Abstract
The inner membrane of the nuclear envelope (NE) was previously shown to contain a Na/Ca exchanger (NCX) tightly linked to GM1 ganglioside that mediates transfer of nucleoplasmic Ca2+ to the NE lumen and constitutes a cytoprotective mechanism. This transfer was initially observed with isolated nuclei and is now demonstrated in living cells in relation to subcellular Ca2+ dynamics. Four cell lines with varying expression of NCX and GM1 in the NE were transfected with cameleon-fluorescent Ca2+ indicators genetically targeted to NE/endoplasmic reticulum (ER) and nucleoplasm to monitor [Ca2+]ne/er and [Ca2+]n respectively. Cytosolic Ca2+ ([Ca2+]cyt) was indicated with fura-2. Thapsigargin caused progressive loss of [Ca2+]ne/er, which was rapidly replaced on addition of extrinsic Ca2+ to those cells containing fully functional NCX/GM1: differentiated NG108–15 and C6 cells. Reduced elevation of [Ca2+]ne/er following thapsigargin depletion occurred in cells containing little or no GM1 in the NE: undifferentiated NG108–15 and NG-CR72 cells. No change in [Ca2+]ne/er due to applied Ca2+ was seen in Jurkat cells, which entirely lack NCX. Ca2+ entry to NE/ER was also blocked by KB-R7943, inhibitor of NCX. [Ca2+]n and [Ca2+]cyt were elevated independent of [Ca2+]ne/er and remained in approximate equilibrium with each other. Ca2+ rise in the ER originated in the NE region and extended to the entire ER network. These results indicate the nuclear NCX/GM1 complex acts to gate Ca2+ transfer from cytosol to ER, an alternate route to the sarcoplasmic/endoplasmic reticulum calcium ATPase pump. They also suggest a possible contributory mechanism for independent regulation of nuclear Ca2+.
Keywords: cameleon calcium indicator, nuclear calcium, nuclear envelope, ER calcium, ganglioside function
Regulation of nuclear Ca2+ is critically important in determining cell viability and a wide range of signaling processes that govern virtually every aspect of cell behavior. The luminal space of the endoplasmic reticulum (ER), a storage site for a major portion of cellular Ca2+, is recognized as playing a major role in such processes and the same applies to the lumen of the nuclear envelope (NE) with which it is continuous (1, 2). The outer membrane of the NE is continuous with the ER and resembles the latter in containing sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA), a type of Ca2+-activated ATPase that pumps cytosolic Ca2+ ([Ca2+]cyt) into the lumen of the NE, and hence of the ER (3). Cytosolic Ca2+ is also transferred into the NE lumen via inositol 1,3,4,5-tetrakisphosphate, receptors for which occur in the outer membrane of the NE (4). The inner nuclear membrane, with unique composition, contains Ca2+-release mechanisms regulated by Ins(1,4,5)P3, cADP-ribose, and NAADP, which regulate input to nucleoplasmic Ca2+ ([Ca2+]n) through transfer from the NE (5–7). Some aspects of nuclear Ca2+, in particular its capacity for independent regulation, have been controversial because of the existence of nuclear pore complexes that appeared to allow free diffusion of Ca2+ between cytosol and nucleoplasm (8). However, some studies have suggested the existence of cytoplasm-nucleoplasm Ca2+ gradients (9, 10), and there is growing evidence for independent Ca2+ regulation in that organelle (11). Adding to the story was the discovery of a mechanism for Ca2+ flux between NE and nucleoplasm, mediated by sodium-calcium exchanger (NCX) activity in the inner membrane of the NE of neural- and certain other cells; analyses that revealed the latter exchanger included immunoprecipitation/immunoblotting (IP/IB), immunocytochemistry, RT-PCR, and 45Ca2+ uptake by the NE of isolated nuclei (12, 13). A key feature of this NCX was its potentiation by GM1 ganglioside with which it forms a high affinity complex that survives SDS/PAGE. This property distinguishes it from NCX isoforms of the plasma membrane, which do not form such a complex with GM1, although a looser association with possible effect on activity was not excluded.
