Abstract
This work investigated associations of age-related brain atrophy and functional neural networks identified using multivariate analyses of BOLD fMRI data in young and elder participants (young, N=37; mean age=25; elders, N=15; mean age=74). Two networks were involved in retaining increasing loads of verbal information in working memory. Network utilizations were used to test associations between function and indices of grey matter volume changes using voxel based morphometry. Global changes in brain volume were not associated with the secondary network. Lower regional grey matter volume in the left pre-central gyrus within the primary network was associated with increased secondary network utilization independent of age group. Decreased regional grey matter volume was associated with increased age only in the elders. Increased secondary network expression was associated with increased slope of reaction times across memory load, in the elders. These results support the theory of neural compensation, that elder participants recruit additional neural resources to maintain task performance in the face of age-related decreases in regional grey matter volume.
Keywords: functional MRI, voxel based morphometry, multivariate linear modeling, aging, path analysis
Introduction
The current work investigated the association of age-related changes in whole and regional brain volume on previously identified functional neural networks. In an earlier study, blood-oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) data were used to identify and compare brain networks employed by groups of healthy young and healthy elder participants during the performance of a delayed item recognition task (Zarahn, Rakitin, Abela, Flynn, & Stern, 2007). This well studied task consisted of three experimental phases for each task trial; a 3 s stimulus phase in which a set of one, three, or six letters constituting a memory set was presented, a 7 s retention phase without secondary processing or manipulation of the memory set, and a 3 s probe phase that presented a comparison letter for a speeded recognition decision. Multivariate Linear Modeling (MLM) (Worsley, Poline, Friston, & Evans, 1997; Zarahn et al., 2007) was used to extract latent spatial covariance patterns, or “networks,” underlying whole-brain BOLD signal change associated with the increase in working memory load as a function of memory set-size for each experimental phase.
Only during the retention phase of this task were multiple (two) load-dependent networks identified. The young group mainly expressed the primary information retention network while the elder group expressed both. The elder participants therefore recruited extra resources to retain the same amount of information as their younger counterparts as reflected by the elder group’s high performance accuracy even at the highest load levels. These findings are consistent with the network reorganization hypotheses of aging: either dedifferentiation or neural compensation. Dedifferentiation is defined as recruitment of differential networks of brain regions between age groups resulting in a detrimental effect on cognitive function (Cabeza, Daselaar, Dolcos, Prince, Budde et al., 2004a) and suggests that the differential recruitment of brain regions by older adults to perform a task does not improve their performance relative to younger adults (Baltes & Lindenberger, 1997). Neural compensation is a model predicting that the recruitment of extra resources, as a means of compensation for age-related neurocognitive decline (Cabeza, 2002; Cabeza, Anderson, Locantore, & McIntosh, 2002), such as normal age-associated atrophy, has a beneficial result on performance relative to the absence of recruitment of these extra resources (Scarmeas & Stern, 2004; Stern, Habeck, Moeller, Scarmeas, Anderson et al., 2005).
In relating the expression of these neural networks to behavior, an interaction was found between the degree of secondary network expression, encompassing the extra resources, and age group in predicting an increased slope across load levels of reaction time (RT): greater secondary network expression among the elderly participants was only associated with increased slope across load levels of RT. There were no significant relationships between network expressions and task accuracy across increasing load levels. These findings could be consistent with the dedifferentiation hypothesis; however, the result would also be consistent with the neural compensation hypothesis if the expression of the secondary network was compensatory for effects of age-related atrophy within the primary network. We previously noted that an index of age-related brain atrophy was needed to test this idea further (Zarahn et al., 2007).
The advent of structural imaging studies over the past two decades have documented the loss of brain tissue that occurs with normal aging (Brickman & Buchsbaum, In press; Raz, Lindenberger, Rodrigue, Kennedy, Head et al., 2005; Raz & Rodrigue, 2006)(for review, see (Raz, Rodrigue, & Haacke, 2007)). These changes may be most prominent in prefrontal areas (Raz et al., 2006; Zimmerman, Brickman, Paul, Grieve, Tate et al., 2006) although age-related atrophic changes are widely distributed throughout the cortex and subcortex (Raz et al., 2007). Importantly, age-associated atrophic changes are related to performance on standardized tests of cognitive abilities (Brickman, Habeck, Ramos, Scarmeas, & Y., In press; Brickman, Habeck, Zarahn, Flynn, & Stern, 2007; Brickman, Zimmerman, Paul, Grieve, Tate et al., 2006; Cook, Leuchter, Morgan, Conlee, David et al., 2002; Gunning-Dixon & Raz, 2003; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998; Tisserand, van Boxtel, Pruessner, Hofman, Evans et al., 2004; Zimmerman et al., 2006), suggesting that such changes in cognition are at least partially mediated by structural changes that occur with normal aging.
The nature of the interplay between functional networks and brain volume remains unclear. The widespread decrease in whole brain volume that comes with advancing age (Fotenos, Snyder, Girton, Morris, & Buckner, 2005) may affect all functional resources globally causing a need for neural compensation. A significant association between a functional network and an index of whole brain volume change suggests neural compensation. An alternative hypothesis is that decreased regional brain volume is associated with a functional network. A decrease in grey matter volume specifically within the primary network may negatively impact its function and induce the need for extra resources. Therefore, even in the presence of widely distributed grey matter volume decreases, there may be differences in the functional sensitivity to underlying grey matter volume decreases within the primary network. This scenario would be supported by a significant association between regional grey matter volume decreases within the primary functional network and the expression of the secondary network that is beyond what is attributed to whole brain volume changes. Whole brain or regional grey matter volume measures associated with the secondary network expression would lend support to the secondary network’s association with neural compensation.
