Abstract
Gamma-aminobutyric acid A (GABAA) receptors are ligand-gated ion channels responsible for mediation of fast inhibitory action of GABA in the brain. Preliminary reports have demonstrated altered expression of GABA receptors in the brains of subjects with autism suggesting GABA/glutamate system dysregulation. We investigated the expression of four GABAA receptor subunits and observed significant reductions in GABRA1, GABRA2, GABRA3, and GABRB3 in parietal cortex (Brodmann's Area 40(BA40)), while GABRA1 and GABRB3 were significantly altered in cerebellum, and GABRA1 was significantly altered in superior frontal cortex (BA9). The presence of seizure disorder did not have significant impact on GABAA receptor subunit expression in the three brain areas. Our results demonstrate that GABAA receptors are reduced in three brain regions that have previously been implicated in the pathogenesis of autism, suggesting widespread GABAergic dysfunction in the brains of subjects with autism.
Keywords: GABRA1, GABRA2, GABRA3, GABRB3, autism, brain
Autism is a severe neurodevelopmental disorder characterized by social deficits, language abnormalities and repetitive behavior (APA, 1994). A limited number of reports have demonstrated abnormalities involving the glutamate and GABAergic systems in brain, blood, and platelets of subjects with autism (Blatt et al., 2001; Dhossche et al., 2002; Fatemi, 2008). Our laboratory has demonstrated that brain levels of glutamic acid decarboxylase 65 and 67kDa proteins (GAD65/67), the rate limiting enzyme responsible for normal conversion of glutamate to GABA in the brain, were significantly decreased in cerebellum (GAD65) and parietal cortex (GAD67) (Fatemi et al., 2002a). Yip et al. (2007) similarly reported a significant decrease in GAD67 mRNA in autistic cerebellum, thereby confirming our early findings (Fatemi et al., 2002a).
GABAA receptors are ligand-gated ion channels responsible for mediation of fast inhibitory action of GABA in the brain (Brandon et al., 2000). GABAA receptors are also the sites for clinical action of benzodiazepines, barbiturates, and anesthetics. GABAA receptors are divided into multiple subunits, e.g., α1-α6, β1-β4, γ1- γ4, δ, ε, π, θ, and ρ1-ρ2, producing multiple GABAA receptor isoforms (Ma et al., 2005; Brandon et al., 2000). GABAA receptor expression varies according to developmental timetables of the brain, suggesting different roles for different types of receptors. Mutations of various subunits cause various phenotypes, e.g., β3 gene knockouts produce a model of Angelman syndrome (Homanics et al., 1997).
Separate studies by Blatt et al. (2001) and Samaco et al. (2005) have shown a significant decrease in GABAA receptor binding sites (3H-flunitrazepam-labeled benzodiazepine binding sites and 3H-muscimol-labeled binding sites) and GABAA receptor β3 (GABRB3) subunit protein level, respectively, in brain tissues of subjects with autism when compared with controls. Recently, Guptill et al. (2007) extended the earlier work by Blatt et al. (2001) to show that the decrease in 3H-flunitrazepam-labeled benzodiazepine binding sites was due to a decrease in binding site number (Bmax) rather than altered affinity to ligand binding (Kd). In contrast, Purcell et al. (2001) showed increases in GABAA protein and GABAA receptor α5 mRNA levels in brains of subjects with autism. GABAA receptor α2 (GABRA2) has been linked with autism, based on the genotype-pedigree disequilibrium test (Ma et al., 2005) and a case study of an autistic patient, which revealed three copies of the GABRA2 gene (Kakinuma et al., 2007). While no previous reports have linked GABAA receptor α1 (GABRA1) or GABAA receptor α3 (GABRA3) to autism, both have been linked to schizophrenia (Ohnuma et al., 1999; Hakak et al., 2001; Inada et al., 2008), and it has previously been demonstrated that a number of genes, e.g., glutamic acid decarboxylase 1 and 2, are associated with multiple disorders (Akbarian et al., 1995; Guidotti et al., 2000; Fatemi et al., 2002a, 2005). Thus, these GABAA receptors may vary differentially in autism.
