Abstract
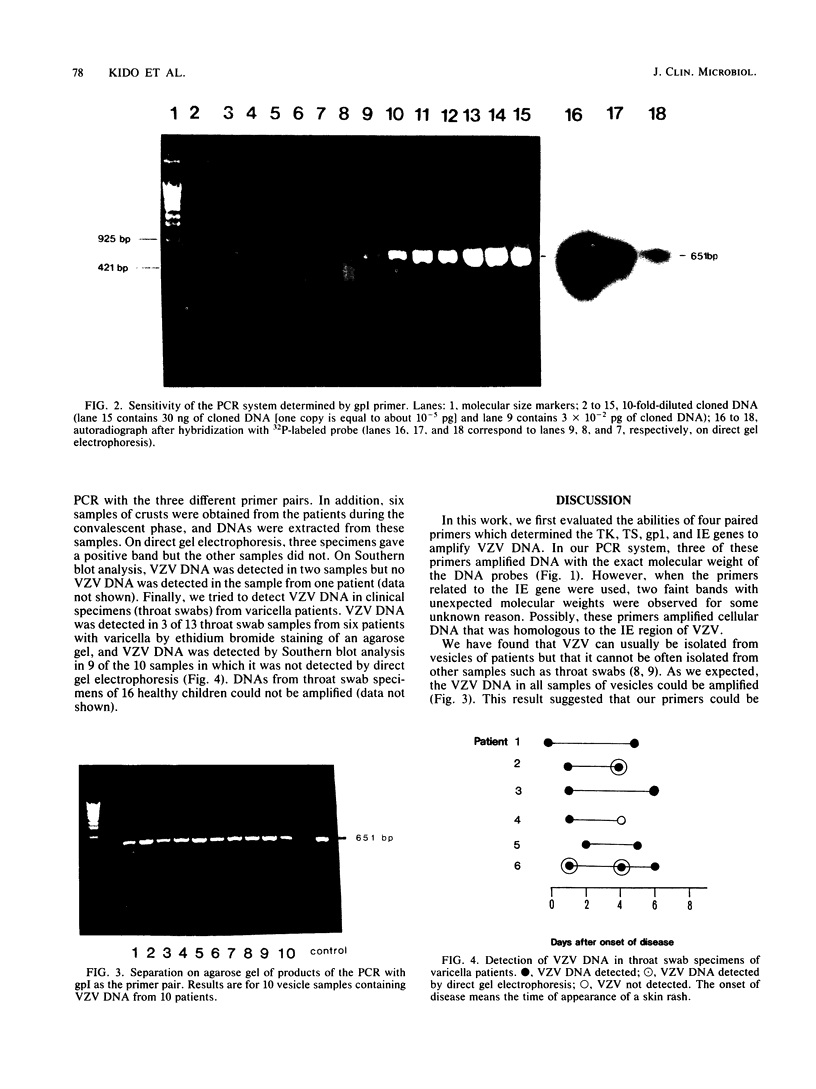
A polymerase chain reaction system for the detection of varicella-zoster virus was established. Of 25 nucleotides, 4 oligonucleotide pairs (regions of thymidine kinase, thymidylate synthetase, glycoprotein I, and immediate early gene) were synthesized. The first three oligonucleotide pairs could be used as primers on the basis of specific DNA amplification. Varicella-zoster virus DNA was amplified by this polymerase chain reaction system in 20 of 20 vesicle samples, 5 of 6 crusts, and 12 of 13 throat swabs collected from patients with clinical varicella.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Itakura N., Hiroishi Y., Hirose S., Nagai T., Ozaki T., Yazaki T., Yamanishi K., Takahashi M. Viremia is present in incubation period in nonimmunocompromised children with varicella. J Pediatr. 1985 Jan;106(1):69–71. doi: 10.1016/s0022-3476(85)80468-6. [DOI] [PubMed] [Google Scholar]
- Crescenzi M., Seto M., Herzig G. P., Weiss P. D., Griffith R. C., Korsmeyer S. J. Thermostable DNA polymerase chain amplification of t(14;18) chromosome breakpoints and detection of minimal residual disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Kitamura K., Hayakawa Y., Takahashi M., Kojima A., Sato S., Yamanishi K. Transcription mapping of glycoprotein I (gpI) and gpIV of varicella-zoster virus and immunological analysis of the gpI produced in cells infected with the recombinant vaccinia virus. Microbiol Immunol. 1989;33(4):299–312. doi: 10.1111/j.1348-0421.1989.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Ozaki T., Ichikawa T., Matsui Y., Nagai T., Asano Y., Yamanishi K., Takahashi M. Viremic phase in nonimmunocompromised children with varicella. J Pediatr. 1984 Jan;104(1):85–87. doi: 10.1016/s0022-3476(84)80596-x. [DOI] [PubMed] [Google Scholar]
- Ozaki T., Matsui Y., Asano Y., Okuno T., Yamanishi K., Takahashi M. Study of virus isolation from pharyngeal swabs in children with varicella. Am J Dis Child. 1989 Dec;143(12):1448–1450. doi: 10.1001/archpedi.1989.02150240070019. [DOI] [PubMed] [Google Scholar]
- Trlifajová J., Bryndová D., Rýc M. Isolation of varicella-zoster virus from pharyngeal and nasal swabs in varicella patients. J Hyg Epidemiol Microbiol Immunol. 1984;28(2):201–206. [PubMed] [Google Scholar]