In the current study extra resources were recruited only during the retention period of the task as evidenced by the finding of multiple networks. Therefore, to investigate the implications of requiring extra resources, we tested the neural compensation hypothesis by investigating the relationship between functional neural network expression and whole brain and regional grey matter volume changes and then related the results to behavioral performance. Our first hypothesis was that whole brain volume decreases would be associated with the expression of the secondary functional network. The second hypothesis was that regional grey matter volume within the primary network would be associated with the expression of the secondary functional network. Results were related to performance to assess the association among function, structure and behavioral performance using a path analysis.
Materials and Methods
Study Participants
The current study used a subset of the participants in the original analysis (Zarahn et al., 2007) for whom T1-weighted anatomical images were available. Thirty-seven (out of 40) young and nine (out of 18) elder participants had T1-weighted anatomical images collected at the time of the functional scanning session. Six of the elders had T1-weighted anatomical images acquired at a later date on the same scanner with the same scanning sequence when they returned to the facility to participate in another study within 4 months. Therefore, data were available for thirty-seven healthy, young participants (29 men and 8 women; mean (± s.d.) age = 25.0 ± 3.9; mean (± s.d.) years of education = 15.6 ± 1.4; all right handed), and 15 healthy, elderly participants (7 men and 8 women; mean (± s.d.) age = 74.5 ± 6.9; mean (± s.d.) years of education = 15.5 ± 2.4; all right handed). All participants were screened with structured medical, neurological, psychiatric, and neuropsychological evaluations to ensure that they had no neurological or psychiatric disease or cognitive impairment. The screening procedure included a detailed interview that excluded individuals with a self-reported history of major or unstable medical illness, significant neurological history (e.g. epilepsy, brain tumor, stroke), history of head trauma with loss of consciousness for greater than 5 min, history of Axis I psychiatric disorder (Association, 1994). Individuals taking psychotropic medications were excluded. Global cognitive functioning was assessed with a modified version of the Folstein Mini Mental State Examination (mMMS: (Stern, Sano, Paulson, & Mayeux, 1987)), which has a maximum score of 57. All participants were classified as non-demented and without clinically significant cognitive impairment, although the elder group had lower scores than the young group (young mean (± s.d.) mMMS total = 55.2 ± 1.5; elder mean (± s.d.) mMMS total = 53.3 ± 2.6, t (18.1) = 2.68, p = .015)). IQ was estimated with the American version of the New Adult Reading Test (NART: (Nelson & O’Connell, 1978)). The elder NART scores were significantly lower than the young (young mean (± s.d.) NARTIQ = 120.4 ± 6.1; elder mean (± s.d.) NARTIQ = 116.1 ± 6.8; t (50) = 2.20, p < .05) although IQs were above average for both groups. Although the group differences for mMMS and NARTIQ were significant they were not clinically meaningful.
The participants in the young age group were also included in previous reports that examined load-dependent patterns of retention delay fMRI signal (Habeck, Rakitin, Moeller, Scarmeas, Zarahn et al., 2005; Zarahn, Rakitin, Abela, Flynn, & Stern, 2005).
Behavioral Task Independent Variables
Working memory in young and elder participants was examined using a delayed item recognition (DIR) task in which 1, 3 or 6 letters were visually presented for three seconds. This was followed by a seven second period in which the screen was blank and participants were required to retain the information without secondary processing or manipulation of the memory set (Zarahn et al., 2007). Following the delay was a probe phase where one letter was presented to the participants for three seconds and they had to decide whether it was in the initial set of letters. The task included basic elements of a common short-term memory task (Sternberg, 1966), modified for use in functional imaging studies with the addition of a delay period, alteration of the temporal dynamics of the task, and inclusion of variable inter-trial intervals (Habeck, Rakitin, Moeller, Scarmeas, Zarahn et al., 2004; Rypma & D’Esposito, 1999). Behavioral data from this sample included measures of slope and intercepts for reaction times and accuracy using the discriminability measure, dL, across load levels.
MRI data acquisition
During the performance of each block of the delayed letter recognition task, 207 BOLD images (Kwong, Belliveau, Chesler, Goldberg, Weisskoff et al., 1992; Ogawa, Menon, Tank, Kim, Merkle et al., 1993), were acquired with an Intera 1.5 Tesla Phillips MR scanner equipped with a standard quadrature head coil, using a gradient echo echo-planar (GE-EPI) pulse sequence (TE/TR = 50 ms/3000 ms; flip angle = 90; 64×64 matrix, in-plane voxel size = 3.124mm×3.124 mm; slice thickness=8mm (no gap); 17 trans-axial slices per volume). Four additional GE-EPI excitations were performed before the task began, at the beginning of each run, to allow transverse magnetization immediately after radio-frequency excitation to approach its steady-state value; the images corresponding to these excitations were discarded. A high resolution T2-weighted, fast spin echo image was acquired from each participant for spatial normalization purposes (TE/TR =100 ms/2000 ms; flip angle = 90, 256×256 matrix; in-plane voxel size = 0.781mm×0.781 mm; slice thickness=8mm (no gap); 17 trans-axial slices per volume). A T1-weighted spoiled gradient image was acquired for voxel-based morphometry (VBM) analyses (107 slices; 256 × 256 grid; FOV = 230 mm by 160.5 mm by 183.28mm).
Task stimuli were back-projected onto a screen located at the foot of the MRI bed using an LCD projector which participants viewed via a mirror system located in the head coil. All participants wore MR compatible glasses as needed to have vision at their best corrected acuity (manufactured by Safe Vision, LLC. Webster Groves, MO). Responses were made on a LUMItouch response system (Photon Control Company) using the index fingers of either hand. Task administration and collection of RT and accuracy data were controlled using PsyScope 1.2.5 (Macwhinney, Cohen, & Provost, 1997) running on a Macintosh G3 iBook. Task onset was electronically synchronized with the MRI acquisition computer. A Carnegie Mellon Button Box (New Micros, Inc. Dallas, TX) provided digital input-output for the response system and synchronization with the MRI acquisition computer, as well as millisecond accurate timing of responses.