Initial evidence that this NCX/GM1 complex contributes to selective regulation of Ca2+ within the nucleus came from studies with isolated nuclei involving Ca2+ transfer from nucleoplasm to NE lumen (12). A more detailed examination of this phenomenon is provided in the present study, employing whole cells transfected with genetically encoded cameleon Ca2+ sensors developed in the laboratory of R. Tsien (14, 15); these monitor Ca2+ dynamics in the nucleoplasm and NE/ER ([Ca2+]ne/er) compartments. An approximation of [Ca2+]cyt was obtained with use of fura-2, a ratiometric Ca2+ indicator widely used to determine intracellular Ca2+ exclusive of NE/ER. Monitoring of Ca2+ in these 3 compartments over time facilitated a more comprehensive assessment of coordinated cellular Ca2+ changes in the presence or absence of nuclear NCX/GM1. This was accomplished by comparing Ca2+ dynamics in various cell lines, which either do or do not express this complex. A pharmacological inhibitor of NCX provided additional data. The results provide conclusive evidence for NCX/GM1-mediated Ca2+ transfer from nucleoplasm to NE and subsequently to the ER network, thus providing a mechanistic alternative to the SERCA pump for transfer of cytosolic Ca2+ to the ER. Implications regarding independent regulation of nuclear Ca2+ are discussed.
Results
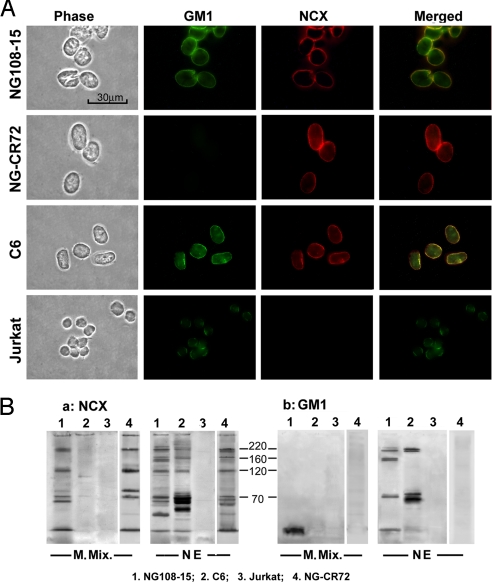
Expression of GM1 and NCX, or absence thereof, in the nuclei of 4 cell lines was verified in one series of experiments by immunocytochemistry. Purified nuclei were double stained with cholera toxin B subunit linked to FITC and anti-NCX antibody (Ab) plus goat anti-mouse IgG linked to Texas red. This revealed colocalization of GM1 and NCX in the NE of differentiated NG108–15 and C6 cells (Fig. 1A); NG-CR72 cells were shown to contain NCX but no GM1 in the NE, while Jurkat cells expressed GM1 but no NCX at that locus. This was confirmed by IP/IB analysis in which lysates of the NEs were reacted with mouse monoclonal antibody (mAb) to NCX plus protein-L-linked beads, followed by SDS-PAGE and electrophoretic transfer to PVDF membrane and IB with rabbit polyclonal anti-NCX Ab plus second Ab linked to HRP. NE of differentiated NG108–15 and C6 cells again showed expression of NCX, which appeared in regions corresponding to 70-, 120-, 160-, and 220 kDa (Fig. 1Ba). The 120-kDa band corresponds to the mature protein, considered a conformational isomer to the 160-kDa protein, while the 70-kDa and 220-kDa bands correspond to proteolytic and multimeric products, respectively (see Discussion). No bands appeared for Jurkat cells, a cell line devoid of NCX (16). Controls for NG108–15 cells were obtained by omitting the first Ab that revealed no staining (not shown), consistent with absence of staining for Jurkat cells known not to express NCX. A parallel PVDF was blotted with cholera toxin B-HRP, revealing GM1 that comigrated with NCX in 3 of those regions (Fig. 1Bb). This was attributed to tenacious association of GM1 with these NCX species (12, 16). The pattern of nuclear NCX on SDS/PAGE was generally similar, albeit with differences in intensity, to that of plasma membrane NCX as depicted in membrane mixture (see M. Mix. in Fig. 1Ba). NG-CR72 cells showed similar NCX pattern as NG108–15 cells in both NE and plasma membrane. C6 cells, despite clear expression of NCX in the NE, showed no evidence of NCX in the plasma membrane (M. Mix.), as previously reported (17). As previously described (12), NCX band from the plasma membrane did not comigrate with GM1 but may have formed a looser association, as suggested by the appearance of GM1 at the migration front (see Fig. 1Bb). Jurkat cells were unique in showing no NCX in either NE or plasma membrane as previously reported (16), even though GM1 was detected in the NE (apparently subtending other functions).
Fig. 1.