Here, we extend our previous investigations of the GABAergic system in autism to measure expression of GABAA subunits in a well-characterized group of age, sex, and postmortem-interval (PMI) matched brain samples from subjects with autism and matched controls in three brain areas that are involved in the pathology of autism: parietal cortex (Brodmann's area 40 (BA40)), superior frontal cortex (BA9), and cerebellum.
Methods
Tissue Preparation
All experimental procedures were approved by the Institutional Review Board of the University of Minnesota School of Medicine. Postmortem blocks of parietal cortex (Brodmann's BA40), superior frontal cortex (BA9), and cerebellum (lobar origin unknown) were obtained from the Autism Research Foundation and various brain banks (NICHD Brain and Tissue Bank for Developmental Disorders; TARF; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program). These samples, which have been used by our laboratory previously, are some of the most well-characterized and most-studied brain collections used by multiple groups (for review, see Palmen et al., 2004). Before being frozen, donated brains were sectioned in half, dissected by anatomists and placed in labeled bags. All samples were stored at −80°C until use. Samples were derived from three groups of subjects (cerebellum: N=5−7 from subjects with autism, N=7−9 from control subjects; BA9: N=4−6 from subjects with autism, N=3 from control subjects; BA40: N=6−8 from subjects with autism, N=5−6 from control subjects). Consent from next of kin was given to the respective institutions. DSM-IV diagnoses were established prior to death by neurologists and psychiatrists using information from all available medical records and from family interviews. Details regarding the subject selection, diagnostic process, and tissue processing were collected by the Autism Research Foundation. Samples were matched for age, gender, and postmortem interval (PMI). All demographic information is listed in Table 1. Seven out of nine subjects with autism had seizure disorder, and all subjects with autism displayed varying degrees of mental retardation (personal communication from Dr. Margaret Bauman). None of the controls had any known history of neuropsychiatric disorders, seizure disorder, or mental retardation.
Table 1.
Demographic Data for Subjects with Autism and Controls
| Case | Dx | Sex | Age | PMI (Hrs.) | Ethnicity | Medication History | Cause of Death | MR* | Seizure* | Brain Areas |
|---|---|---|---|---|---|---|---|---|---|---|
| B1078 | Autistic | M | 22 | 14.3 | Caucasian | Dilantin, Tegretol, Phenobarbital, Theodure | Asphyxia | Yes | Yes | A40 |
| B1045 | Autistic | M | 28 | 16.3 | Caucasian | Cefobid, Urecholine, Duracef | Cardiac arrest | Yes | Yes | Cer, A40 |
| B5000 | Autistic | M | 27 | 8.3 | Caucasian | Synthroid | Drowning | Yes | No | Cer |
| B1401 | Autistic | F | 21 | 20.6 | Caucasian | Tetracycline | Pneumonia, sepsis | Yes | Yes | Cer, A9, A40 |
| B1664 | Autistic | M | 20 | 15 | Caucasian | Vitamins B, C | Perforation of ulcer; asphyxia | Yes | Yes | Cer, A9, A40 |
| B2825 | Autistic | M | 19 | 9.5 | Caucasian | None | Seizure | Yes | Yes | Cer, A9, A40 |
| B3511 | Autistic | M | 29 | 15 | Caucasian | None | Hit by train | Yes | Yes | Cer, A9, A40 |
| B3845 | Autistic | M | 30 | 28.4 | Caucasian | Mellaril, Phenobarbital, Dilantin | Shock; acute pancreatitis | Yes | Yes | A9, A40 |
| B1484 | Autistic | M | 19 | 15 | Caucasian | None | Burns | Yes | No | A9, A40 |
| B3829 | Control | M | 22 | 24.3 | Caucasian | None | MVA | No | No | Cer |
| B4267 | Control | M | 26 | 20 | African-American | None | MVA | No | No | Cer |
| B4268 | Control | M | 30 | 22 | African-American | None | Cardiomyopathy | No | No | Cer, A40 |
| B4269 | Control | M | 28 | 24 | Caucasian | Lidocaine 12.0 mg/L found in blood | Areteriosclerotic cardiovascular disease | No | No | Cer, A9, A40 |
| B4272 | Control | M | 19 | 17 | Caucasian | None | Accident; chest injuries | No | No | Cer |
| B4275 | Control | M | 20 | 16 | Caucasian | None | Accident | No | No | Cer, A9, A40 |
| B4279 | Control | F | 20 | 21 | Caucasian | None | MVA | No | No | Cer |
| B4362 | Control | M | 30 | 20 | African-American | None | MVA | No | No | Cer, A9 |
| B4101 | Control | M | 24 | 5 | Unknown | None | Gun shot wound | No | No | Cer, A40 |
| B4271 | Control | M | 19 | 21 | African-American | EtOH, Advil, Amoxapine | Epiglottitis | No | No | A40 |
| B4756 | Control | M | 56 | 23 | Unknown | None | Myocardial infarction | No | No | Cer |
| B4363 | Control | M | 21 | 9 | Caucasian | None | MVA | No | No | Cer, A40 |
Dx, diagnosis; Hrs, hours; PMI, postmortem interval; M, male; F, female; EtOH, alcohol; MVA, motor vehicle accident; MR, Mental retardation
Communication from Dr. M. Bauman.
