Abstract
Determining the transient chemical properties of the intracellular environment can elucidate the paths through which a biological system adapts to changes in its environment, for example, the mechanisms that enable some obligate anaerobic bacteria to survive a sudden exposure to oxygen. Here we used high-resolution Fourier transform infrared (FTIR) spectromicroscopy to continuously follow cellular chemistry within living obligate anaerobes by monitoring hydrogen bond structures in their cellular water. We observed a sequence of well orchestrated molecular events that correspond to changes in cellular processes in those cells that survive, but only accumulation of radicals in those that do not. We thereby can interpret the adaptive response in terms of transient intracellular chemistry and link it to oxygen stress and survival. This ability to monitor chemical changes at the molecular level can yield important insights into a wide range of adaptive responses.
Keywords: Desulfovibrio, hydrogen bond, synchrotron FTIR spectromicroscopy, oxygen stress, cellular water
Significant progress has been made at the biochemical and genetic levels in our understanding of how some environmentally and medically important obligate anaerobes can survive temporarily a sudden exposure to oxygen molecules (1–6). However, our understanding at a cellular molecular level of the actual capacity and mechanisms of how these anaerobes survive remains incomplete (7). The cellular chemical environment fundamentally comprises the nexus between external stimuli and internal biochemical regulatory mechanisms—and affects many properties of cellular adaptive response as well. Determining this transient chemical environment in vivo is critical for achieving a more coherent understanding of how some obligate anaerobes adapt to the extreme fluctuations in oxygenation. Such knowledge is seldom complete because it is difficult to make in vivo molecular measurements without disturbing cells. In almost all previous studies, cellular chemistry of oxygen-stress adaptive response has been determined by measuring intermediate reaction products in cell extracts taken at selected times (8–10); notably, ongoing changes within a live cell are seldom measured directly. The instability of many of the intermediates greatly complicates measurements of cell extracts and their analyses.
Here, we present results of our using the non-invasive synchrotron radiation-based Fourier transform infrared (FTIR) spectromicroscopy approach (11) to determine the cellular chemical environment by continuously monitoring the dynamics of hydrogen bonding in cellular water in vivo. More than 70% of the cellular constituents are highly polar water molecules, and their hydrogen bonding is a useful reflection of the cellular chemical environment because it responds “instantaneously” to ions and other species in their surroundings (12, 13). The infrared spectrum of OH stretch vibrations has been widely used to characterize the dynamics of hydrogen-bonding structures in pure water (13–23). These infrared spectroscopy studies have revealed distinct shifts in vibration frequencies and changes in spectral shapes and intensities induced by the presence of ions and small molecules (e.g., radicals, small organic acids, and hydrogen gas) in water; similar small molecules are expected to be in cellular water during functional metabolism of the oxygen-stress adaptive response.
In this study, we investigated the dynamics of cellular chemical environment in a model oxygen-stress adaptive response system, namely that of the strictly anaerobic sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough during transient exposure to air. Sulfate-reducing bacteria are of particular interest because of their importance in cycling and transformation of essential nutrients and minerals (24, 25) and of their links to different pathogenesis (26, 27) in environments where extreme fluctuations in oxygen concentrations occur. Among sulfate-reducing bacteria, genome sequencing has shown that D. vulgaris has developed well-defined protective enzymatic oxygen-defense systems (5, 24). The bacteria can even survive very high levels of oxygen in their natural environment (28, 29), but the mechanism remains elusive. We demonstrate here that molecular information provided by real-time, in vivo FTIR measurements of the transient cellular chemical environment is critical for advancing a fundamental understanding of how these obligate anaerobes adapt to extreme changes during air exposure, by providing direct observations of molecular events measured in the same cells over time.
Results and Discussion
Identification of D. vulgaris Cells That Can Survive Temporarily in Atmospheric Oxygen.
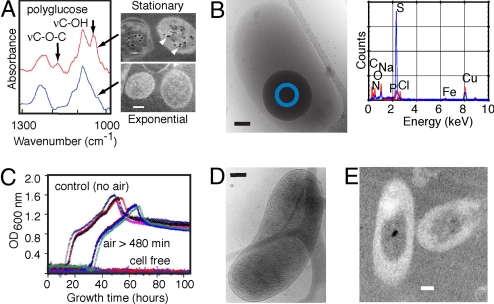
We first conducted microscopic and spectroscopic analyses to establish at a whole cell level the molecular identity of D. vulgaris cells that can survive transient exposure to atmospheric oxygen. This identity enabled us to select the appropriate cells for the real-time FTIR measurements of the oxygen-stress survival response. Fluorescence microscopy images of 2 nucleic acid stains show that most stationary-phase (but not exponential-phase) D. vulgaris can survive short-term oxygen exposure. Subsequent electron microscopy images reveal that stationary-phase (but not exponential-phase) D. vulgaris accumulates polyglucose (Fig. 1A) and stores elemental sulfur particles (Fig. 1B). Their FTIR spectrum shows distinctly the non-glycosidic polyglucose vibration (υC−OH) band between 1,055 and 1,045 cm−1, and the glycosidic linkage vibration (υC−O−C) at ≈1,175 cm−1 (30). These cells can survive air exposure for hours and resume growth when returned to anaerobic conditions (Fig. 1C), despite significant changes in cellular structures and contents (Figs. 1 D and E).
Fig. 1.
