiate post-error behavioral adjustment in young adult social drinkers (Ridderinkhof et al., 2002). The present findings of greater error-related activity thus appeared to characterize a complementary profile of cerebral responses in PAD during a stage of early abstinence. Taken together, a contrasting pattern of decreased prefrontal activity during response inhibition and post-error behavioral adjustment and increased frontal including anterior cingulate activity during errors described our cohort of PAD during the stop signal task. These results add to the evidence that prefrontal cortical function may play a critical role in the shaping of alcohol dependence.
Neural processes of risk taking and alcohol dependence
Compared to HC subjects, PAD showed less activation during risk-taking decisions in a number of cortical and subcortical structures. For instance, PAD showed less activation of the medial orbitofrontal cortex (mOFC), an area implicated in prediction error signaling and the detection of contingency change (Blair, 2007). Interestingly, the mOFC (in contrast to the lateral OFC) has also been shown to play a role in processing positive and rewarding information (Liu et al., 2007; Nieuwenhuis et al., 2005; O'Doherty et al., 2001). Thus, compared to HC subjects, PAD might experience post-go speeding, in contrast to post-go slowing, as a less rewarding event. Alcohol dependence is associated with a down regulation of circuit activity that is “normally” engaged when individuals partake in a risk-taking decision.
Risk taking also activates parietal cortex and the rostral anterior cingulate cortex (rACC), according to a meta-analysis of imaging studies involving decision making (Krain et al., 2006). Compared to HC subjects, PAD showed less activation of the bilateral parietal cortices and the rACC, suggesting that risk taking is a less salient event for the patients. Furthermore, our finding of decreased amygdala activity during risk taking decisions in the stop signal task is also consistent with the literature implicating this subcortical structure in alcohol misuse. For instance, alcohol abuse has also been associated with impaired amygdala processes during aversive learning (Stephens et al., In press). Postmortum studies showed altered serotonergic neurotransmission in the amygdala, suggesting dysfunctional affect regulation in chronic alcoholics (Storvik et al., 2007). Taken overall, these findings suggest that risk taking as a distinct dimension of impulse control does not evoke in PAD cerebral activity documented for healthy individuals. The stop signal task appears to be a useful proxy to examine risk related behavior as well as cognitive control.
Limitations of the study and conclusions
It is important to note a few limitations of the current study. Firstly, although they do not meet criteria of another substance use disorder, many of our PAD used cocaine or other illicit substances. Because of the moderate sample size of the current study, we did not attempt to accommodate this and other clinical factors such as history of trauma and/or mood disorders, which may impact the neural measure of impulse control. Secondly, impulse control can be addressed in a number of behavioral tasks other than the stop signal paradigm. In particular, behavioral tasks incorporating an explicit component of reward, such as the delayed discounting task, would be of tremendous value in elucidating other aspects of impulse control impairment in alcohol dependence (Bjork et al., 2004; Field et al., 2007; Mitchell et al., 2005; Petry, 2001; Petry et al., 2002; Richards et al., 1999; Takahashi et al., 2007; Vuchinich and Simpson, 1998; see also Bickel et al., 2007 for a review). Thirdly, we conducted the fMRI study during a relatively early stage of abstinence in the PAD. Although the patients were free of symptoms and signs of acute alcohol withdrawal, they might continue to experience evolution of other alcohol-related mood states such as anxiety. Thus, studies would be warranted at a later stage of abstinence to confirm the present findings. Fourthly, because of the small number of women recruited for the study, we did not compare women and men subjects in the current study. However, we have previously noted gender differences in cognitive control during the stop signal task (Li et al., 2006b). It would be important to further explore whether the gender differences in brain activation during the stop signal task manifest in relation to alcohol dependence. Finally, PAD overall did not differ from HC in behavioral measures of impulse control. Therefore, the current results did not ascertain behavioral deficits in impulse control in PAD. Studies of a greater sample size are required in the future to further pursue this issue.
Despite these limitations, the current findings are to our knowledge the first to dissect the component processes of impulse control altered in alcohol dependence within a single behavioral paradigm. We confirmed prefrontal cortical deficits during cognitive control, highlighted a contrasting pattern of error-related activations, and elucidated a distinct dimension of risk taking, which may serve as useful neural markers of alcohol dependence.
ACKNOWLEDGEMENTS
This study was supported by the Yale Interdisciplinary Women's Health Research Scholar Program on Women and Drug Abuse (Mazure), funded by the NIH Office of Research on Women's Health, the Alcoholic Beverage Medical Research Foundation (Li), Clinician Scientist K12 award in substance abuse research (Rounsaville), and NIH grants P50-DA16556 (Sinha), and R01-DA023248 (Li) to Yale University. This project was also funded in part by the State of Connecticut, Department of Mental Health and Addictions Services.
