Abstract
Each hepatitis B virus (HBV) genotype and subgenotype is associated with a particular geographic distribution, ethnicity, and anthropological history. Our previous study showed the novel HBV subgenotypes C6 (HBV/C6) and D6 (HBV/D6), based on the S gene sequences of isolates in Papua, Indonesia. The present study investigated the complete genome sequence of 22 strains from Papua and subjected them to molecular evolutionary analysis. A phylogenetic analysis revealed that 9 out of 22 strains were classified as HBV/C6, 3 strains as HBV/D6, and 9 strains as HBV/B3. A particular strain positioned between HBV/B3 and HBV/B5 remained unclassifiable into any known subgenotypes. This strain showed high homology with HBV/C5 from the Philippines in the core region and was thought to have undergone genetic recombination with HBV/C5. Further studies are needed to determine whether this strain belongs to a new subgenotype of HBV/B. Based on the amino acid alignment, HBV/C6 has subgenotype specific variations (G18V and V47M) in the S region. HBV/C6 strains were more closely related in terms of evolutionary distance to strains from the east Asia and Pacific regions than those found in southeast Asia. HBV/D6 strains were most closely related to strains from the Western countries (HBV/D3) rather than those from Asia and Papua New Guinea. In conclusion, we have confirmed by complete sequence analysis that two novel HBV subgenotypes, HBV/C6 and HBV/D6, are prevalent in Papua, Indonesia.
Hepatitis B virus (HBV) is an etiological agent of chronic liver disease, including chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma, and this poses major health problems worldwide, especially in Asian Pacific countries (7, 10).
HBV strains that infect humans show genetic and antigenic heterogeneity, and eight genotypes, designated A to H, have been identified so far by molecular evolutionary analysis (19). The HBV genotypes have distinct geographical distributions, which are associated with anthropological history (4, 13, 20, 31). Furthermore, previous studies have demonstrated the presence of several subgenotypes within the widely spread genotypes. HBV genotype B (HBV/B) is classified into six subgenotypes, B1 to B6, B1 dominating in Japan, B2 in China and Vietnam, B3 in Indonesia, B4 in Vietnam, B5 in the Philippines, and B6 in the Arctic (3, 16, 23, 24, 26, 27). As for HBV/C, C1 is common in southeast Asia, C2 in east Asia, C3 in Oceania, C4 in Aborigines, and C5 in the Philippines (15, 26). HBV/D has a worldwide distribution, with its highest prevalence in the Mediterranean region, and is classified as D1 to D5 (1, 15, 17). Our previous study revealed novel subgenotypes (HBV/C6 and HBV/D6) based on the S gene sequence of HBV isolates in Papua, Indonesia, where HBV infection is endemic (9).
HBV genotyping with the S gene sequence is, in general, consistent with the genotyping of the full genomic sequence, and therefore, HBV genotypes can be assigned based upon S gene sequences (11, 16, 19). Subgenotype classification, however, may not be applicable to some HBV strains on the basis of the S region sequence alone (9, 14, 15). Accordingly, complete genome sequences are more reliable for the analysis of genotype and subgenotype classification for HBV (14). The data on the complete genome sequences of the HBV strains found in Papua are scant. The present study aimed to evaluate the HBV genotypes and subgenotypes present among the Papuan population using complete genome sequences. In addition, the phylogenetic relatedness of HBV strains isolated from Papua was assessed.
MATERIALS AND METHODS
Source of HBV DNA.
A total of 45 HBsAg-positive serum samples were obtained from blood donors screened at the Red Cross Blood Center, Papua, Indonesia. Twenty-seven (2 HBV/B, 23 HBV/C, and 2 HBV/D) of them were derived from the previous study, in which 2 novel subgenotypes were identified on the basis of S gene sequences (9). To examine more isolates of HBV/B and HBV/D, 18 samples (14 HBV/B and 4 HBV/D) were analyzed for their HBV subgenotypes on the basis of their S gene sequence and enrolled in this study. Ethnically, 13 samples (5 HBV/B and 8 HBV/C) were from Papuan inhabitants, and 9 samples (5 HBV/B, 1 HBV/C, and 3 HBV/D) were from non-Papuan inhabitants (Table 1). The national census showed that about 80% of the population in this area was Papuan. Ethnicity was determined by birthplace through three generations. Informed consent for participation in this study was obtained from each individual.