Microscopic and spectroscopic analyses of Desulfovibrio vulgaris. (A) Left, typical infrared absorption spectra of stationary-phase (red) and exponential-phase (blue) D. vulgaris; Right, transmission electron microscopy (TEM) images of thin sections poststained by the periodic acid thiosemicarbazide-osmium (PATO) method (51) show intracellular polyglucose granules (arrows) in stationary-phase but not exponential-phase D. vulgaris. (B) Left, cryo-electron microscopy (Cryo-EM) image of a stationary-phase cell containing a large, dense ball; Right, energy dispersive X-ray analysis of freeze-dried cells with (blue) and without (red) such structures. Spectra from areas such as marked by the blue circle in the left image show that the particle contains mainly sulfur. (C) Re-growth of D. vulgaris after exposure to air. Note the approximate 20-h lag-time (compared to controls). Different colors represent different viability experiments. (D and E) Cryo-EM (Left) and TEM/PATO (Right) images of D. vulgaris after exposure to air for hours show changes in cell membranes, variation in periplasmic space, mottled appearance of cell contents and decreased number of polyglucose granules compared to the unexposed cell in Figs. 1 A and B. The frequency of cells showing such alterations compared to those with substantially more damage suggests that these cells were still alive. (Scale bars, 0.2 μm.)
FTIR Measurement and Analysis Considerations.
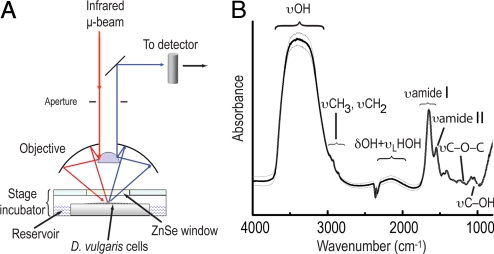
Mid-infrared photons emitted from the synchrotron at the Advanced Light Source in the Lawrence Berkeley National Laboratory (CA, USA) were focused through a 15-μm aperture onto a monolayer of stationary-phase D. vulgaris cells maintained inside an oxygen-free humidified microscope stage chamber (Fig. 2A). In Fig. 2B is an FTIR spectrum typical of small groups of stationary-phase D. vulgaris cells, showing well-resolved vibration bands from polyglucose (30) and other biological macromolecules (31) superimposed on the broad continuum absorption features of the aqueous liquid. To minimize inter-experimental uncertainties, only cells that exhibited spectral features within one standard deviation of the mean (Fig. 2B) were selected for the oxygen-stress adaptive response experiments and the controls. We used the spectrally integrated absorption intensity of the polyglucose υC-OH band between ≈1,055 and 1,045 cm−1 to monitor polyglucose degradation. The intensity of the combination band δOH +υLHOH at ≈2,100 cm−1 tracks changes in water content because biomolecules typically show very little absorbance in the δOH +υLHOH region (31); the intensity of this band represents water concentration in biological samples (32). To detect changes in the hydrogen-bonding structures in cellular water as a measure of transient chemical environment in living D. vulgaris, we first derived the FTIR time-difference spectra (relative to the initial state, t = 0) in the hydride-OH dominated stretch region between 1,900 and 3,800 cm−1 from the measured real-time FTIR spectra. In each time-difference spectrum, we minimize the water-continuum absorbance; a positive absorption band reflects the formation of intermediates while a negative band the depletion of an initial state. To interpret changes observed in the FTIR time-difference spectra and to link them to the presence of ions and other small molecules, we used results from previous infrared simulation studies and infrared measurements on aqueous liquid and water clusters (15–23). Because water molecules simultaneously can be hydrogen donors and acceptors, whether the water be liquid or small clusters, spectral information from vibrational spectra of water clusters can be applied to understand dynamics in liquid or other condensed phases (33, 34) such as water in the cellular environment.
Fig. 2.
FTIR measurement setup. (A) Schematic description of the experimental measurement setup. An all-reflective optics infrared microscope focuses the interferometer-modulated synchrotron infrared microbeam through a 15-μm aperture onto a monolayer (see Methods) of live D. vulgaris. The reflected signals are collected and sent to the detector. The optical density of this thin film is typically 0.05 at the band dominated by the combined water-bending vibration and libration at ≈2,100 cm−1. This is an equivalent to a water-film of ≈1.5-μm thick. This experimental system enables FTIR measurements with a temporal resolution of every minute for up to 4 h; a different experimental arrangement would be needed to investigate changes on a finer temporal scale or a longer duration. (B) Spectral variations in polyglucose-accumulated stationary-phase D. vulgaris in anaerobic atmosphere. The spectrum shows the polyglucose C−OH vibration (υC−OH) band between 1,055 and 1,045 cm−1. Within the hydride-OH dominated stretch region between 1,900 and 3,800 cm−1 are a broad OH stretching (υOH) band between 2,900 and 3,700 cm−1, the combined water OH bending and libration modes (δOH +υLHOH) at ≈2,100 cm−1. Absorption bands between 1,800 and 900 cm−1 are dominated by vibration motion of biomolecules of D. vulgaris. Averaged spectrum (black line) ± 1.0 standard deviation (gray lines); n = 50.
Real-Time FTIR Spectromicroscopy of Oxygen-Stress Adaptive Response in Live D. vulgaris.