Individual-level Time Series Analysis of the BOLD data
All functional image preprocessing and analysis were implemented using the SPM99 program (Wellcome Department of Cognitive Neurology) and in-house developed software written in MATLAB 5.3 (Mathworks, Natick, MA). The following steps were taken in turn for each participant’s GE-EPI data set: data were temporally shifted to correct for the order of slice acquisition, using the first slice acquired in the TR as the reference. All GE-EPI images were realigned to the first volume of the first session. The T2-weighted (structural) image was then coregistered to the first EPI volume using the mutual information coregistration algorithm implemented in SPM99. This coregistered high-resolution image was then used to determine parameters (7×8×7 nonlinear basis functions) for transformation into a standard space defined by the Montreal Neurologic Institute template brain supplied with SPM99. This transformation was then applied to the GE-EPI data, which were resliced using sine-interpolation to 2 mm × 2 mm × 2 mm.
The fMRI data analysis comprised 2 levels of voxel-wise GLMs (Friston, Holmes, Price, Buchel, & Worsley, 1999). In the (participant-separable) first-level GLM, the GE-EPI time series were modeled with regressors representing the expected BOLD fMRI response (implicitly, relative to the blank inter-trial intervals where participants were presented with a black screen) to the 3 DIR trial components of memory set presentation, retention delay, and probe presentation/response, separately for each crossing of the set size and true positive/true negative factors. The regressors were constructed by convolutions of an indicator sequence (i.e., a train of discrete-time delta functions) representing the task trial component onsets, an assumed BOLD impulse response model (as represented by default in SPM99), and a rectangular function of duration dictated by the duration of the assumed neural response (Zarahn, 2000). Two rectangular functions (and hence, 2 regressors) were used for the trial component of memory set presentation: one modeling a relatively brief (400 ms) neural response at the beginning of that trial component, and another modeling a neural response lasting throughout that entire component (3000 ms); the same 2 rectangular functions were used for probe presentation. A single rectangular function of 7000-ms duration was used for the retention delay. For both the memory set presentation and probe presentation trial components, a linear combination (i.e., a contrast) of the 2 parameter estimates was computed that estimated the area under the curve of the neural response for that trial component. For the retention delay trial component, the analogous contrast was simply the coefficient of its single basis function. Linear combinations (i.e., contrasts) of the estimated coefficients of these predictors were computed to yield estimates of the linear effect with respect to set size of the area under the curve of the neural response for each trial component in each participant. The contrast estimate images were then intensity normalized via voxel-wise division by the time-series mean and spatially smoothed with an isotropic Gaussian kernel (full-width-at-half-maximum = 8 mm). The resulting images (3 trial components = 3 images/participant) were used as the dependent variables in a second-level GLM.
Group-level Analysis of BOLD data
The group-level analysis of BOLD image data used multivariate linear modeling (MLM: (Worsley et al., 1997)) to identify significant load-dependent networks, or covariance patterns comprising latent spatial variables within the BOLD image effects of interest, engaged by the young and elderly groups. The MLM analysis performed singular value decomposition to the spatially whitened load dependent effects during the different phases of the task. Sequential latent root testing, using a global F-test and an α level of .05, was used to determine the number of significant spatial patterns. Once calculated, the spatial patterns were multiplied voxelwise by the participant specific load dependent contrast maps that were entered into the MLM analysis, then summed to calculate each participant’s network expression (Zarahn et al., 2007). These network expression scores served as independent measures describing the degree to which each participant used, or expressed, a significant spatial pattern. The results of these analyses were such that all intercept comparisons resulted in a single network being identified. The slope contrasts for the stimulus and probe phases also identified a single network, with the retention phase having two networks being expressed. It is these two networks, referred to as networks 1 and 2, which are the focus of this study. The latent spatial patterns were scaled by the singular values (analogous to SPM t-images) and thresholded at p < .001 with a cluster extent of 50 voxels (Zarahn et al., 2007).
Structural image processing
The T1-weighted anatomical images were first segmented into three tissue types: grey matter, white matter and cerebro-spinal fluid, using the unified segmentation routines in SPM5 (Wellcome Department of Cognitive Neurology) (Ashburner & Friston, 2000, 2005; Good, Johnsrude, Ashburner, Henson, Friston et al., 2001b). The result was a classification for each voxel in the image based on the probability that it belonged to each tissue type. This results in each segment image containing measures of tissue densities in each voxel location. The images were spatially normalized to a standard atlas (MNI-space) using 12 degrees of freedom affine transforms and non-linear warping. Once warped, the images were modulated using the Jacobian determinant. This step converts the density images into measures of absolute volume at each voxel location. (Good, Johnsrude, Ashburner, Henson, Friston et al., 2001a).
The resultant modulated, spatially normalized grey matter probability maps, grey matter volume maps, were intensity thresholded at .1 to include only voxels consisting of at least 10% grey matter (Busatto, Garrido, Almeida, Castro, Camargo et al., 2003). Finally, the images were spatially smoothed with an isometric Gaussian smoothing kernel of 8mm at its FWHM. These grey matter volume maps were used for voxel based statistical parametric mapping using SPM5.
Network expression predictive of normalized whole brain volume
As a measure of whole brain volume, the proportion of intra-cranial volume occupied by grey and white matter was calculated. Total volume of grey matter plus white matter was divided by the sum of grey, white and CSF volume to derive the intra-cranial brain fraction (Chard, Parker, Griffin, Thompson, & Miller, 2002; Fotenos et al., 2005). This measure is termed normalized whole brain volume (nWBV) and was used as a covariate in the analyses.
General linear modeling followed a model reduction approach. An α level of .05 was used to determine if expression of network 2 was associated with nWBV. The full model was a heterogeneous slopes model including nWBV as the dependent variable and group, expression of network, and the interaction of group and network expression as predictors. If an interaction was non-significant, it was dropped and the reduced model was tested.