TABLE 1.
Demographic and virological characteristics of HBV carriers infected with HBV/B, HBV/C, and HBV/D
| Straina | Ethnicity | Age | Sex | Genotype and subgenotypeb
|
Nucleotide length (bp) | |
|---|---|---|---|---|---|---|
| S gene (previous study) | Full genome (this study) | |||||
| 25UC | Papuan | 31 | Male | B3 | B3 | 3,215 |
| 03UC | Papuan | 29 | Male | C6 | C6 | 3,215 |
| 06UC | Papuan | 30 | Male | C6 | C6 | 3,215 |
| 08UC | Papuan | 35 | Male | C6 | C6 | 3,119 |
| 16UC | Papuan | 29 | Male | C6 | C6 | 3,215 |
| 29UC | Papuan | 18 | Male | C6 | C6 | 3,214 |
| 31UC | Papuan | 40 | Male | C6 | C6 | 3,215 |
| 32UC | Papuan | 40 | Male | C6 | C6 | 3,220 |
| 43UC | Papuan | 27 | Male | C6 | C6 | 3,121 |
| 07UC | Non-Papuan | 44 | Male | D6 | D6 | 3,156 |
| 17UC | Non-Papuan | 21 | Male | D6 | D6 | 3,179 |
| 05UC | Non-Papuan | 27 | Male | C6 | C6 | 3,215 |
| 33UC | Non-Papuan | 18 | Male | B3 | B3 | 3,219 |
| P14 | Papuan | 26 | Male | B/ND | B3 | 3,216 |
| P41 | Papuan | 45 | Male | B/ND | B3 | 3,218 |
| P48 | Papuan | 36 | Male | B/ND | B3 | 3,212 |
| P19 | Papuan | 33 | Male | B/ND | B3 | 3,214 |
| P9 | Non-Papuan | 42 | Male | B/ND | B3 | 3,215 |
| P18 | Non-Papuan | 32 | Female | B/ND | B | 3,215 |
| P30 | Non-Papuan | 23 | Male | B/ND | B3 | 3,215 |
| P57 | Non-Papuan | 25 | Male | B/ND | B3 | 3,219 |
| P7 | Non-Papuan | 42 | Male | D/ND | D6 | 3,155 |
The top 13 strains are from our previous study (9). The bottom nine strains are newly added strains.
ND, not determined. For P18, the subgenotype was undetermined.
Complete genome sequencing.
DNA was extracted from 100 μl of serum that had been stored at −80°C using a DNA extractor kit (QIAamp DNA blood mini kit; Qiagen, Tokyo, Japan). The complete HBV genome sequences were determined by the method reported previously (28). In brief, the complete genome of HBV was first amplified as two overlapping fragments, a 3,200-bp amplicon (fragment A) and a 462-bp amplicon (fragment B) that covers the remaining region. Fragment A was then subjected to nested PCR to amplify 11 overlapping fragments. The amplified fragments were sequenced directly using the Big Dye Deoxy Terminator cycle sequencing kit with an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA).
Phylogenetic analysis.
Reference sequences were retrieved from DDBJ/EMBL/GenBank databases. Alignments were done using CLUSTAL X software, the phylogenetic trees were constructed by the neighbor-joining method (22), and bootstrap resampling was performed 1,000 times. These analyses were carried out using the Molecular Evolutionary Genetics Analysis (MEGA) software program (http://www.megasoftware.net). Subgenotypes were assigned as described previously (15, 18, 23, 24, 29).
Evidence for HBV genetic recombination.