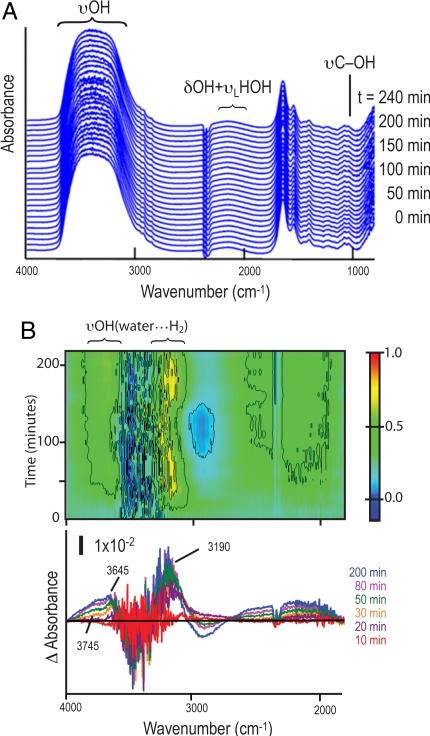
We monitored cells first under anaerobic conditions and then exposed to air. The measurements are shown in Figs. 3 and 4, respectively, with comparisons shown in Figs. 4 C and D. The experiments proceeded as follows. We first made real-time FTIR measurements on a monolayer of D. vulgaris inside the oxygen-free humidified microscope stage chamber [hereafter D. vulgaris(+polyG;-air)] every 5 min for 240 min without interruption. Fig. 3A shows the real-time FTIR spectra. An easy way to analyze and understand the time-difference spectra is to make a 2-dimensional time-frequency contour plot of the difference spectra in the hydride-OH stretch region, as shown in Fig. 3B (negative values are shown in dark blue) with difference spectrum snapshots below. The plot shows, for t < ≈50 min, positive bands at ≈3,190 cm−1, 3,645 cm−1, and a shoulder feature at 3,745 cm−1. These frequencies are in the υOH regions of H-bonding structures of water molecules surrounded by hydrogen gas H2 (i.e., hydrogen hydrates) (14). This increasing positive behavior suggests a temporarily enhanced hydrogen gas production event, which is consistent with the central metabolism of D. vulgaris under anaerobic conditions (24, 35). This spectral information is taken as reference.
Fig. 3.
FTIR analyses of D. vulgaris in anaerobic atmosphere. (A) Real-time FTIR spectra of polyglucose-accumulated stationary-phase D. vulgaris in an anaerobic environment. Sequential spectra are offset vertically for clarity. Since all spectra are derived using air as a reference, the negative spectral feature at ≈2,348 cm−1 (associated with lack of atmospheric CO2) is a marker for an air-free condition throughout this investigation. (B) FTIR time-difference spectra in the hydride-OH dominated stretch region. Top, a 2-dimensional frequency-time contour plot (the time-difference intensities are normalized to the maximum); Bottom, snapshots for selected different time points. Positive bands [labeled as υOH(water···H2)] arise from υOH of water molecules forming H-bonding with H2 (≈3,190; ≈3,640, and ≈3,745 cm−1). The straight black line marks that difference absorbance = 0.
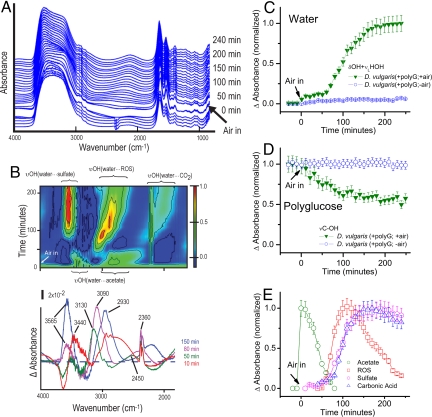
Fig. 4.
FTIR analyses of D. vulgaris during oxygen-stress adaptive response. (A) Typical real-time FTIR spectra of polyglucose-accumulated stationary-phase D. vulgaris transition from an anaerobic to aerobic environment. Sequential spectra are offset upward for clarity. Since all spectra are derived using air as a reference, the abrupt change in the spectral feature at ≈2,348 cm−1 associated with the presence of atmospheric CO2. (B) Corresponding FTIR time-difference spectra in the hydride-OH dominated stretch region. Top, a 2-dimensional frequency-time contour plot (the time-difference intensities are normalized to the maximum); Bottom, snapshots for selected different time points. The dashed line marks that difference absorbance = 0. Positive values arise from υOH of water molecules forming H-bonding with acetate (≈3,440; ≈2,930 cm−1) [labeled as υOH(water···acetate)], reactive oxygen species ROS (≈3,090 cm−1) [labeled as υOH(water···ROS)], sulfate (≈3,565 cm−1) [labeled as υOH(water···sulfate)], and carbonic acid (≈2,450 cm−1) [labeled as υOH(water···CO2)]. The positive absorption feature at ≈2,360 cm−1 is from CO2 in air. (C and D). Typical time course of infrared intensity (normalized by the maximum value) of water and polyglucose content. (Bars, ±10% error.) (E) Transient chemistry as seen by the time-course of difference absorbance normalized by the maximum value for each species. (Bars, ±10% error.)
Using the same methodology, we then examined the survival of similar D. vulgaris [D. vulgaris(+polyG;+air)] in atmospheric oxygen. We began by making FTIR measurements of D. vulgaris in an anaerobic atmosphere for 30 min before introducing sterile air at t = 0. Unlike the quiescent FTIR spectra from D. vulgaris(+polyG;-air) (Fig. 3A), these spectra show dramatic variations over time (Fig. 4A). The 2-dimensional time-frequency contour plot of the time-difference spectra in the hydride-OH stretch region is shown in Fig. 4B with time-difference spectrum snapshots below.