SDS-PAGE and Western Blotting
Brain tissue (∼40 mg per subject) was cut and placed on ice in lysis buffer (3 μl/mg of tissue) [20mM Tris pH 8.0, 0.2 mM EDTA, 150 mM NaCl, 3% Igepal.NP40 (v/v), 1% sodium deoxycholate (w/v), 0.1% SDS (w/v), 50 μl/ml leupeptin, 0.2 mM PMSF, 1 mM sodium orthovanadate, and aprotinin (Sigma, St. Louis, MO; A6279, 30μl/ml buffer)]. Tissue samples were homogenized using a Kontes hand pestle (Kimble-Kontes, Vineland, NJ, USA) while the temperature was maintained at 4°C. Following homogenization, an additional 1 μl of PMSF (0.2 mM) was added to each sample, and the samples were incubated on ice for 30 min. The homogenates were centrifuged for 20 min at 10,000 × g at 4°C. Supernatants were collected and assayed for total protein using the Bradford method (BioRad, Richmond, CA). Samples were stored at −86°C until used. Samples were mixed with denaturing SDS sample buffer (20% glycerol, 100 mM Tris pH 6.8, 0.05% w/v Bromophenol blue, 2.5% SDS (w/v), 5% β-mercaptoethanol) and denatured by heating at 100°C for 5 minutes. SDS polyacrylamide gels were prepared with standard Laemmli solutions (BioRad) (resolving: 10%, stacking: 5%). Sixty μg of protein per lane was loaded onto the gel and electrophoresed for 15 min at 75V followed by 55 min at 150V at room temperature (RT). The proteins were electrotransferred onto nitrocellulose membranes for 2 hr at 300mAmp at 4°C. Protein blots were blocked with 0.2% I-Block (Tropix, Bedford, MA, USA) in PBS with 0.3% Tween 20 for 1hr at RT. The blots were then incubated with anti-GABAA receptor alpha 1 (GABRA1) (06−868, Upstate Biotechnology Inc., (Lake Placid, NY) 1:2,000), anti-GABAA receptor alpha 2 (GABRA2) (GAA21-P, Alpha Diagnostic International Inc. (San Antonio, TX) 1:1,000), anti-GABAA receptor alpha 3 (GABRA3) (GAA31-P, Alpha Diagnostic International Inc. (San Antonio, TX) 1:1,000), or anti-GABAA receptor beta 3 (GABRB3) (NB300−199, Novus Biologicals (Littleton, CO) 1:1,000), for 20 hr at 4°C. Blots were subsequently washed with 0.3% Tween-PBS for 30 minutes, then incubated in secondary antibody for 1 hr at RT (A-9169, Sigma, goat anti-rabbit IgG 1:80,000). Blots were washed twice for 15 minutes with 0.3% Tween-PBS. The immune complexes were visualized using the ECL Plus detection system (Amersham Pharmacia Biotech, Arlington Hts., IL) and exposed to Hyperfilm ECL (Amersham Pharmacia Biotech). Sample densities were analyzed blind to nature of diagnosis using a BioRad densitometer and the BioRad Multi Analyst software. The molecular weights of approximately 56 kDa (GABRB3), 55 kDa (GABRA3), and 51 kDa (GABRA1 and GABRA2) immunoreactive bands were quantified with background subtraction. Results obtained are based on at least two independent experiments.