HBV genetic recombination was investigated using the bootscan analysis implemented in the SimPlot software program (2, 6, 8, 12, 21, 32). Four sequences were used to detect phylogenetically informative sites: the putative recombinant sequence (P18; HBV/B), two consensus sequences of the parental genotypes (D23678 for HBV/B and AB241109 for HBV/C), and a consensus sequence as an outgroup (X75658 for HBV/F) (27, 30). Informative sites were identified where two sequences were shared.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank databases under accession no. AB493827 to AB493848.
RESULTS
Genotypes and subgenotypes of HBV based on the complete genome sequences and phylogenetic relatedness.
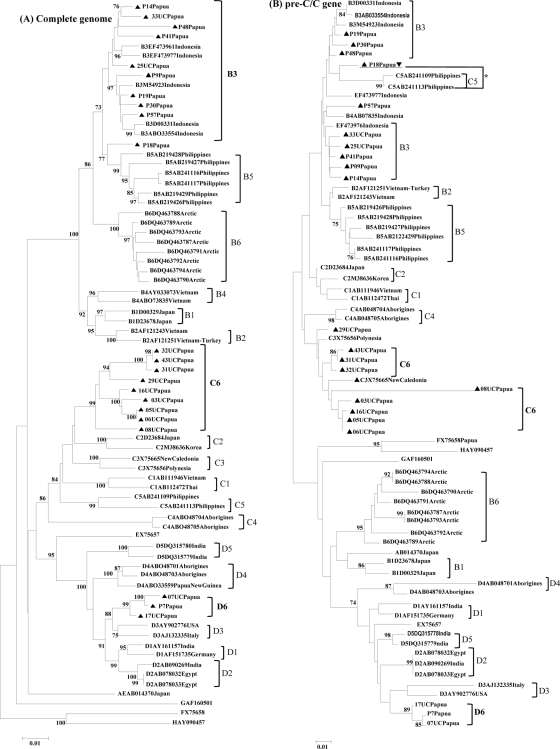
Of 45 serum samples obtained from blood donors in Papua, HBV complete genome sequences were successfully determined for 22 samples (10 HBV/B, 9 HBV/C, and 3 HBV/D; 21 males and 1 female; mean age, 31.5 years; age range, 18 to 45 years). Their demographic and genetic characteristics are summarized in Table 1. Phylogenetic analyses of the complete genome sequences of the 22 strains were conducted by comparing them with the complete genome sequences of 52 HBV strains from DDBJ/EMBL/GenBank (Fig. 1A). They were classified as HBV/B (10 strains), HBV/C (9 strains), and HBV/D (3 strains).
FIG. 1.
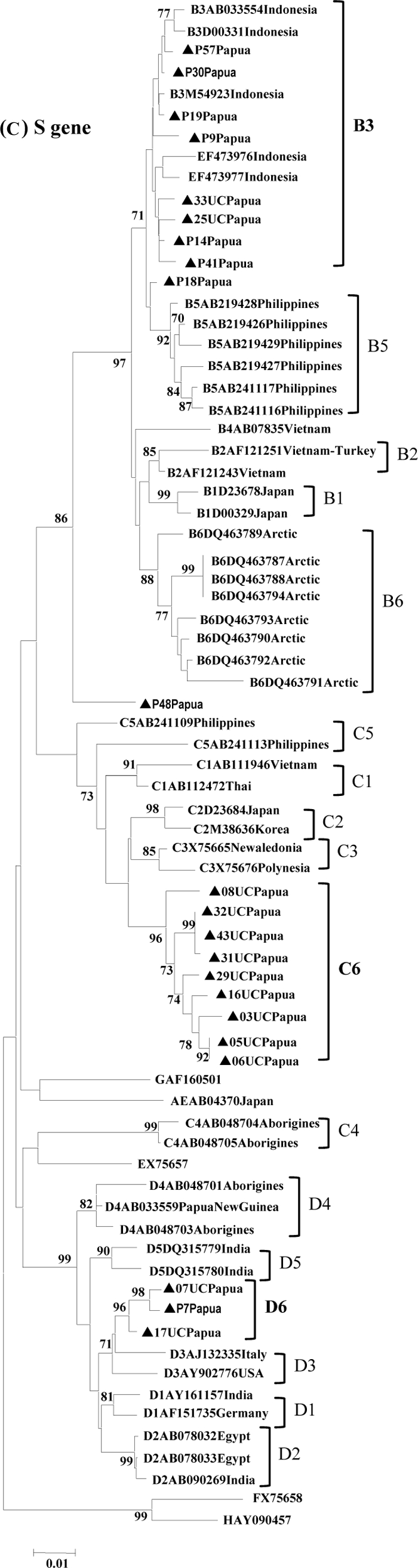
Phylogenetic trees of HBV strains isolated from 22 blood donors in Papua along with 52 reference strains. (A) Complete genome; (B) pre-C/C gene; (C) S gene. The number in the tree indicates the bootstrap reliability. The lengths of the horizontal bars indicate the number of nucleotide substitutions per site. *, HBV/C5 including P18 in this study cluster in the same branch as HBV/B due to the genetic recombination with