Spectrally integrated absorption intensities of water and polyglucose bands are shown in Figs. 4 C and D. Under anaerobic conditions, the intensity of the combined water OH bending and libration modes (blue circles in Fig. 4C) and the polyglucose C−OH vibration band (blue circles in Fig. 4D) exhibit little change, but after air exposure they exhibit a multiphasic pattern. There is a small but reproducible “jump” in the water band intensity (green inverted triangles in Fig. 4C) upon air exposure, which can result from a contribution due to periplasmic oxygen reduction to water by cytochrome c3 with existing intracellular H2 (36, 37), since we observed H2 was produced during anaerobic metabolism (see Fig. 3B). Then, from t > 0 to < ≈50 min, there is a substantial decrease in the polyglucose band intensity (Fig. 4D) but little change in the water band intensity (Fig. 4C). Polyglucose oxidation by D. vulgaris(+polyG;+air) may contribute to this substantial polyglucose decline. We performed an independent carbohydrate analysis of similar D. vulgaris exposed to humid air which showed a more than 30% decrease in carbohydrate in cells in the first hour of air exposure. At t > ≈50 min, the water band intensity increases abruptly (Fig. 4C); the rate of polyglucose disappearance slows at later times (t > ≈100 min) (Fig. 4D). For an elucidation of the mechanism(s) underlying this behavior, it is crucial to analyze the time-difference spectra in the hydride-OH region carefully.
As seen in Fig. 4B, between t >0 and < ≈50 min there are 2 large increasingly positive absorption bands, and the band positions are in the region of the υOH of a water molecule H-bonding with a carboxylic acid or carboxylate: the band between ≈3,500 and 3,300 cm−1 corresponds to the υOH of a water molecule H-bonding loosely with the alcohol OH group (19), and the band between≈3,000 and 2,600 cm−1 corresponds to the υOH of a water molecule tightly H-bonded to a carboxyl (C = O) group (19). Polyglucose oxidation by desulfovibrios to acetate (a 2-carbon carboxylate) (9, 38) may contribute to these 2 large increasingly positive bands. The significant increase with time in the peak intensity of acetate observed (labeled as green circles in Fig. 4E), and the concurrent decrease in the polyglucose band intensity (inverted green triangles in Fig. 4D) suggest that D. vulgaris(+polyG;+air) oxidizes polyglucose rapidly to acetate, which it initially accumulates.
The noticeable disappearance of these 2 acetate υOH bands at t > ≈50 min (Fig. 4B) implies disappearance of acetate (see also green circles in Fig. 4E), even though polyglucose degradation continues for a time (green inverted triangles in Fig. 4D). Meanwhile, a new broad υOH band begins to appear at frequencies between ≈2,750 and 2,550 cm−1 (Fig. 4B) in the region of a water molecule H-bonded with CO2 (39) (see reference list in ref. 39) to form carbonic acid (blue triangles in Fig. 4E). The most likely explanation of this co-incidence at t > ≈50 min (compare the green circles for acetate and the blue triangles for CO2 in Fig. 4E) is a conversion of acetate to CO2 (with increasing water content in cells; see inverted green triangles in Fig. 4C). This co-incidence suggests an onset of an adenosine triphosphate (ATP)-generating pathway, possibly via C1 intermediates (40). Other pathways would be the tricarboxcylic acid (TCA) cycle or the glyoxylate shunt, although they are less likely to occur since D. vulgaris lacks the genes for the production of key enzymes required in these cycles (24, 41).
Also appearing at t > ≈50 min is an intense positive υOH band between ≈3,200 and 3,030 cm−1 (Fig. 4B), which is typical of a water molecule that forms a strong ionic H-bond with species such as the superoxide anion (16), hydroxyl radicals (15, 17, 18), and the hydroperoxyl radicals (22). This suggests that the formation of ROS exceeds their removal by the protective enzymes and other mechanisms in D. vulgaris(+polyG;+air) at this time (red squares in Fig. 4E).
At t = ≈70 min, a striking new υOH band begins to appear between ≈3,630 and 3,520 cm−1 in the spectral region of water molecules H-bonded to sulfate anions (23). Its notable persistence in intensity (pink hexagons in Fig. 4E) is consistent with previous observation that D. vulgaris can oxidize its accumulated elemental sulfur and other reduced sulfur compounds (42, 43). It has been suggested that the oxidation is by means of an ATP/adenyl sulfate (ATP/APS) pathway that couples the sulfate ion formation with oxygen reduction to water (42, 43). Considering that sulfate formation is an acidification reaction that produces protons (H+), it is interesting that this acidification process coincides with the disappearance of ROS (compare the pink hexagons to the red squares in Fig. 4E) and the increase of water content (green inverted triangles in Fig. 4C).
There is increasing spectral complexity after t = ≈70 min, as suggested by the pattern of the contours in Fig. 4B. The complexity includes progressive band broadening (≈100 cm−1) and a redshift (≈250 cm−1) in the existing υOH band of the water···ROS system (between ≈3,200 and 3,030 cm−1) as well as the water···CO2 system (at frequencies < ≈2,650 cm−1). This may arise partly from an increase in water molecules available to H-bond with either other anions or neutral species in D. vulgaris(+polyG;+air), or other more complicated factors that distort the spectral character (44, 45). For longer times (t > ≈150 min), the intensity of the υOH band of the water···ROS declines (red squares in Fig. 4E), which suggests an improved ROS removal in D. vulgaris(+polyG;+air).
Confirmation.