Network expression predictive of regional grey matter volume
General linear modeling was used to determine if expression of network 2 was associated with regional grey matter volume changes within network 1 after accounting for nWBV and group membership. Grey matter volume maps served as the dependent measures and age-group, nWBV and network expressions were covariates. Hierarchical tests were again conducted with the full model containing all main effects and the interactions between group and the two continuous covariates. If an interaction was non-significant it was removed and the reduced model was tested. Significant regions of grey matter volume differences were identified using a one-tailed unconnected height threshold of p < .005 and an extent threshold of 20 voxels and restricted to only those voxels identified in the previous study as being significant members of network 1. This lenient unconnected threshold was chosen based on the hypothesis of significant effects within the highly restricted search space chosen for these analyses. Any identified significant clusters were averaged across voxels for use in subsequent analyses.
Statistical analyses were conducted using the SPSS software package release 15.0.0 (SPSS Inc. Chicago, Illinois) and voxel based morphometry was conducted with SPM5.
Results
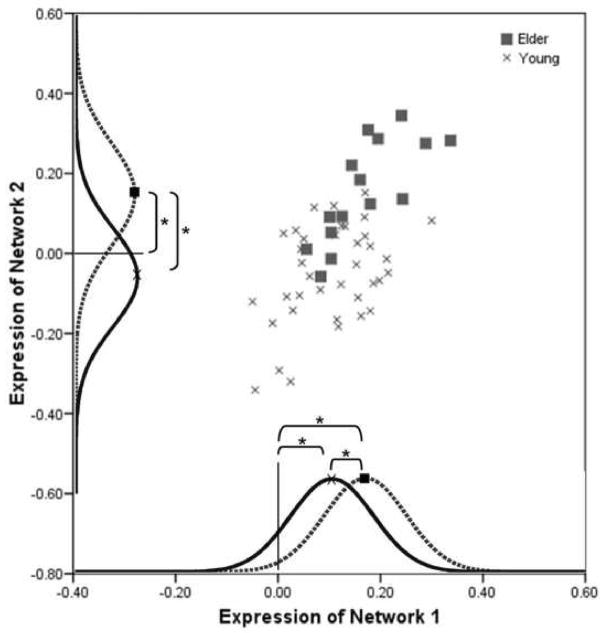
Figure 1 illustrates the expressions for both networks as previously calculated and described (Zarahn et al., 2007). Network 1 consisted of regions that increased with memory load in the midline cerebellum, left insula/inferior frontal gyrus, left hippocampus, right middle/superior frontal gyri, left inferior/middle frontal gyri into the pre-central gyrus, left inferior/superior parietal lobule, right cingulate gyrus into medial/superior frontal gyri and left medial/superior gyri. Network 1 also included regions in the midline cingulate, left medial/superior temporal gyri, right medial frontal gyrus and left cingulate gyrus where signal decreased with increased working memory load. Network 2 included the right parahippocampal gyrus, which showed increased activity with increased working memory load. It is important to note that the regional areas of the networks were defined using an arbitrary threshold. Therefore, while network 2 is defined as containing the right parahippocampal gyrus it in fact includes all other regions of the brain to various degrees. The threshold identified those regions that had the greatest contribution to the covariance maps. As previously described, at more lenient extent thresholds the parahippocampal gyrus was in fact bilaterally involved in network 2 (Zarahn et al., 2007).
Figure 1.
Expressions of network 1 versus network 2 for the young and elder groups. Expressions for both networks were significantly correlated. Expressions of network 1 significantly differed between groups and were significantly different from zero for each group. Expressions of network 2 significantly differed between groups and were significantly different from zero for only the elder group. Curves are only used to represent the significant differences in the data. The solid curve represents the young group and the dashed the elder group. Note: * p < .01.
Expression of network 1 was significantly greater in the elder participants than in the young participants. Expression of network 2 was also significantly greater in the elders than the young participants, (see Table 1 for results from the original sample and the smaller sample used in the current study). The expression of network 1 was significantly different from zero for both the young (t (36) = 7.97, p < .0001) and the elder group (t (14) = 8.19, p < .0001). However, the expression of network 2 was significantly different from zero for the elder group (t (14) = 4.74, p < .0001), but not the young group (t (36) = 1.70, p = .098). Figure 1 shows the expression scores for both networks and both groups.
Table 1.
Network Expressions and Performance Measures
| Total N = 58 |
Total N = 52 |
|||||
|---|---|---|---|---|---|---|
| Young (Y) | Elder (E) | Young (Y) | Elder (E) | |||
| N = 40 |
N=18 |
N = 37 |
N=15 |
|||
| Source | mean(s.d.) | mean(s.d.) | t(Y>E)a | mean(s.d.) | mean(s.d.) | t(Y>E)b |
| Network 1 | 0.18(0.14) | 0.29(0.15) | 2.53* | 0.19(0.14) | 0.30(0.14) | 2.55* |
| Network 2 | −0.078(0.22) | 0.26(0.26) | 5.18** | −0.057(0.21) | 0.28(0.23) | 5.15** |
| Intercept dL | 6.21(1.18) | 6.06(1.17) | 0.44 | 6.23(1.19) | 6.19(1.15) | 0.92 |
| Slope dL | −0.04(0.26) | −0.22(0.43) | 1.68c | −0.027(0.26) | −0.27(0.44) | 2.02d |
| Intercept RT | 806(180) | 875(176) | 1.35 | 804(185) | 884(188) | 1.41 |
| Slope RT | 59.0(30.8) | 85.7(35.7) | 2.91** | 56.6(29.5) | 90.0(35.6) | 3.48** |
Note: df = 56, except as noted.
df = 50, except as noted.
df = 22.9.
df = 18.3.
p <.05.
p <.01.