HBV/B over the pre-C/C gene. Isolates from the database are indicated with accession numbers, and relevant country names are added to each HBV/B, HBV/C, and HBV/D strain. The nucleotide sequence accession numbers used as references in the phylogenetic trees are as follows: for HBV/B, EF473976, EF473977, M54923, AB033554, D00331, AB219426 through AB219429, AB241116, AB241117, DQ463791, DQ463792, DQ463787 through DQ463794, AY033073, AB073835, D00329, D23678, AF121243, and AF121251; for HBV/C, D23684, M38636, X75656, X75665, AB111946, AB112472, AB241109, AB241113, AB048704, and AB048705; for HBV/D, DQ315779, DQ315780, AB048701, AB048703, AB033559, AY161157, AF151735, AB090269, AB078032, AB078033, AJ132335, and AY902776; for HBV/A, AB014370; for HBV/E, EX75657; for HBV/F, FX75658; for HBV/G, GAF160501; and for HBV/H, HAY090457.
HBV/B in Papua.
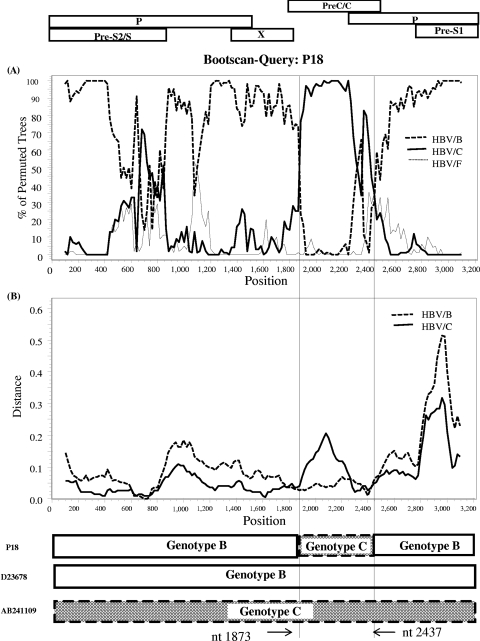
Nine of the 10 HBV/B strains were grouped into subgenotype B3, which formed a cluster including previously reported Indonesian isolates (Fig. 1A). The remaining one strain (P18) was distinctly positioned between HBV/B3 and HBV/B5 and had a high homology with HBV/C5 in the core region (Fig. 1B). SimPlot analysis was applied to determine any possible recombination and its sites between HBV/B and HBV/C5. The bootscan analysis revealed that the P18 strain had undergone a recombination event with HBV/C5 in the pre-C/C region (Fig. 2A). The recombination breakpoints were estimated at nucleotides 1873 and 2437, respectively. To further confirm the recombination event, we performed analysis using a Web-based genotyping resource (Genotyping tool, NCBI [http://www.ncbi.nih.gov/projects/genotyping/formpage.cgi]). The result obtained was consistent with the data from the SimPlot analysis (data not shown).
FIG. 2.