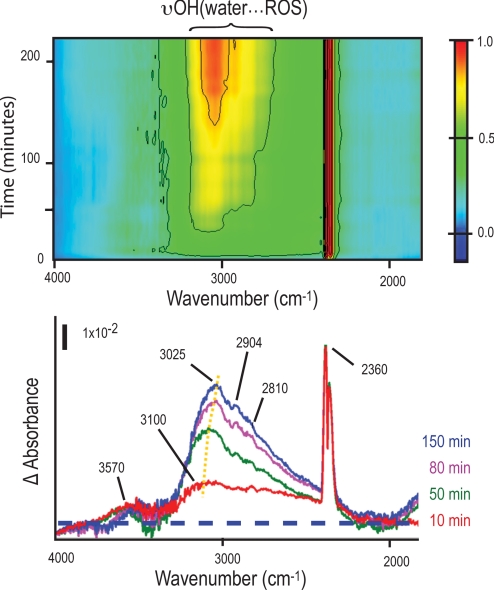
To confirm our results, we therefore used the same method and examined how the FTIR spectra in the hydride-OH dominated stretch region would differ in D. vulgaris that had not accumulated intracellular polyglucose granules [hereafter D. vulgaris(−polyG;+air)]. Unlike the D. vulgaris(+polyG;+air), a positive broad υOH feature appeared immediately in the frequency range associated with the ionic H-bond with anionic ROS (15–18, 22) (Fig. 5Upper). The feature broadened by several hundreds of cm−1 toward lower frequencies, and reflects an initial disordered ensemble of many different OH stretching vibration modes. The ROS formation continued to exceed their removal with air exposure time, as demonstrated by the monotonically increasing intensity and gradual red-shifts of the vibration modes (centered at ≈3,100 cm−1 initially to ≈3,050 cm−1 at later times). The immediate positive feature detected at ≈3,570 cm−1 (the red trace in Fig. 5 Lower) is within the spectral region of water molecules H-bonded to sulfate anions (23). This small positive value is consistent with our earlier observation that D. vulgaris in exponential phase accumulates little elemental sulfur. Little growth was detected when these D. vulgaris were returned to oxygen-free conditions.
Fig. 5.
Typical FTIR difference spectra show reactive oxygen species (ROS) build-up in polyglucose-deficient exponential-phase D. vulgaris. Top, a 2-dimensional frequency-time contour plot (the time-difference intensities are normalized to the maximum); Bottom, snapshots for selected different time points. The peak centered at ≈3,100 cm−1 and other local maxima centered at ≈2,904 cm−1 and 2,810 cm−1 are at frequencies typical of the υOH of water molecules H-bonding with hydroxyl and hydroperoxyl radicals [labeled as υOH(water···ROS)]. The feature at ≈3,570 cm−1 is at a frequency typical of the υOH of water molecules H-bonded to sulfate anions. Yellow dots mark the red-shift of ≈75 cm−1 of υOH of hydroxyl radical band peak. The dashed line marks that difference absorbance = 0. The positive absorption feature at ≈2,360 cm−1 is from CO2 in air.
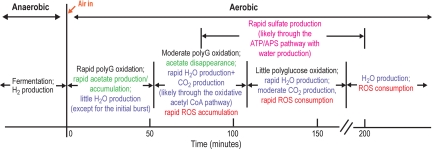
Conclusions and Implications.
Our interpretation of the υOH bands in the FTIR time-difference spectra along with the time-course of the polyglucose υC-OH and the water (δOH +υLHOH) band intensities is summarized in Fig. 6. Considering the complexity of a living bacterial system, the consistency of the spectral features and the agreement with the putative events of oxygen-stress adaptive response is striking. We have thus demonstrated both the efficacy of using the hydrogen bonding in water of living cells to profile intracellular chemical environment and their significant consequences for understanding functional metabolic controls in “obligate” anaerobic bacteria that can survive oxygen-stress transiently at the chemical level, by providing direct observations of molecular events measured in the same cells over time. Together, these high-resolution synchrotron radiation-based FTIR experiments have revealed a remarkable sequence of well-orchestrated mechanisms that some D. vulgaris use to temporarily survive oxygen exposure. When extending this approach to other adaptive-response cellular systems, the experimental design and interpretation of the data should be straightforward in cases where transient chemistry is dominated by ions or other small chemical species. Even in more complex cases, we anticipate that the interpretation of infrared spectroscopic data in terms of the hydrogen-bonding structure of cellular water will open the door to investigations of chemical and molecular structural changes in living bacteria and other cellular systems over the course of their stress-adaptive response.
Fig. 6.
A summary of the evolving cellular chemical environment and possible survival mechanisms inside the same living D. vulgaris during its transient oxygen-stress and adaptive response, as revealed by the real-time high-resolution synchrotron FTIR measurements and analyses. Polyglucose is labeled as PolyG.
Materials and Methods
Bacterial Strains and Culture.
Desulfovibrio vulgaris Hildenborough (ATCC 29579) was obtained from the American Type Culture Collection. All D. vulgaris used in this study were cells of second passage grown on a defined lactate sulfate medium (LS4D medium) soft agar plate (1.0% wt/vol). The LS4D medium was based on Postgate's medium C. To prepare stationary-phase D. vulgaris population, we grew D. vulgaris (at high cell density) anaerobically on soft LS4D agar until the growth of some bacterial colonies reached confluence. Most cells from the confluent colonies exhibited infrared spectral characteristics typical of polyglucose-containing D. vulgaris (red trace in Fig. 1A). To prepare exponential-phase D. vulgaris population, we grew D. vulgaris (at low cell density) anaerobically on LS4D agar until the growth of bacterial colonies first became visible. Cells from these microcolonies mostly did not exhibit infrared spectral characteristics typical of polyglucose-containing D. vulgaris (blue trace in Fig. 1A).