Statistical Analysis
All protein measurements for subjects with autism and control subjects were normalized against β-actin (Table 2). Possible confounding variables were compared between controls and autistics. Neither age nor PMI were found to be statistically different between groups (t(40)=0.94, P=0.35, t(40)=0.38, P=0.71, respectively) with effect sizes for the differences of 0.33 or smaller. We also examined gender and found no significant difference between the two groups (chi-square=0.91, P=0.34). Given this overall lack of difference on potential confounds, we chose to conduct group comparisons (independent group t-tests) without covariates. Significance criteria was set at P<0.05, and all tests were two-tailed. There was a significant difference on seizure status between groups. None of the controls had seizures whereas 75% of the subjects with autism did (chi-square=28.6, P<0.001). Therefore, we conducted a second analysis examining subjects with autism comorbid with seizure disorder vs. controls using independent group t-tests. Significance criteria was again set at P<0.05, and all tests were two-tailed.
Table 2.
Expression of GABRA1-GABRA3 and GABRB3 in Cerebellum, BA40, and BA9 in Subjects with Autism vs. Controls
| Cerebellum | Control | Autistic | Change | P* |
|---|---|---|---|---|
| GABRA1 / β-Actin | 1.350 ± 0.470 | 0.504 ± 0.356 | ↓ 63% | 0.007 |
| GABRA2 / β-Actin | 0.073 ± 0.028 | 0.053 ± 0.025 | ↓ 27% | 0.21 |
| GABRA3 / β-Actin | 0.216 ± 0.100 | 0.142 ± 0.073 | ↓ 34% | 0.17 |
| GABRB3 / β-Actin | 0.061 ± 0.022 | 0.030 ± 0.013 | ↓ 51% | 0.008 |
| BA40 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABRA1 / β-Actin | 0.955 ± 0.305 | 0.458 ± 0.266 | ↓ 52% | 0.018 |
| GABRA2 / β-Actin | 0.214 ± 0.054 | 0.131 ± 0.060 | ↓ 39% | 0.033 |
| GABRA3 / β-Actin | 0.166 ± 0.039 | 0.072 ± 0.049 | ↓ 57% | 0.005 |
| GABRB3 / β-Actin | 0.039 ± 0.011 | 0.024 ± 0.006 | ↓ 38% | 0.006 |
| BA9 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABRA1 / β-Actin | 0.260 ± 0.050 | 0.090 ± 0.062 | ↓ 65% | 0.012 |
| GABRA2 / β-Actin | 0.159 ± 0.041 | 0.092 ± 0.042 | ↓ 42% | 0.057 |
| GABRA3 / β-Actin | 0.127 ± 0.006 | 0.193 ± 0.083 | ↑ 52% | 0.236 |
| GABRB3 / β-Actin | 0.040 ± 0.002 | 0.041 ± 0.009 | ↑ 2.5% | 0.853 |
two-tailed independent group t-test
Results
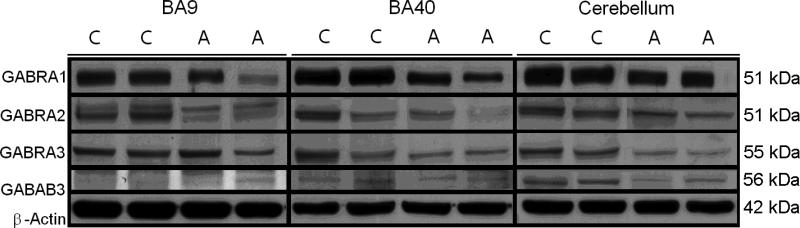
All GABAA western-blotting experiments were normalized against β-actin and are shown as ratios of the various GABAA subunits to β-actin. GABRA1 and GABRB3 were decreased in cerebella obtained from subjects with autism vs. matched controls: GABRA1 (51 kDa) was decreased by 63%, P<0.007; and GABRB3 (56 kDa) was decreased by 51%, P<0.008 (Figure 1, Table 2).