The participants also differed in nWBV; the elders had significantly smaller nWBV (young mean (± s.d.) = 0.795 ± 0.040, elders = 0.644 ± 0.407, t (50) = 12.258, p < .0001). The full model that tested whether network 2 predicted nWBV included group, expression of network 2 and their interaction as predictors. Their interaction was not significant (F (1, 48) = 0.228, p = .635) and was thus removed to produce the reduced model. In the reduced model, the elder group had significantly lower measures of nWBV than the young group (F (1, 49) = 90.982, p < .0001) but the main effect of secondary network expression was not associated with nWBV (F (1, 49) = 0.251, p = .619).
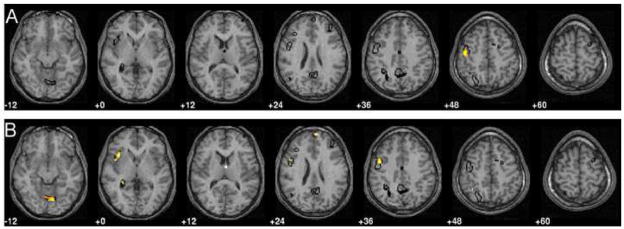
The VBM analyses were restricted to only network 1. The full model which included group, nWBV, network 2 expression and the interactions of group with nWBV and network expression had no significant clusters for either of the two interactions when thresholded at an unconnected p < .005, extent = 10 (the equivalent F value for this height threshold was: F (1, 45) = 8.71). The interactions were removed and the reduced model demonstrated the significant results listed in Table 2 and shown in Figure 2. Expression of network 2 was significantly associated with a single cluster of increased regional grey matter volume in the left pre-central gyrus, Brodmann area 6. Group effects were more widespread; the young group had greater grey matter volume than the elders in the right thalamus, right superior medial frontal regions (BA 10), left hippocampus, left inferior parietal cortex (BA 40), right vermis, left insula and left supplementary motor cortex (BA 6).
Table 2.
Significant clusters related to regional gray matter volume
| Effect | Coordinates |
Z-score | Extent (voxels) | Brain Area | ||
|---|---|---|---|---|---|---|
| X | y | z | ||||
| Decreased exp. N2 | −48 | −8 | 46 | 3.32 | 91 | L Pre-central (BA 6) |
|
| ||||||
| Group (young > elder) | 4 | 62 | 26 | 4.16 | 38 | R Sup. Med. Front. (BA 10) |
| −40 | −50 | 46 | 3.69 | 46 | L Inf. Parietal (BA 40) | |
| 12 | −4 | 18 | 3.67 | 12 | R Caudate | |
| −38 | 18 | −2 | 3.55 | 95 | L Insula | |
| −46 | 6 | 34 | 3.56 | 130 | L Pre-central (BA 6) | |
| −32 | −38 | −2 | 3.55 | 27 | L Hippocampus | |
| 6 | −74 | −12 | 3.41 | 92 | R Cerebellum | |
Figure 2.
Overlays show an outline in black of the primary retention network. This defined the search space for the voxel-based morphometry analyses, where the height threshold was t = 2.86, p < .005, uncorrected and extent threshold of 20 voxels. A) Results related to expression of the secondary network in the negative direction, increased expression related to decreased grey matter volume. B) Results related to group differences, young > elders. Note: Left is left. Values under slices represent position in z-direction in MNI coordinates.
Behavioral Results
Behavioral performance results were comparable in the full original sample and the smaller sample used in the current analyses. Results differed only numerically with all significant results in the original sample remaining significant in the smaller sample. In the current data set, performance only significantly differed between groups for the slope RT measure; among the older adults reaction times were relatively slower with increasing set-size, see Table 1. All subsequently reported results are from the reduced data set.
The VBM finding was used to relate the structural/functional relationship to the behavioral outcome of slope RT. The grey matter volume values from the cluster in the left pre-central gyrus associated with the expression of network 2 were averaged to create a regional grey matter volume measure rGMV, Hierarchical ANOVA models were used to test the relationship of structural changes (nWBV and rGMV), functional network expressions and age to performance (slope RT).
In the previous work, a significant association was found between network 2 expression and slope RT; therefore, this was again modeled with the additional inclusion of network 1. This analysis tested whether group differences in slope RT were associated with the differential expression of the two networks. The full model included group as a fixed variable and the interactions between group and the network expressions. The interaction between group and network 1 was non-significant and was dropped from the model (F (1, 46) = 0.106, p = .746). In this reduced model network 1 was non-significant (F (1, 47) = 2.282, p = .138) and dropped leading to the final reduced model where the group by network 2 interaction significantly predicted slope RT, see Table 3 and Figure 3. This interaction showed a significant positive relationship between network 2 and slope RT in the elder group and a slight negative relationship in the young group.
Table 3.
Analysis of variance for functional, structural and behavior associations
| Model of Slope RT | ||||
| Source | cf | F | B (young/elder) | Observed Power |
|
| ||||
| Group (Gr) | 1 | 0.381 | 8.392 | .093 |
| Network 2 (N2) | 1 | 4.405* | −3.644 | .539 |
| N2* Gr | 1 | 4.822* | −3.644/165.069 | .576 |
| Error | 48 | (900.395) | ||
|
| ||||
| Model of Network 2 | ||||
| Source | cf | F | B (young/elder) | Observed Power |
|
| ||||
| Group | 1 | 2.404 | −.068 | .330 |
| Network 1 | 1 | 16.296** | .713 | .977 |
| rGMV | 1 | 8.370** | −.716 | .809 |
| Error | 48 | (0.010) | ||
|
| ||||
| Model of rGMV | ||||
| Source | cf | F | B (young/elder) | Observed Power |
|
| ||||
| Group (Gr) | 1 | 5.266 | 0.375 | .614 |
| Age | 1 | 3.875 | 0.001 | .488 |
| Gr* Age | 1 | 5.466* | 0.001/−0.007 | .630 |
| Error | 48 | (0.003) | ||
Note: Values enclosed in parentheses represent mean square errors. Two values for B relate to the parameter estimates for the two groups from a significant interaction.
p <.05.
p <.01
Figure 3.