Preparation of D. vulgaris Monolayers.
To ensure that each D. vulgaris cell in the FTIR experiment was in contact with atmospheric oxygen, we prepared μm-thick layers of D. vulgaris. We transferred D. vulgaris cells onto a LS4D-treated gold-coated glass wafer. An additional mist of liquid LS4D was applied to the replica-printed wafer, which was then incubated for an additional 24 h to facilitate migration of mobile cells to form monolayers on the LS4D-treated gold-coated glass wafer under suitable conditions. To assess the morphological quality of the monolayers, the wafer was placed in a custom microscope stage chamber (filled with nitrogen gas), and was observed by oblique illumination microscopy. Before the FTIR experiment, any excess (moving) LS4D medium was removed by wicking.
High-Resolution FTIR Spectromicroscopy.
We built a high-humidity microscope stage chamber (Fig. 2A) that allows one to maintain a constant μm-thick layer of live D. vulgaris, overcoming the water interference during high-resolution FTIR spectromicroscopy measurements. All measurements were performed with a Nicolet Magna 760 FTIR bench and a Nicolet Nic-Plan IR microscope equipped with a microscope stage chamber at the infrared beamline of the Advanced Light Source (Lawrence Berkeley National Laboratory, CA, USA; http://infrared.als.lbl.gov/). Each spectrum represents an average of 64 scans over a wavenumber range of 4,000 to 650 cm−1 at a spectral resolution of 4 cm−1 with an absorption peak position accuracy of 1/100 cm−1. As the beam current of the synchrotron decreases with time between electron refills (9 h), the beam intensity decreases proportionally. Appropriate baseline removal took this into account. All data processing was performed using Thermo Electron's Omnic 7.2 (http://www.thermo.com/) and Origin 6.0 (http://www.originlab.com/). Time-difference spectra are calculated [using Thermo Electron's Omnic 7.2 software (http://www.thermo.com/)] from experimental spectra after subtraction of culture medium/liquid water absorbance (46). The time-difference spectra calculation was performed using the water factor approach (47) to minimize absorbance of the water continuum. Analysis of time difference spectra is described in the main text.
Carbohydrate Analysis.
Cell pellets were collected from air exposure experiments and were re-suspended in 1 mL of 0.7% NaCl (wt/vol). Protein concentrations were determined with the Lowry assay using BSA (Pierce Biochemicals) as the standard (48). Hexose sugars were measured using the colorimetric cysteine-sulfuric acid method (49) with glucose as the standard.
Live/Dead Fluorescence Microscopy for Distinguishing Live and Dead Bacteria.
The LIVE/DEAD BacLight bacterial viability kit (Molecular Probes) in combination with a fluorescence microscope (Zeiss) was used. Here, cells were exposed to air for t = 0, 30, 240, and 480 min; and the level of internalized red fluorescing propidium relative to green fluorescing SYTO9 allowed for differentiation between D. vulgaris with intact cytoplasmic membranes (bright green) and D. vulgaris with damaged cytoplasmic membranes (red/yellow) (50).
Electron Microscopy.
For conventional TEM/PATO samples, D. vulgaris were fixed, embedded, thin sectioned, and post-stained by the periodic acid thiosemicarbazide-osmium (PATO) exactly as previously described (51). Preparation and imaging were conducted at the Robert D. Ogg Electron Microscope Lab at the University of California, Berkeley, CA (http://em-lab.berkeley.edu/EML/index.php). Cryo-EM samples were made by placing 5-μL aliquots of the D. vulgaris suspension onto lacey carbon grids (Ted Ted Pella 01881) that were pretreated by glow-discharge, then blotting and plunging into liquid ethane. Images were recorded on a JEOL-3100 electron microscope operated at 300 kV by a Gatan 795 CCD camera at a magnification of 30,000×. Samples to be used for EDX analysis were freeze-dried in the microscope's airlock and then examined in an FEI CM-200 microscope equipped with a Link EDX detector.
Re-Growth Experiment.
The re-growth experiments were to obtain growth curves of control (anaerobic) versus air-exposed cells when returned to oxygen-free conditions. Stressed and control cells grown from the oxygen stress experiment were removed from the culture bottles after 24 h of either N2 or air purge. Ten microliters culture was added to 100 μL LS4D medium in wells of an empty microplate. Eight replicate wells were used for each culture. Cells were incubated in the Omnilog (Biolog) anaerobically at 30 °C for 100 h. Increase in cell density was quantified by recording increased opacity caused by the accumulation of FeS precipitates. The Omnilog uses a scanning technology, which is analogous to a turbidity measurement, to record the increase in density in Omnilog (OL) units.
Acknowledgments.