(A) Location of the recombination event in the viral genome of the HBV/B P18 strain determined by the SimPlot program and bootscanning analysis. The sequence of the P18 strain was compared with three representative HBV isolates, HBV/B (GenBank accession no. D23678), HBV/C (accession no. AB241109), and outgroup HBV/F (accession no. X75658) over the full genome with a 200-bp window size, a 20-bp step size, 100 bootstrap replicates, gap stripping, and neighbor-joining analysis. (B) Genetic distances of the P18 strain from HBV/B (GenBank accession no. D23678) and HBV/C (accession no. AB241109) over the complete genome, with a window size of 200 bp and a step size of 20 bp. The dotted vertical lines show the breakpoints of recombination.
A previous study from Nusa Tenggara, Indonesia, reported the presence of the novel HBV subgenotype B7 (18). Strains of this subgenotype, however, are genetically close to the HBV/B3 strains in this study. Five HBV/B3 strains in particular (25UC, 33UC, P14, P41, and P48) showed high homology with the strains described as HBV/B7 (Fig. 1A), with 1.9 to 2.9% divergence. To confirm the relationship, we also analyzed each open reading frame, core (Fig. 1B), large S (pre-S1 to S gene) (Fig. 1C), P, and X gene (data not shown). The results were mostly consistent with that obtained for the complete genome sequence analysis. No specific mutations in the small S gene, including residues 124 to 147 of HBsAg of the HBV/B strains, were found in the strains from Papua or those from Nusa Tenggara (see Fig. S1 in the supplemental material). Therefore, the HBV/B strains from Nusa Tenggara, which were previously reported to be a novel subgenotype (18), can be included in HBV/B3 along with the strains in this study.
HBV/C in Papua.
All nine HBV/C strains formed a novel cluster, separated from the other HBV/C strains (C1 to C5) based on the complete genome sequences (Fig. 1A). This result confirms our previous observation and proposal of a novel subgenotype HBV/C6 based on the S gene analysis (9). With a high divergence from other subgenotypes, specific amino acid substitutions in the small S gene, G18V and V47M, were found (see Fig. S1 in the supplemental material). These nine strains in Papua were shown to be more closely related to the HBV/C3 strains from New Caledonia and the HBV/C2 strains from east Asia than to the HBV/C1 strains from southeast Asia (Fig. 1A to C).
HBV/D in Papua.
All three HBV/D strains also formed a novel cluster, separated from the other HBV/D strains (D1 to D5) with significant bootstrap values, based on the complete genome sequences (Fig. 1A). This result again confirms our previous observation and proposal of the novel subgenotype HBV/D6 based on S gene analysis (9). The three strains in Papua were shown to be closely related to the HBV/D3 strains from the Western countries (Fig. 1A).
Divergences of the entire nucleotide sequences among HBV/C6 and HBV/D6 strains.
Divergences in the entire genome sequences of the novel subgenotypes HBV/C6 and HBV/D6 were examined by comparing with the reference sequences of the other subgenotypes within a given genotype. The seven HBV/C6 strains showed 0.1 to 3.2% divergence from each other. On the other hand, they showed divergences of 6.2 to 6.7% with HBV/C5, 6.6 to 6.9% with HBV/C4, 4.4 to 4.9% with HBV/C3, 4.7 to 5.1% with HBV/C2, and 6.2 to 6.6% with HBV/C1. Similarly, the three HBV/D6 strains showed 1.4 to 2.0% divergence from each other but showed divergences of 4.7 to 6.3% with HBV/D5, 4.1 to 5.6% with HBV/D4, 3.3 to 4.7% with HBV/D3, 3.8 to 5.3% with HBV/D2, and 3.4 to 4.8% with HBV/C1.