Figure 1.
Representative samples of GABRA1 (51 kDa), GABRA2 (51 kDa), GABRA3 (55 kDa), GABRB3 (56 kDa), and β-Actin (42 kDa) in BA9, BA40, and cerebellum of subjects with autism (A) and matched controls (C).
In BA40, we found all GABAA subunits significantly decreased in subjects with autism when compared with controls. GABRA1 was decreased by 50%, P<0.018; GABRA2 (51 kDa) was decreased by 39%, P<0.033; GABRA3 (55 kDa) was decreased by 57%, P<0.005; and GABRB3 was decreased by 52%, P<0.006 (Figure 1, Table 2). In contrast, only GABRA1 was reduced significantly in BA9 (65%, P<0.012) (Figure 1, Table 2).
There was a significant difference on seizure status between subjects with autism and controls (chi-square=28.6, P<0.001). However, presence of seizure disorder in subjects with autism did not have an impact on the observed significant reductions in protein levels of GABAA receptor subunits (Table 3). Subjects with autism and seizure disorder showed significant reductions in protein levels of GABRA1 (63%, P<0.007) and GABRB3 (51%, P<0.015) in the cerebellum (Table 3) when compared to controls. In BA40, there were significant reductions in protein levels of GABRA1 (52%, P<0.018), GABRA2 (45%, P<0.017), GABRA3 (56%, P<0.010), and GABRB3 (38%, P<0.009) in subjects with autism and seizure disorder when compared to controls (Table 3). Finally, in BA9, there was a significant reduction in GABRA1 protein (65%, P<0.012) in subjects with autism and seizure disorder when compared to controls (Table 3).
Table 3.
The Impact of Seizure on Expression of GABRA1-GABRA3 and GABRB3 in Cerebellum, BA40, and BA9 in Subjects with Autism vs. Controls
| Cerebellum | Control | Autistic | Change | P* |
|---|---|---|---|---|
| GABRA1 / β-Actin | 1.350 ± 0.470 | 0.504 ± 0.356 | ↓ 63% | 0.007 |
| GABRA2 / β-Actin | 0.073 ± 0.028 | 0.052 ± 0.029 | ↓ 29% | 0.240 |
| GABRA3 / β-Actin | 0.216 ± 0.100 | 0.138 ± 0.083 | ↓ 36% | 0.200 |
| GABRB3 / β-Actin | 0.061 ± 0.022 | 0.030 ± 0.014 | ↓ 51% | 0.015 |
| BA40 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABRA1 / β-Actin | 0.955 ± 0.305 | 0.458 ± 0.266 | ↓ 52% | 0.018 |
| GABRA2 / β-Actin | 0.214 ± 0.054 | 0.118 ± 0.055 | ↓ 45% | 0.017 |
| GABRA3 / β-Actin | 0.166 ± 0.039 | 0.073 ± 0.053 | ↓ 56% | 0.010 |
| GABRB3 / β-Actin | 0.039 ± 0.011 | 0.024 ± 0.006 | ↓ 38% | 0.009 |
| BA9 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABRA1 / β-Actin | 0.260 ± 0.050 | 0.090 ± 0.062 | ↓ 65% | 0.012 |
| GABRA2 / β-Actin | 0.159 ± 0.041 | 0.096 ± 0.045 | ↓ 40% | 0.098 |
| GABRA3 / β-Actin | 0.127 ± 0.006 | 0.193 ± 0.083 | ↑ 52% | 0.236 |
| GABRB3 / β-Actin | 0.040 ± 0.002 | 0.043 ± 0.008 | ↑ 7.5% | 0.597 |
two-tailed independent group t-test
Discussion
We observed that GABRA1−3 and GABRB3 were significantly reduced in BA40, while GABRA1 and GABRB3 were significantly reduced in cerebellum, and GABRA1 was significantly reduced in BA9 of subjects with autism. These results were specific for GABAA subunits, as β-actin was unchanged. While there was a significant difference between subjects with autism and control subjects regarding presence of seizure, it did not impact the observed reductions of GABAA subunits in the three brain areas studied. All subjects with autism had varying degrees of mental retardation, which may also have had an impact on levels of GABAA subunits.