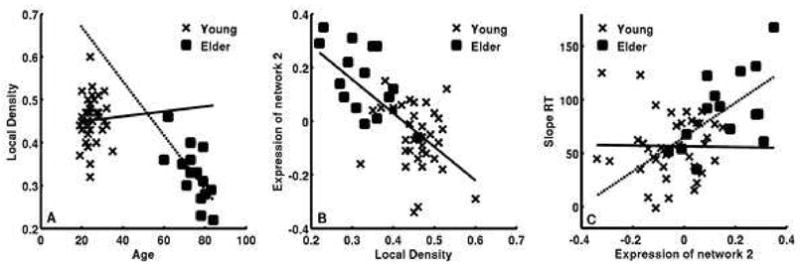
Scatter plots of the significant effects in relating the structural, functional and behavioral findings using ANOVA models. A) There was a significant interaction by group between age and regional grey matter volume. B) There was a significant main effect over group between regional grey matter volume and expression of network 2. C) There was a significant interaction by group between expression of network 2 and slope RT. These results were combined and tested in a path analysis.
The rGMV finding in the left supplementary motor area suggests that decreased grey matter volume in this region may be associated with increased slope RT due to motor slowing (Yordanova, Kolev, Hohnsbein, & Falkenstein, 2004). This alternative interpretation would suggest that increased slope RT was associated with the structural integrity of the left pre-central gyrus and unrelated to the identified functional networks. An alternative model tested the association between group, rGMV and their interaction to predict slope RT. The interaction was non-significant and was dropped from the model F (1, 48) = 2.600, p = .113). In the reduced model rGMV was also non-significant (F (1, 49) = 0.542, p = .465); therefore rGMV was not associated with slowing task performance independent of the functional networks.
The VBM analysis was restricted to areas comprising network 1; therefore, to test for the impact of grey matter volume on the network, rGMV was used as a predictor of network 1 expression together with group and their interaction. The interaction was non-significant and dropped (F (1, 48) = 0.131, p = .719). In the reduced model there was no significant association between network 1 and group (F (1, 49) = 0.904, p = .346) or rGMV (F (1, 49) = 1.580, p = .215). The model to predict network 2 included network 1, rGMV, group and the interaction of group with rGMV and network 1. Both interactions were non-significant and dropped from the model (group by rGMV: F (1, 46) = 0.045, p = .833; group by network 1: F (1, 46) = 1.268, p = .266). In the reduced model both rGMV and network 1 significantly predicted network 2 while the group effect was non-significant, see Table 3 and Figures 1 and 3. Network 2 was positively correlated with network 1 and negatively correlated with rGMV.
The measure rGMV was associated with network 2 independent of age group raising the question of whether individual differences in grey matter volume in the region were simply associated with normal variability and thus not related to any age-related effects. Therefore, a model tested rGMV’s association with group, age and their interaction. The interaction in this model was significant; see Table 3 and Figure 3, in the young group there was a small positive association between age and rGMV while in the elders there was a strong negative association between advancing age and rGMV.
A path analysis was performed to combine the results of these hierarchical models into one complete structure. The path model included slope RT predicted by network 2, network 2 predicted rGMV and finally rGMV predicted by age. The paths between the variables that had significant interactions with group were estimated for both the young and elder groups and included the path between age and rGMV and the path between network 2 and slope RT. The remaining path that showed no interaction with group was fixed and estimated for the entire participant pool. This was the path between rGMV and network 2. The path model fit the data well, p = .855, RSMEA = 0.00, X2(10) = 5.50, comparative fit index (CFI) = 1, see Figure 4 for a diagram of the path and the estimated coefficients. Path analyses were conducted using LISREL 8.80 (Jöreskog & Sörbom, 1993)
Figure 4.
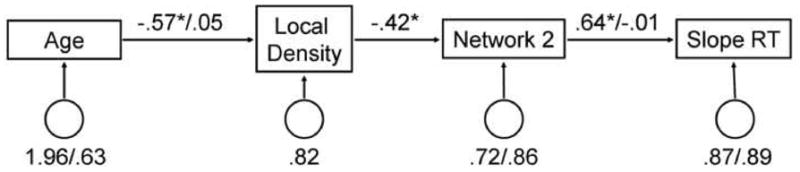
Path analysis results relating the expressions of network 2, regional grey matter volume within the primary network, age and their association with the behavioral measure, slope RT. Values on the paths represent standardized regression parameter estimates, circles represent error variances. Two numbers represent the values for each group (elder/young) and a single number represents an equivalent association across age groups. Model fit values were: χ2(8) = 5.89, p = .66, RMSEA = 0.00 and CFI = 1.00
Discussion
Previous work identified two functional networks that were expressed to different degrees in young and elder groups for load related effects of retaining verbal information over a period of seconds (Zarahn et al., 2007). The primary network was significantly expressed by both groups but expressions were approximately 60 percent greater in the elder participants than the younger participants. The elder group also significantly expressed a secondary network, while the young participants, as a group, did not. These results demonstrated two ways in which age-related changes impacted the retention of verbal information: the elder participants used the primary functional network to a greater degree than the younger participants and also recruited a secondary network.
We found that these differences in expression of the functional networks used for information retention were not related to differences in normalized whole brain volume. In contrast, they were associated with regional grey matter volume in an area of the primary functional network. Thus, normalized whole brain volume was not related to the differential expression of the secondary network while a regional difference in grey matter volume was.