DISCUSSION
In our previous report on HBV in Papua, Indonesia, we provisionally proposed novel subgenotypes HBV/C6 and HBV/D6, and other ambiguous subgenotypes of HBV/B, based on the S gene sequence variations (9). Needless to say, HBV phylogenetic analysis based on the complete genome sequences is more reliable than that of the S gene alone (14, 20). In this study, therefore, we performed complete genome sequence analysis and confirmed the presence of novel subgenotypes HBV/C6 and HBV/D6 in Papua (Fig. 1A). Subgenotypes are determined, in general, on the basis of sequence divergence from the complete genome sequence by 4% or greater (16, 19, 23). The HBV/C6 and HBV/D6 strains in this study showed ca. 4% or greater divergence from the complete genome sequence in comparison with other existing subgenotypes HBV/C1 to HBV/C5 and HBV/D1 to HBV/D5, respectively. In addition to the new subgenotypes, a unique HBV/B strain that has recombination with HBV/C5 in the pre-C/C region (P18) was found (Fig. 1 and 2). This strain was distinctly positioned between HBV/B3 and HBV/B5.
Of the 10 HBV/B isolates examined, 9 were classified as HBV/B3, together with the strains from the other place (Nusa Tenggara) in Indonesia (18). This HBV/B3 cluster can now be divided into two groups, although high homology is indicated between the two groups. We suggest that the strains from Nusa Tenggara can be considered a subgroup of HBV/B3 due to its comparatively low divergence (1.9 to 3.0%) from the other HBV/B3 strains. In general, subgenotypes are divided according to 4% or greater difference in the entire nucleotide sequences (16, 19, 23).
Recombination between HBV genotypes is a common event in countries where different genotypes are prevalent. Moreover, the recombination between HBV/B and HBV/C was found frequently in southeast Asia, and the breakpoint of recombination has been reported to be in the pre-C/C region (5, 8, 12, 25, 27, 30). Consistent with the previous reports, we found in this study the possible recombination in the pre-C/C region of the P18 strain (HBV/B) with HBV/C (Fig. 2). It is increasingly accepted that recombination between genotypes generates novel variants that contribute to the genetic diversity of HBV. Thus, genetic recombination is a significant and relatively frequent event in the evolution of HBV (26).
HBV/D was also found in parts of Asia and in aboriginal populations in Papua New Guinea (28). The phylogenetic position of HBV/D6 (7UC, 17UC, and P7 strains) appears to be closer to HBV/D3 strains isolated from the Western countries than those from Asia and Papua New Guinea (Fig. 1). The significance of this relationship, however, remains unclear.
In conclusion, HBV genotypes B, C, and D are prevalent in Papua, Indonesia. We confirmed the presence of the novel subgenotypes HBV/C and HBV/D on the basis of the complete genome analysis. Our study will lead to interesting findings on the genetic variety of HBV, as well as its clinical relevance in Papua, Indonesia, a multiethnic nation.
Supplementary Material
Acknowledgments
This study was supported by a grant-in-aid through the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Published ahead of print on 22 April 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Banerjee, A., F. Kurbanov, D. Sibnarayan, P. K. Chandra, Y. Tanaka, M. Mizokami, and R. Chakravarty. 2006. Phylogenetic relatedness and genetic diversity of hepatitis B virus isolates in Eastern India. J. Med. Virol. 781164-1174. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan, R., S. N. Kazim, M. Kumar, J. Bhattacharjee, N. Krishnamoorthy, and S. K. Sarin. 2008. Identification and characterization of genotype A and D recombinant hepatitis B virus from Indian chronic HBV isolates. World J. Gastroenterol. 146228-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devesa, M., C. L. Loureiro, Y. Rivas, F. Monsalve, N. Cardona, M. C. Duarte, F. Poblete, M. F. Gutierrez, C. Botto, and F. H. Pujol. 2008. Subgenotype diversity of hepatitis B virus American genotype F in Amerindians from Venezuela and the general population of Colombia. J. Med. Virol. 8020-26. [DOI] [PubMed] [Google Scholar]