Neuroanatomical studies have revealed structural abnormalities throughout the brain of subjects with autism including frontal (BA9) and parietal (BA40) cortices and cerebellum (for review, see Bauman and Kemper, 1994, 2005). Cerebellar structural abnormalities include loss of granular and Purkinje cells (Ritvo et al., 1986; Bauman and Kemper, 1994) and atrophy of Purkinje cells (Fatemi et al., 2000, 2002b). The cerebellar abnormalities may be responsible for the dysfunctions within the motor system associated with autism (reviewed in Nayate et al., 2005). Several studies also indicate that the parietal cortex may be abnormal in autism (Courchesne et al., 1993; Saitoh and Courchesne, 1998). Courchesne et al. (1993) reported on the reduction in volumes of the parietal lobes in some autistic subjects. Abnormalities of the parietal cortex in autism may be associated with disturbances of visuospatial-integration, impaired language, and slowed attention shift between and within modalities (Townsend et al., 1996; Haas et al., 1996). Abnormalities of the frontal cortex, including early growth abnormalities (Carper et al., 2002; Carper and Courchesne, 2005) and minicolumn maldevelopment (Casanova et al., 2002; Buxhoeveden et al., 2004), are likely to contribute to the serious deficiencies in cognition, language, and emotional functions associated with autism (reviewed in Courchesne and Pierce, 2005). Our results demonstrate reductions in GABAA subunits in these three regions, suggesting widespread GABAergic dysfunction in the brains of individuals with autism. Interestingly, GABRB3 knockout mice display significant decreases in surface area of cerebellar vermal lobules II - VII compared to control mice (Delorey et al., 2008). It may be that the observed changes in GABAA subunits may contribute to similar gross abnormalities in subjects with autism.
While there is evidence of a strong genetic component to autism (Persico et al., 2006; Steffenburg et al., 1989), to date there have not been consistent findings for any specific gene. However, abnormalities in the 15q11-q13 locus are present in 1−4% of subjects with autism (McCauley et al., 2004; Schroer et al., 1998). There are several potential gene targets in this locus, including the GABAA receptor gene cluster, which consists of three genes for the receptor's subunits: GABRB3, GABRA5, and GABRG3. Investigations of these genes has yielded mixed results, although GABRB3 seems to yield the most potential. While an association with a GABRB3 has been demonstrated in the Korean population (Kim et al., 2006), no association was found in the Japanese population (Tochigi et al., 2007). It has previously been shown that mice that are deficient in GABRB3 display epilepsy, as well as learning and memory deficits (DeLorey et al., 1998, 2008). Samaco et al. (2005) have demonstrated reduced GABRB3 expression in BA9 (Samaco et al., 2005). Samaco et al. (2005) also observed reductions in GABRB3 protein in mice deficient for MECP2, which codes methyl-CpG-binding protein 2 (MeCP2). This protein acts as a transcriptional repressor for methylated gene constructs, suggesting that epigenetic changes could lead to a reduction in GABRB3 (Samaco et al., 2005). More recently, Hogart et al. (2007) observed that while GABRB3 is normally biallelically expressed, in some samples from subjects with autism, GABRB3 was monoallelically expressed, and this correlated with a reduction of GABRB3 protein (Hogart et al., 2007). Moreover, chromatin immunopercipitation experiments revealed two locations within the 5’ end of the GABRB3 gene, where MeCP2 binds and these regions are methylated, suggesting that MeCP2 is a positive regulator of GABRB3 (Hogart et al., 2007). Taken together, these results suggest epigenetic dysregulation for GABRB3 in subjects with autism. Further studies are required to investigate possible epigenetic regulation of GABAA receptor expression outside of the 15q11-q13 locus. While we did not observe decreased GABRB3 in BA9, we did observe reductions in BA40 and cerebellum, again suggesting a potential role for this subunit in the etiology and pathogenesis of autism.