We thank Dr. K. McDonald and Ms. R. Zalpuri at the Robert D. Ogg Electron Microscope Lab, UC Berkeley, and Dr. Z. Lee at the National Center for Electron Microscopy, Lawrence Berkeley National Laboratory, for technical assistance; Ms. D. Joyner for technical assistance; Drs. H. Bechtel, Z. Hao, J. Jansson, C. Jansson, M.C. Martin, W. McKinney and J. Zhou for discussion and comments on this work and the manuscript. This work was supported by the U.S. Department of Energy Office of Biological and Environmental Research's Structural Biology Program, and Genomics:GTL Program through contract DE-AC02–05CH11231 with Lawrence Berkeley National Laboratory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Fay P. Oxygen relations of nitrogen-fixation in Cyanobacteria. Microbiol Rev. 1992;56:340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradshaw DJ, Marsh PD, Watson GK, Allison C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun. 1998;66:4729–4732. doi: 10.1128/iai.66.10.4729-4732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cypionka H. Oxygen respiration by Desulfovibrio species. Annu Rev Microbiol. 2000;54:827–848. doi: 10.1146/annurev.micro.54.1.827. [DOI] [PubMed] [Google Scholar]
- 4.Baughn AD, Malamy MH. Molecular Basis for Aerotolerance of the Obligately Anaerobic Bacteroides Spp. In: Nakano MM, Zuber P, editors. Strict and Facultative Anaerobes: Medical and Environmental Aspects. New York: CRC Press; 2004. pp. 161–169. [Google Scholar]
- 5.Dolla A, Fournier M, Dermoun Z. Oxygen defense in sulfate-reducing bacteria. J Biotechnol. 2006;126:87–100. doi: 10.1016/j.jbiotec.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Brioukhanov AL, Netrusov AI. Aerotolerance of strictly anaerobic microorganisms and factors of defense against oxidative stress: A review. Appl Biochem Micro+ 2007;43:567–582. [PubMed] [Google Scholar]
- 7.Muyzer G, Stams AJM. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 2008;6:441–454. doi: 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- 8.Fuseler K, Cypionka H. Elemental sulfur as an intermediate of sulfide oxidation with oxygen by Desulfobulbus Propionicus. Arch Microbiol. 1995;164:104–109. [Google Scholar]
- 9.Van Niel EWJ, et al. The role of polyglucose in oxygen-dependent respiration by a new strain of Desulfovibrio salexigens. Fems Microbiol Ecol. 1996;21:243–253. [Google Scholar]
- 10.Fareleira P, et al. Response of a strict anaerobe to oxygen: Survival strategies in Desulfovibrio gigas. Microbiology. 2003;149:1513–1522. doi: 10.1099/mic.0.26155-0. [DOI] [PubMed] [Google Scholar]
- 11.Holman H-YN, Martin MC. Synchrotron radiation infrared spectromicroscopy: a non-invasive molecular probe for biogeochemical processes. In: Sparks D, editor. Advances in Agronomy. Vol 90. New York: Elsevier; 2006. pp. 79–127. [Google Scholar]
- 12.Woutersen S, Bakker HJ. Resonant intermolecular transfer of vibrational energy in liquid water. Nature. 1999;402:507–509. [Google Scholar]
- 13.Cowan ML, et al. Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O. Nature. 2005;434:199–202. doi: 10.1038/nature03383. [DOI] [PubMed] [Google Scholar]
- 14.Okumura M, Yeh LI, Myers JD, Lee YT. Infrared-spectra of the solvated hydronium ion - vibrational predissociation spectroscopy of mass-selected H3O+. (H2O)n. (H2)m. J Phys Chem-Us. 1990;94:3416–3427. [Google Scholar]
- 15.Nelander B. The peroxy radical as hydrogen bond donor and hydrogen bond acceptor: A matrix isolation study. J Phys Chem A. 1997;101:9092–9096. [Google Scholar]
- 16.Weber JM, et al. Isolating the spectroscopic signature of a hydration shell with the use of clusters: Superoxide tetrahydrate. Science. 2000;287:2461–2463. doi: 10.1126/science.287.5462.2461. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhuri C, et al. Infrared spectra and isomeric structures of hydroxide ion-water clusters OH− (H2O)(1–5): A comparison with H3O+ (H2O)(1–5) Mol Phys. 2001;99:1161–1173. [Google Scholar]
- 18.Robertson WH, et al. Spectroscopic determination of the OH− solvation shell in the OH−. (H2O)(n) clusters. Science. 2003;299:1367–1372. doi: 10.1126/science.1080695. [DOI] [PubMed] [Google Scholar]
- 19.Max JJ, Chapados C. Infrared spectroscopy of aqueous carboxylic acids: Comparison between different acids and their salts. J Phys Chem A. 2004;108:3324–3337. [Google Scholar]
- 20.Shin JW, et al. Infrared signature of structures associated with the H+(H2O)(n) (n=6 to 27) clusters. Science. 2004;304:1137–1140. doi: 10.1126/science.1096466. [DOI] [PubMed] [Google Scholar]
- 21.Headrick JM, et al. Spectral signatures of hydrated proton vibrations in water clusters. Science. 2005;308:1765–1769. doi: 10.1126/science.1113094. [DOI] [PubMed] [Google Scholar]
- 22.Iyengar SS. Dynamical effects on vibrational and electronic spectra of hydroperoxyl radical water clusters. J Chem Phys. 2005;123 doi: 10.1063/1.2006674. 084310–084311. [DOI] [PubMed] [Google Scholar]
- 23.Bush MF, Saykally RJ, Williams ER. Evidence for water rings in the hexahydrated sulfate dianion from IR spectroscopy. J Am Chem Soc. 2007;129:2220–2223. doi: 10.1021/ja068357b. [DOI] [PubMed] [Google Scholar]