- 4.Furusyo, N., N. Kubo, H. Nakashima, K. Kashiwagi, and J. Hayashi. 2004. Relationship of genotype rather than race to hepatitis B virus pathogenicity: a study of Japanese and Solomon Islanders. Am. J. Trop. Med. Hyg. 70571-575. [PubMed] [Google Scholar]
- 5.Huy, T. T. T., A. A. Sall, J. M. Reynes, and K. Abe. 2008. Complete genomic sequence and phylogenetic relatedness of hepatitis B virus isolates in Cambodia. Virus Genes 36299-305. [DOI] [PubMed] [Google Scholar]
- 6.Laoi, B. N., and B. Crowley. 2008. Molecular characterization of hepatitis B virus (HBV) isolates, including identification of a novel recombinant, in patients with acute HBV infection attending an Irish hospital. J. Med. Virol. 801554-1564. [DOI] [PubMed] [Google Scholar]
- 7.Lee, W. M. 1997. Hepatitis B virus infection. N. Engl. J. Med. 3371733-1745. [DOI] [PubMed] [Google Scholar]
- 8.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lusida, M. I., V. E. Nugrahaputra, Soetjipto, R. Handajani, M. Nagano-Fujii, M. Sasayama, T. Utsumi, and H. Hotta. 2008. Novel subgenotypes of hepatitis B virus genotypes C and D in Papua, Indonesia. J. Clin. Microbiol. 462160-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merican, I., R. Guan, D. Amarapuka, M. J. Alexander, A. Chutaputti, R. N. Chien, S. S. Hasnian, N. Leung, L. Lesmana, P. H. Phiet, H. M. Sjalfoellah Noer, J. Sollano, H. S. Sun, and D. Z. Xu. 2000. Chronic hepatitis B virus infection in Asian countries. J. Gastroenterol. Hepatol. 151356-1361. [DOI] [PubMed] [Google Scholar]
- 11.Mizokami, M., T. Nakano, E. Orito, Y. Tanaka, H. Sakugawa, M. Mukaide, and B. H. Robertson. 1999. Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett. 45066-71. [DOI] [PubMed] [Google Scholar]
- 12.Morozov, V., M. Pisareva, and M. Groudinin. 2000. Homologous recombination between different genotypes of hepatitis B virus. Gene 26055-65. [DOI] [PubMed] [Google Scholar]
- 13.Mulyanto, F. Tsuda, A. T. Karossi, S. Soewignjo, Roestamsjah, D. Sumarsidi, R. H. Trisnamurti, Sumardi, Surayah, L. Z. Udin, Melani-Wikanta, K. Kanai, and S. Mishiro. 1997. Distribution of the hepatitis B surface antigen subtypes in Indonesia: implications for ethnic heterogeneity and infection control measures. Arch. Virol. 1422121-2129. [DOI] [PubMed] [Google Scholar]
- 14.Nagasaki, F., H. Niitsuma, J. G. Cervantes, M. Chiba, S. Hong, T. Ojima, Y. Ueno, E. Bondoc, K. Kobayashi, M. Ishii, and T. Shimosegawa. 2006. Analysis of the entire nucleotide sequence of hepatitis B virus genotype B in the Philippines reveals a new subgenotype of genotype B. J. Gen. Virol. 871175-1180. [DOI] [PubMed] [Google Scholar]
- 15.Norder, H., A. M. Couroucé, P. Coursaget, J. M. Echevarria, S. D. Lee, I. K. Mushahwar, B. H. Robertson, S. Locarnini, and L. O. Magnius. 2004. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 47289-309. [DOI] [PubMed] [Google Scholar]
- 16.Norder, H., A. M. Couroucé, and L. O. Magnius. 1994. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology 198489-503. [DOI] [PubMed] [Google Scholar]
- 17.Norder, H., and L. O. Magnius. 1993. Complete nucleotide sequences of six hepatitis B viral genomes encoding the surface antigen subtypes ayw4, adw4q-, and adrq- and their phylogenetic classification. Arch. Virol. Suppl. 8189-199. [DOI] [PubMed] [Google Scholar]