The occurrence of seizure disorders with autism has been estimated anywhere from 4% to 44% (Tuchman and Rapin, 2002). This wide range is thought to be due to the heterogeneity of clinical populations (Canitano, 2007). Eplieptiform activity interferes with cognition by causing disturbances of vigilance, shifting attention, and sudden language difficulties (Binnie, 1993); phenomena that may also occur in children with autism and epilepsy. Alterations in all GABA receptors may partially explain the seizure disorders associated with autism. One of our subjects with autism died from seizures, and, in total, seven of our subjects with autism were comorbid with seizure disorders (Table 1). Whether the reduction in GABAA subunits is due to seizure or whether reduced levels of GABAA subunits contribute to seizure is not yet known. Future studies should include additional brain areas associated with seizure disorder, including the thalamus.
There are multiple ways in which the observed reductions GABAA receptor protein may have occurred: 1) Reduction in GABAA receptor mRNA, resulting in reduction of GABAA receptor protein; 2) Posttranslational modification, leading to a reduction in GABAA receptor protein; or 3) Epigenetics. It has been demonstrated that Reelin expression is reduced following hypermethylation of the Reln gene promoter (Abdolmaleky et al., 2005; Grayson et al., 2005, 2006). As described above, there is strong evidence of epigenetic control of GABRB3 expression in autism (Samaco et al., 2005; Hogart et al., 2007), and it is possible that other epigenetic mechanisms may play a role in expression of GABAA subunit genes.
Our results are the first to demonstrate systematic changes in GABAA subunit expression in superior frontal cortex, parietal cortex, and cerebellum of subjects with autism. Limitations of the study include: 1) Limited sample size; 2) Presence of mental retardation in subjects with autism; 3) Presence of seizure disorder in the majority of subjects with autism; 4) Lack of adequate samples from younger patients; and 5) Heterogeneity of samples, indicating presence of different biological etiologies for various subtypes of autism. Future studies should expand the number of brain regions to include other areas involved in the pathology of autism, including hippocampus and amygdala, use of techniques such as qRT-PCR to verify our results, and include data from children with autism and matched controls in order to account for neuroanatomical changes in childhood that are associated with autism.
Acknowledgments
Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program and is gratefully acknowledged. Grant support by National Institute of Child Health and Human Development (#5R01HD052074−01A2) to SHF is gratefully acknowledged.
Footnotes
There have been no changes in any of the author affiliations subsequent to the time of the study.
References
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. APA Press; Washington, DC: 1994. [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism. In: Bauman MM, Kemper T, editors. The neurobiology of autism. Johns Hopkins University Press; Baltimore, MD: 1994. pp. 19–145. [Google Scholar]
- Bauman ML, Kemper TL. Structural Brain Anatomy in Autism: What is the Evidence? In: Bauman M, Kemper T, editors. The neurobiology of autism. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 121–135. [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. Journal of Autism and Developmental Disorders. 2001;31:537–544. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptors structure and function. In: Martin D, Olsen R, editors. GABA in the nervous system: the view at fifty years. Lippincott, Williams and Wilkins; Philadelphia, PA: 2000. pp. 233–244. [Google Scholar]
- Brandon NJ, Smart TG, Moss SJ. Regulation of GABAA Receptors by protein phosphorylation. In: Martin D, Olsen R, editors. GABA in the nervous system: the view at fifty years. Lippincott, Williams and Wilkins; Philadelphia, PA: 2000. pp. 191–206. [Google Scholar]
- Brucato FH, Levin ED, Mott DD, Lewis DV, Wilson WA, Swartzwelder HS. Hippocampal long-term potentiation and spatial learning in the rat: effects of GABAB receptor blockade. Neuroscience. 1996;74:331–339. doi: 10.1016/0306-4522(96)00131-5. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Semendeferi K, Schenker N, Courchesne E. Decreased cell column spacing in autism.. Program and abstracts of the Society for Neuroscience 34th Annual Meeting; San Diego, California. 2004. Abstract 582.6. [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Carper R, Courchesne E. Localized enlargement of the frontal lobe in autism. Biological Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Press GA, Young-Courchesne R. Parietal lobe abnormalities detected with MR in patients with infantile autism. American Journal of Roentgenology. 1993;160:387–393. doi: 10.2214/ajr.160.2.8424359. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta 3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. Journal of Neuroscience. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behavioural Brain Research. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, Martinez J. Elevated plasma gama-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for GABA hypothesis of autism. Medical Science Monitor. 2002;8:PR1–PR6. [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Earle J, Kist DA, Realmuto GR, Thuras PD, Merz A. Reduced Purkinje cell size in autistic cerebellum. Biological Psychiatry. 2000;47:S128. [Google Scholar]