The VBM analysis identified one cluster in the left pre-central region of the supplementary motor area (SMA, Brodmann area 6) that was significantly associated with the expression of network 2 after accounting for age group and nWBV differences. Therefore, lower grey matter volume in this region was associated with load dependent signal increases in network 2. The left pre-central location has been repeatedly implicated in the retention of verbal working memory information and is hypothesized to be involved with sub-vocal rehearsal of information (Altamura, Elvevag, Blasi, Bertolino, Callicott et al., 2007; Muller & Knight, 2006; Paulesu, Frith, & Frackowiak, 1993; Smith & Jonides, 1999). Decreased grey matter volume in this region could be interfering with elder participants’ ability to accurately rehearse information sub-vocally. An alternative possibility is that the left pre-central finding is related to age-related motor slowing (Yordanova et al., 2004).
Our measure of slope RT across loads provides evidence that does not support this alternative hypothesis. Calculation of the intercept and slope of reaction times across increasing cognitive loads separated the effects of motor slowing and cognitive slowing. The intercept effect is related to baseline RT, or what the estimated RT is with no cognitive load. The slope effect is defined as being orthogonal to this index of motor slowing and related to cognitive slowing (correlation between slope and intercept RT is r(52) = −.14, p = .31). The current study found no significant difference between groups on the intercept of RT; however, there was a significant group effect for the slope RT. We also found no relationship between the grey matter volume in the left pre-central region and the slope RT measure which would support the alternative hypothesis.
The finding that reduced grey matter volume in the left pre-cenral gyrus was related to increased expression of network 2 was found independent of age group. The young group however expressed network 2 in the negative direction while the elders expressed it in the positive direction. Therefore, the underlying association between grey matter volume in the left pre-central gyrus and network 2 may differ between the two groups. Support for this was previously presented with the significant interaction between group and network 2 predicting slope RT (Zarahn et al., 2007). The directionality of these relationships were opposite in the two groups and straddled the zero point for network 2 expression. That is, decreased expression of network 2 was associated with increased slope RT in the young while increased expression of network 2 was associated with increased slope RT in the elders. Age was subsequently found to interact with group to predict the individual differences in regional grey matter volume with a significant negative relationship in the elders. Based on this finding we conclude that the relationship within the young group between slope RT and regional grey matter volume is associated with inter-participant variability and in the elders it is due to advancing age.
The path analysis integrated the series of ANOVA models into one compact structure that summarizes the main findings of this study. The paths demonstrate that advanced age is negatively associated with grey matter volume of the left pre-central gyrus among elder adults while there is no association for younger adults. For both groups, network 2 expression increased with decreased grey matter volume in the left pre-central gyrus; however, in the young group the result was smaller magnitude of expression while in the elder group it was greater magnitude of expression. This dichotomy is a key result and we feel is related to the differential expression of the secondary network. The younger participants with larger grey matter volume suppress the network more than the young participants with smaller grey matter volume resulting in increased expression of network 2 with decreased grey matter volume. In the elders decreased grey matter volume is related to increased expression of the functional network.
Based on the associations between age-related grey matter volume decreases in the primary functional network and secondary network expression and the subsequent effect on performance we propose that the secondary functional network is compensatory. The primary network is affected by age-related grey matter volume decline and accurate retention of information in working memory is particularly sensitive to the grey matter volume in the left pre-central gyrus. To compensate for this age-related effect the elder participants need extra resources, namely the secondary network.
The dedifferentiation hypothesis offers an alternative explanation for age-associated regional differences in the networks required to perform a task (Baltes et al., 1997). One situation where our data would support the dedifferentiation hypothesis would be if our region of reduced grey matter volume was involved in inhibitory functions in our DIR task. It is plausible that decreased function, resulting from decreased grey matter volume, in the left pre-central gyrus could result in a decreased ability to inhibit interference or distractions. Thus decreased inhibition would result in a breakdown of optimal functional organization, dedifferentiation. A recent study teased apart brain regions associated with inhibition and working memory and demonstrated that the left pre-central gyrus was related to verbal working memory tasks and not involved in any of their three inhibitory tasks (McNab, Leroux, Strand, Thorell, Bergman et al., 2008).
Additionally, the two functional networks were related such that expression of network 2 increased as a function of network 1. This strong correlation between the two networks argues against the idea of dedifferentiation which represents a breakdown of optimal functional organization (Logan, Sanders, Snyder, Morris, & Buckner, 2002) and reduced ability to achieve task-related focal activation (Cabeza, Anderson et al., 2002). While increased network expression being associated with poorer performance supports the hypothesis of dedifferentiation, results from our incorporation of indices of grey matter volume change provide support for the neural compensation hypothesis. Neural compensation as described here is the situation where the effects of aging or grey matter volume changes alter functional brain networks resulting in the use of networks that would not be engaged in unaffected individuals. This situation presumes that the altered network compensates for an inability to respond to increases in task difficulty as an unaffected person’s brain would (Stern et al., 2005). We argue that in our sample of elder participants the expression of the secondary network meets the criteria for neural compensation, such that expression of the secondary network varies as a function of grey matter volume in the primary network.
Furthermore, we speculate that the secondary network is supporting performance in the face of age-related grey matter volume declines within the primary network. This view of grey matter volume decline related compensation differs from that of Cabeza et al. 2002 where compensation “counteracts age-related neurocognitive decline” (Cabeza, Daselaar, Dolcos, Prince, Budde et al., 2004b).
Age-related grey matter volume decrease may impair an elder participant’s ability to rely on a recollection strategy for information retention. Therefore, the elder participants may be forced to rely on information familiarity. Aging has previously been found to impair recollection strategies of information retention while not impairing familiarity strategies (Parkin & Walter, 1992). The parahippocampal gyrus, the main region of the compensatory network, has previously been associated with familiarity and linked to age-related effects on working memory (Cabeza et al., 2004a).