- 18.Nurainy, N., D. H. Muljono, H. Sudoyo, and S. Marzuki. 2008. Genetic study of hepatitis B virus in Indonesia reveals a new subgenotype of genotype B in east Nusa Tenggara. Arch. Virol. 1531057-1065. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto, H., F. Tsuda, H. Sakugawa, R. I. Sastrosoewignjo, M. Imai, Y. Miyakawa, and M. Mayumi. 1988. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J. Gen. Virol. 692575-2583. [DOI] [PubMed] [Google Scholar]
- 20.Orito, E., T. Ichida, H. Sakugawa, M. Sata, N. Horiike, K. Hino, K. Okita, T. Okanoue, S. Iino, E. Tanaka, K. Suzuki, H. Watanabe, S. Hige, and M. Mizokami. 2001. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology 34590-594. [DOI] [PubMed] [Google Scholar]
- 21.Robertson, D. L., B. H. Hahn, and P. M. Sharp. 1995. Recombination in AIDS viruses. J. Mol. Evol. 40249-259. [DOI] [PubMed] [Google Scholar]
- 22.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4406-425. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto, T., Y. Tanaka, J. Simonetti, C. Osiowy, M. L. Borresen, A. Koch, F. Kurbanov, M. Sugiyama, G. Y. Minuk, B. J. McMahon, T. Joh, and M. Mizokami. 2007. Classification of hepatitis B virus genotype B into 2 major types based on characterization of a novel subgenotype in Arctic indigenous populations. J. Infect. Dis. 1961487-1492. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto, T., Y. Tanaka, E. Orito, J. Co, J. Clavio, F. Sugauchi, K. Ito, A. Ozasa, A. Quino, R. Ueda, J. Sollano, and M. Mizokami. 2006. Novel subtypes (subgenotypes) of hepatitis B virus genotypes B and C among chronic liver disease patients in the Philippines. J. Gen. Virol. 871873-1882. [DOI] [PubMed] [Google Scholar]
- 25.Simmonds, P., and S. Midgley. 2005. Recombination in the genesis and evolution of hepatitis B virus genotypes. J. Virol. 7915467-15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugauchi, F., H. Kumada, S. A. Acharya, S. M. Shrestha, M. T. Gamutan, M. Khan, R. G. Gish, Y. Tanaka, T. Kato, E. Orito, R. Ueda, T. Miyakawa, and M. Mizokami. 2004. Epidemiological and sequence differences between two subtypes (Ae and Aa) of hepatitis B virus genotype A. J. Gen. Virol. 85811-820. [DOI] [PubMed] [Google Scholar]
- 27.Sugauchi, F., E. Orito, T. Ichida, H. Kato, H. Sakugawa, S. Kakumu, T. Ishida, A. Chutaputti, C. L. Lai, R. Ueda, Y. Miyakawa, and M. Mizokami. 2002. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J. Virol. 765985-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugauchi, F., M. Mizokami, E. Orito, T. Ohno, H. Kato, S. Suzuki, Y. Kimura, R. Ueda, L. A. Butterworth, and W. G. E. Cooksley. 2001. A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: complete genome sequence and phylogenetic relatedness. J. Gen. Virol. 82883-892. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka, Y., E. Orito, M. F. Yuen, M. Mukaide, F. Sugauchi, K. Ito, A. Ozasa, T. Sakamoto, F. Kurbanov, C. L. Lai, and M. Mizokami. 2005. Two subtypes (subgenotypes) of hepatitis B virus genotype C: a novel subtyping assay based on restriction fragment length polymorphism. Hepatol. Res. 33216-224. [DOI] [PubMed] [Google Scholar]
- 30.Tran, T. T., T. N. Trinh, and K. Abe. 2008. New complex recombinant genotype of hepatitis B virus identified in Vietnam. J. Virol. 825657-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utsumi, T., Y. Yano, B. X. Truong, Y. Tanaka, M. Mizokami, Y. Seo, M. Kasuga, M. Kawabata, and Y. Hayashi. 2007. Molecular epidemiological study of hepatitis B virus infection in two different ethnic populations from the Solomon Islands. J. Med. Virol. 79229-235. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Z., Z. Liu, G. Zeng, S. Wen, Y. Qi, S. Ma, N. V. Naoumov, and J. Hou. 2005. A new intertype recombinant between genotypes C and D of hepatitis B virus identified in China. J. Gen. Virol. 86985-990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.