- Fatemi SH, Halt A, Stary J, Kanodia R, Schulz SC, Realmuto G. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in parietal and cerebellar cortices of autistic subjects. Biological Psychiatry. 2002a;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, Merz A. Purkinje cell size is reduced in cerebellum of patients with autism. Cellular and Molecular Neurobiology. 2002b;22:171–175. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophrenia Research. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. The hyperglutamatergic hypothesis of autism. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008 doi: 10.1016/j.pnpbp.2007.11.004. In Press. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, Kundakovic M, Sharma RP. The human reelin gene: transcription factors (+), repressors (-) and the methylation switch (+/−) in schizophrenia. Pharmacology and Therapeutics. 2006;111:272–86. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E, DiGiorgi Gerevini V. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of General Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Haas RH, Townsend J, Courchesne E, Lincoln AJ, Schreibman L, Young-Courchesne R. Neurologic abnormalities in infantile autism. Journal of Child Neurology. 1996;11:84–92. doi: 10.1177/088307389601100204. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11−13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Human Molecular Genetics. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CE, Korpi ER, Makela R, Brilliant MH, Hagiwara N, Ferguson C, Snyder K, Olsen RW. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proceedings of the National Academy of Sciences of the USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Koga M, Ishiguro H, Horiuchi Y, Syu A, Yoshio T, Takahashi N, Ozaki N, Arinami T. Pathway-based association analysis of genome-wide screening data suggest that genes associated with the gamma-aminobutyric acid receptor signaling pathway are involved in neuroleptic-induced, treatment-resistant tardive dyskinesia. Pharmacogenetics and Genomics. 2008;18:317–323. doi: 10.1097/FPC.0b013e3282f70492. [DOI] [PubMed] [Google Scholar]
- Kakinuma H, Ozaki M, Sato H, Takahashi H. Variation in GABA-A subunit gene copy number in an autistic patient with mosaic 4p duplication. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007 doi: 10.1002/ajmg.b.30663. In Press. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kim JH, Park M, Cho IH, Yoo HJ. Association of GABRB3 polymorphisms with autism spectrum disorders in Korean trios. Neuropsychobiology. 2006;54:160–165. doi: 10.1159/000098651. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. American Journal of Human Genetics. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2004;131:51–59. doi: 10.1002/ajmg.b.30038. [DOI] [PubMed] [Google Scholar]
- Nayate A, Bradshaw JL, Rinehart NJ. Autism and Asperger's disorder: are they movement disorders involving the cerebellum and/or basal ganglia? Brain Research Bulletin. 2005;67:327–334. doi: 10.1016/j.brainresbull.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends in Neurosciences. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, Ritvo A. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC autopsy research repot. American Journal of Psychiatry. 1986;143:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Courchesne E. Magnetic resonance imaging study of the brain in autism. Psychiatry and Clinical Neurosciences. 1998;52:S219–S222. doi: 10.1111/j.1440-1819.1998.tb03226.x. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Human Molecular Genetics. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer RJ, Phelan MC, Michaelis RC, Crawford EC, Skinner SA, Cuccaro M, Simensen RJ, Bishop J, Skinner C, Fender D, Stevenson RE. Autism and maternally derived aberrations of chromosome 15q. American Journal of Medical Genetics. 1998;76:327–336. doi: 10.1002/(sici)1096-8628(19980401)76:4<327::aid-ajmg8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Kato C, Koishi S, Kawakubo Y, Yamamoto K, Matsumoto H, Hashimoto O, Kim SY, Watanabe K, Kano Y, Nanba E, Kato N, Sasaki T. No evidence for significant association between GABA receptor genes in chromosome. Journal of Human Genetics. 2007;52:985–989. doi: 10.1007/s10038-007-0207-5. [DOI] [PubMed] [Google Scholar]
- Townsend J, Harris DL, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. Journal of the International Neuropsychological Society. 1996;2:541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathologica. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]