The younger adults, in the absence of age-related grey matter volume decrease, would have the ability to utilize both strategies of information retention, recollection and familiarity (Dennis, Kim, & Cabeza, 2007). Having the means of both strategies at their disposal, it is plausible that the younger participants would utilize the more reliable strategy and suppress the less reliable strategy. This age-associated difference could explain the negative expressions for the secondary network found in the current study in many of the younger participants. Tasks employing distracting stimuli that interrupt sub-vocal rehearsal could test the possibility of a strategy shift in the face of increased task difficulty (Gazzaley, Sheridan, Cooney, & D’Esposito, 2007). If the working memory task with interruptions in the retention period described by Gazzaley et al. (2007) was performed as an imaging experiment, it would be interesting if such a hypothesized strategy shift was reflected by a decrease in task related signal change in left pre-central gyrus and an increase in parahippocampal gyrus.
Alternatively, the compensatory network may be related to information storage. Cabeza et al. (2004), demonstrated parahippocampal activity in a group of young participants engaged in a working memory paradigm with a relatively long, 12 seconds, retention period (Cabeza, Dolcos, Graham, & Nyberg, 2002). They posited that this region was employed to keep an index of transiently stored working memory representations needed to access long-term memory (Cabeza et al., 2004a; Cabeza, Dolcos et al., 2002). The current study used 7 second retention intervals, which, for the elder participants, may have been long enough for them to employ the strategies demonstrated in young participants at longer delay periods.
The Zarahn et al. study and the current sample had dissimilar number of males and females and this disparity was accounted for in the calculation of the functional network expressions (Zarahn et al., 2007). Sex differences have been reported in the literature in regional grey matter volume (Good et al., 200la) and normalized whole brain volume in elder populations (Fotenos et al., 2005). In the study by Fotenos et al. (2005) the authors report their post-hoc means for their elder sample as being .739 and .723 respectively for females and males. In our sample when nWBV was tested in a 2×2 ANOVA including age group, sex and their interaction there was no significant interaction or main effect of sex., results not shown Post-hoc t-tests within age group found no significant gender differences in the elders (male: .659 (0.047); female: .630 (0.031); p = .18) or the youngs (male: .798 (0.032); female: .787 (0.063), p = .50). Sex differences were tested for in the VBM analyses and did not alter the finding of the grey matter volume within the left pre-central gyrus being significantly related to the expression of network 2.
Conclusion
In summary, this study revealed a location in the primary information retention network in which decreased grey matter volume was associated with the increased recruitment of additional functional resources. Based on the relationship of this finding to behavioral performance, our results provide evidence that the additional resources were compensatory for age-related grey matter volume changes in the primary information retention network.
Acknowledgments
This study was supported by National Institute on Aging grant RO1 AG26158 awarded to Y.S.
Footnotes
Disclosure statement: The authors hereby declare that they have no current, past, or anticipated conflicts of interest, whether real or potential. All participants supplied written informed consent in accord with Columbia University Medical Center IRB procedures.
References
- Altamura M, Elvevag B, Blasi G, Bertolino A, Callicott JH, Weinberger DR, et al. Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res. 2007;154(2):103–114. doi: 10.1016/j.pscychresns.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS. Alzheimer’s disease and normal aging: Neurostructures. Learning and Memory: A Comprehensive Review (In press) [Google Scholar]
- Brickman AM, Habeck C, Ramos MS, Scarmeas N, Y S. A forward application of age associated gray and white matter networks. Human Brain Mopping. doi: 10.1002/hbm.20452. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging. 2007;28(2):284–295. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Garrido GEJ, Almeida OP, Castro CC, Camargo CHP, Cid CG, et al. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer’s disease. Neurobiology of Aging. 2003;24(2):221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, Mclntosh AR. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. NeuroImage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and Task-specific Age Effects on Brain Activity during Working Memory, Visual Attention and Episodic Retrieval. Cereb Cortex. 2004a;14(4):364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004b;14(4):364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and Differences in the Neural Correlates of Episodic Memory Retrieval and Working Memory. NeuroImage. 2002;16(2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Chard DT, Parker GJ, Griffin CM, Thompson AJ, Miller DH. The reproducibility and sensitivity of brain tissue volume measurements derived from an SPM-based segmentation methodology. J Magn Reson Imaging. 2002;15(3):259–267. doi: 10.1002/jmri.10064. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan ML, Conlee EW, David S, Lufkin R, et al. Cognitive and physiologic correlates of subclinical structural brain disease in elderly healthy control subjects. Arch Neurol. 2002;59(10):1612–1620. doi: 10.1001/archneur.59.10.1612. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia. 2007;45(14):3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Sheridan MA, Cooney JW, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21(5):532–539. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude L, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. NeuroImage. 2001a;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001b;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2004;18(3):306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, et al. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2005;23(2–3):207–220. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sorbom D. L1SREL 8: Structural equation modeling with the S1MPL1S command language. Hillsdale, NJ: Erlbaum; 1993. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc NatlAcadSci USA. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Macwhinney B, Cohen J, Provost J. The PsyScope experiment-building system. Spat Vis. 1997;11(1):99–101. doi: 10.1163/156856897x00113. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neurcpsychologia. 2008;46(11):2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: Contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14(2):234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Bicphys J. 1993;64(3):803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychol Aging. 1992;7(2):290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362(6418):342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neurcpsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N YAcadSci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci U S A. 1999;96(11):6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer’s disease. Curr Neurol Neurosci Rep. 2004;4(5):374–380. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and Executive Processes in the Frontal Lobes. Vol. 283. 1999. pp. 1657–1661. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15(4):394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: validity and reliability. Neurology 1987 [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14(9):966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the Response of PET and fMRI Data Using Multivariate Linear Models. Neurolmage. 1997;6(4):305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Hohnsbein J, Falkenstein M. Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor-generation processes: evidence from high-resolution event-related potentials. Brain. 2004;127(2):351–362. doi: 10.1093/brain/awh042. [DOI] [PubMed] [Google Scholar]
- Zarahn E. Testing for neural responses during temporal components of trials with BOLD fMRI. Neuroimage. 2000;11(6 Pt 1):783–796. doi: 10.1006/nimg.2000.0560. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cereb Cortex. 2005;15(3):303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28(5):784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]