- 24.Heidelberg JF, et al. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat Biotechnol. 2004;22:554–559. doi: 10.1038/nbt959. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner LK, et al. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries. Sediment Geol. 2006;185:131–145. [Google Scholar]
- 26.Chapple ILC. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. J Clin Pathol-Cl Mol. 1996;49:M247–M255. doi: 10.1136/mp.49.5.m247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffy M, et al. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum. 2002;45:384–388. doi: 10.1007/s10350-004-6187-z. [DOI] [PubMed] [Google Scholar]
- 28.Risatti JB, Capman WC, Stahl DA. Community structure of a microbial mat - the phylogenetic dimension. P Natl Acad Sci USA. 1994;91:10173–10177. doi: 10.1073/pnas.91.21.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minz D, et al. Diversity of sulfate-reducing bacteria in oxic and anoxic regions of a microbial mat characterized by comparative analysis of dissimilatory sulfite reductase genes. Appl Environ Microb. 1999;65:4666–4671. doi: 10.1128/aem.65.10.4666-4671.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stams FJM, Veenhuis M, Weenk GH, Hansen TA. Occurrence of polyglucose as a storage polymer in desulfovibrio species and desulfobulbus-propionicus. Arch Microbiol. 1983;136:54–59. [Google Scholar]
- 31.Naumann D. Infrared Spectroscopy in Microbiology. In: Meyers RA, editor. Encycloedia of Anal Chem. Chichester: John Wiley & Sons Ltd; 2000. pp. 102–131. [Google Scholar]
- 32.Potts RO, Guzek DB, Harris RR, Mckie JE. A non-invasive, in vivo technique to quantitatively measure water concentration of the stratum-corneum using attenuated total-reflectance infrared-spectroscopy. Arch Dermatol Res. 1985;277:489–495. doi: 10.1007/BF00510068. [DOI] [PubMed] [Google Scholar]
- 33.Vernon MF, et al. Infrared vibrational predissociation spectroscopy of water clusters by the crossed laser-molecular beam technique. J Chem Phys. 1982;77:47–57. [Google Scholar]
- 34.Castleman AW, Keesee RG. Clusters - bridging the gas and condensed phases. Acc Chem Res. 1986;19:413–419. [Google Scholar]
- 35.Tsuji K, Yagi T. Significance of hydrogen burst from growing cultures of Desulfovibrio vulgaris, Miyazaki, and the role of hydrogenase and cytochrome c3 in energy-production system. Arch Microbiol. 1980;125:35–42. [Google Scholar]
- 36.Baumgarten A, Redenius I, Kranczoch J, Cypionka H. Periplasmic oxygen reduction by Desulfovibrio species. Arch Microbiol. 2001;176:306–309. doi: 10.1007/s002030100329. [DOI] [PubMed] [Google Scholar]
- 37.Fitz RM, Cypionka H. Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol. 1991;155:444–448. [Google Scholar]
- 38.Santos H, et al. Aerobic metabolism of carbon reserves by the obligate anaerobe Desulfovibrio gigas. Biochem Bioph Res Co. 1993;195:551–557. doi: 10.1006/bbrc.1993.2081. [DOI] [PubMed] [Google Scholar]
- 39.Brucato JR, Palumbo ME, Strazzulla G. Carbonic acid by ion implantation in water/carbon dioxide ice mixtures. Icarus. 1997;125:135–144. [Google Scholar]
- 40.Schauder R, et al. Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric-acid cycle. Arch Microbiol. 1986;145:162–172. [Google Scholar]
- 41.Tang Y, et al. Pathway confirmation and flux analysis of central metabolic pathways in Desulfovibrio vulgaris hildenborough using gas chromatography-mass spectrometry and Fourier transform-ion cyclotron resonance mass spectrometry. J Bacteriol. 2007;189:940–949. doi: 10.1128/JB.00948-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kramer M, Cypionka H. Sulfate formation via ATP sulfurylase in thiosulfate-disproportionating and sulfite-disproportionating bacteria. Arch Microbiol. 1989;151:232–237. [Google Scholar]
- 43.Fuseler K, Krekeler D, Sydow U, Cypionka H. A common pathway of sulfide oxidation by sulfate-reducing bacteria. Fems Microbiol Lett. 1996;144:129–134. [Google Scholar]
- 44.Lawrence CP, Skinner JL. Vibrational spectroscopy of HOD in liquid D2O. III. Spectral diffusion, and hydrogen-bonding and rotational dynamics. J Chem Phys. 2003;118:264–272. [Google Scholar]
- 45.Yamashita T, Takatsuka K. Hydrogen-bond assisted enormous broadening of infrared spectra of phenol-water cationic cluster: An ab initio mixed quantum-classical study. J Chem Phys. 2007;126 doi: 10.1063/1.2434778. 074304. [DOI] [PubMed] [Google Scholar]
- 46.Mantele W. Reaction-induced infrared difference spectroscopy for the study of protein function and reaction-mechanisms. Trends Biochem Sci. 1993;18:197–202. doi: 10.1016/0968-0004(93)90186-q. [DOI] [PubMed] [Google Scholar]
- 47.Max JJ, Chapados C. Infrared spectroscopy of aqueous carboxylic acids: Malic acid. J Phys Chem A. 2002;106:6452–6461. [Google Scholar]
- 48.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 49.Chaplin MF. Monosaccharides. In: Chaplin MF, Kennedy JF, editors. Carbohydrate analysis. Oxford: IRL Press; 1986. pp. 1–4. [Google Scholar]
- 50.Leuko S, Legat A, Fendrihan S, Stan-Lotter H. Evaluation of the LIVE/DEAD BacLight kit for detection of extremophilic archaea and visualization of microorganisms in environmental hypersaline samples. Appl Environ Microb. 2004;70:6884–6886. doi: 10.1128/AEM.70.11.6884-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kropinski AM, Ghiorse WC, Greenberg EP. The intracellular polyglucose storage granules of Spirochaeta aurantia. Arch Microbiol. 1988;150:289–295. [Google Scholar]