REFERENCES
- Akine Y, Kato M, Muramatsu T, Umeda S, Mimura M, Asai Y, Tanada S, Obata T, Ikehira H, Kashima H, Suhara T. Altered brain activation by a false recognition task in young abstinent patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1589–1597. doi: 10.1111/j.1530-0277.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Schweinsburg A, Paulus MP, Brown SA, Tapert S. Examining personality and alcohol expectancies using functional magnetic resonance imaging (fMRI) with adolescents. J Stud Alcohol. 2005;66:323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelson P, Tisseyre F. The time-course of preparation with regular and irregular foreperiods. Quat J Exp Psychol. 1968;20:297–300. doi: 10.1080/14640746808400165. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Dysfunctions of medial and lateral orbitofrontal cortex in psychopathy. Ann N Y Acad Sci. 2007;1121:461–479. doi: 10.1196/annals.1401.017. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Hutton S, Joyce E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci. 2005;17:417–420. doi: 10.1176/jnp.17.3.417. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-P. Region of interest analysis using an SPM toolbox; Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2−6, 2002.2002. [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacol. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Eyler LT, Drummond SP, Braun DR, Tapert SF. Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. Am J Drug Alcohol Abuse. 2007;33:13–19. doi: 10.1080/00952990601082605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, Ploetz KV, Northoff G. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics-A fMRI study. Hum Brain Mapp. doi: 10.1002/hbm.20634. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Rev Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the Taste of Alcohol Elicits Activation of the Mesocorticolimbic Neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: a cognitive-motivational theory of personality vulnerability to alcoholism. Behav Cogn Neurosci Rev. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) American Psychiatric Association; Washington DC: 1995. 1995. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Polone J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiol. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser SM, Flor H, Braus DF, Buchholz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grüsser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Klein S, Heinz A, Mann K, Braus DF. Reduced fMRI activation of an occipital area in recently detoxified alcohol-dependent patients in a visual and acoustic stimulation paradigm. Addict Biol. 2007;12:117–121. doi: 10.1111/j.1369-1600.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Alcohol and error processing. Trends Neurosci. 2003;26:402–404. doi: 10.1016/S0166-2236(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karch S, Jäger L, Karamatskos E, Graz C, Stammel A, Flatz W, Lutz J, Holtschmidt-Täschner B, Genius J, Leicht G, Pogarell O, Born C, Möller HJ, Hegerl U, Reiser M, Soyka M, Mulert C. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: simultaneous acquisition of ERPs and BOLD responses. J Psychiatr Res. 2008;42:734–745. doi: 10.1016/j.jpsychires.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kok A, Ridderinkhof KR, Ullsperger M. The control of attention and actions: Current research and future developments. Brain Res. 2006;1105:1–6. doi: 10.1016/j.brainres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Alcohol and frontal lobe impairment: fascinating findings. Addiction. 2008;103:736–737. doi: 10.1111/j.1360-0443.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1970;49:467–477. [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop signal task – neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006a;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition in a stop signal task. NeuroImage. 2006b;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Krystal JH, Mathalon DH. Fore-period effect and stop signal processing time. Exp Brain Res. 2005c;167:305–309. doi: 10.1007/s00221-005-0110-2. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Sinha R. Frontolimbic dysfunctions in patients with cocaine dependence – neuroimaging evidence and a cognitive analytic perspective. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing in a stop signal task. J Cognit Neurosci. 2008a;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Chao HH-A, Sinha R, Paliwal P, Constable RT, Lee TW, Zhang S. Error-specific medial cortical and subcortical activity during the stop signal task – a functional magnetic resonance imaging study. Neuroscience. 2008b;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Sinha R, Lee T-W. The subcortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008c;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Chao HH-A, Lee TW. The neural correlates of speeded compared to delayed responses in a stop signal task: an indirect analogue of risk taking and association with an anxiety trait. Cerebral Cortex. doi: 10.1093/cercor/bhn132. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. In: Inhibitory Processes in Attention, Memory and Language. Dagenbach D, Carr TH, editors. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Meda SA, Calhoun VD, Astur RS, Turner BM, Ruopp K, Pearlson GD. Alcohol dose effects on brain circuits during simulated driving: An fMRI study. Hum Brain Mapp. doi: 10.1002/hbm.20591. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Rogier BM, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. NeuroImage. 2005;25:1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Aust N Z J Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Parks MH, Morgan VL, Pickens DR, Price RR, Dietrich MS, Nickel MK, Martin PR. Brain fMRI activation associated with self-paced finger tapping in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2003;27:704–711. doi: 10.1097/01.ALC.0000062759.14944.CF. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Rabbit PMA. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Drexler A, Hausmann A, Hinterhuber H, Kurz M. Executive function and memory in relation to olfactory deficits in alcohol-dependent patients. Alcohol Clin Exp Res. 2006;30:1355–1362. doi: 10.1111/j.1530-0277.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, Hommer DW. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Ehlis AC, Plichta MM, Boutter HK, Metzger FG, Fallgatter AJ. Altered frontal brain oxygenation in detoxified alcohol dependent patients with unaffected verbal fluency performance. Psychiatry Res. 2007;156:129–138. doi: 10.1016/j.pscychresns.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Scheurich A. Neuropsychological functioning and alcohol dependence. Curr Opin Psychiatry. 2005;18:319–323. doi: 10.1097/01.yco.0000165602.36671.de. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L. Functional magnetic resonance imaging in studies of neurocognitive effects of alcohol use on adolescents and young adults. Int J Adolesc Med Health. 2006;18:3–7. doi: 10.1515/ijamh.2006.18.1.3. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Anterior cingulate activation in aggressive children and adolescents during affective stimulation: Association with temperament traits. J Psychiatr Res. doi: 10.1016/j.jpsychires.2006.01.006. In Press. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. doi: 10.1098/rstb.2008.0097. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storvik M, Tiihonen J, Haukijärvi T, Tupala E. Amygdala serotonin transporters in alcoholics measured by whole hemisphere autoradiography. Synapse. 2007;61:629–636. doi: 10.1002/syn.20420. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Furukawa A, Miyakawa T, Maesato H, Higuchi S. Two-month stability of hyperbolic discount rates for delayed monetary gains in abstinent inpatient alcoholics. Neuroendocrinol Lett. 2007;28:131–136. [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004a;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004b;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tseng YC, Li C-SR. The effects of response readiness and error monitoring on saccade countermanding. The Open Psychol J. 2008;1:18–25. [Google Scholar]
- Uekermann J, Daum I. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat. Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Woodrow H. The measurement of attention. Psychol Monogr. 1914;17:1–158. [Google Scholar]
- Wrase J, Grüsser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Beck A, Ströhle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]