Expression of NCX and GM1. (A) Nuclei of NG108–15 (differentiated), NG-CR72, C6, and Jurkat cells were isolated, and fixed. GM1 and NCX in the NE were revealed by double staining with CtxB-FITC and anti-NCX Ab (second Ab-linked to Texas red). Images show coexpression of NCX and GM1 in the NE of NG108–15 and C6 cells; NG-CR72 cells showed NCX but no GM1 while Jurkat cells showed GM1 but no NCX. (B) NCX in NE and non-nuclear membrane mixture (M. Mix.) was immunoprecipitated with mouse anti-NCX mAb (C2C12), subjected to SDS/PAGE and electrophoretic transfer to PVDF; it was then blotted with rabbit polyclonal anti-NCX Ab (a). NG108–15 and NG-CR72 cells gave bands corresponding to known NCX pattern in the NE and non-nuclear M. Mix. (e.g., plasma membrane) while C6 cells expressed NCX only in the NE. Jurkat cells were devoid of NCX in both fractions. Parallel blot using CtxB-HRP (b) revealed tight association of GM1 with major NCX bands in the NE of NG108–15 and C6 cells, but not GM1-deficient NG-CR72 cells or NCX-deficient Jurkat cells.
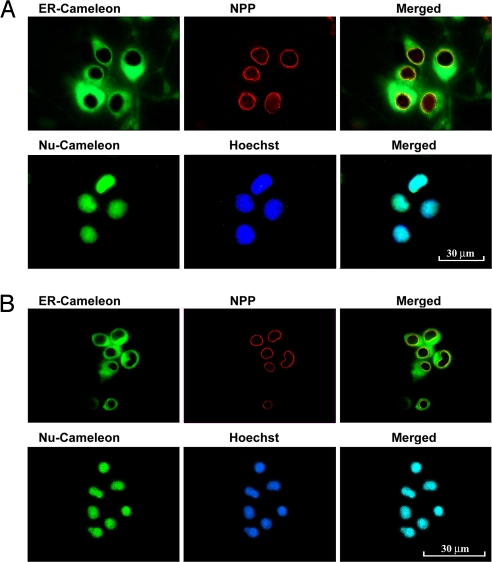
The above cells were transfected with Nu- and ER-cameleons genetically targeted to nucleoplasm and NE/ER lumen, respectively. Fluorescent images of these cameleons expressed in NG108–15 and Jurkat cells are shown in Fig. 2 A and B, respectively, together with markers for NE (nuclear pore protein) and nucleoplasm (Hoechst-33342). ER-cameleon revealed the ER network to pervade the entire extranuclear region and extend into the NE; Nu-cameleon was restricted to nucleoplasm that coincided with staining by Hoechst. Similar patterns were obtained with the other 2 cell lines (not shown). Our approach was to sequentially monitor [Ca2+]ne/er and [Ca2+]n with the above cameleons, in addition to [Ca2+]cyt as indicated by fura-2. As mentioned, the latter represented an approximation in lieu of direct measurement of [Ca2+]cyt; such measurements included [Ca2+]n, with which [Ca2+]cyt appeared to be in equilibrium.
Fig. 2.
Targeted expression of cameleon indicators in NE/ER and nucleoplasm. (A) NG108–15 cells and (B) Jurkat cells were transfected with ER- or Nu-cameleon. ER-cameleon-expressing cells were immunostained with antinuclear pore protein (NPP) Ab and second antibody with Texas red, showing expression of cameleon protein in the NE and throughout the ER. Nu-cameleon-expressing cells were fixed and stained with nuclear-specific dye Hoechst 33342, showing restricted expression in the nucleoplasm.
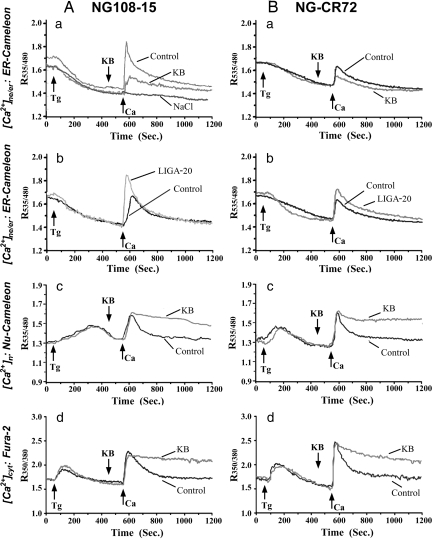
Calcium monitoring in NG108–15 and NG-CR72 cells was started in Ca2+-free buffer containing thapsigargin (Tg) to deplete [Ca2+]ne/er, followed at the indicated times by CaCl2 (0.5 mM) + KCl (50 mM) (Fig. 3 traces labeled “Control”). This K+-induced depolarization caused a surge of Ca2+ influx (indicated at Ca arrow) leading to elevation of Ca2+ in all intracellular compartments. Elevation of [Ca2+]ne/er was significantly greater (Table 1) in differentiated NG108–15 cells (see Fig. 3Aa), which possess NCX and high levels of GM1 in the NE, compared to [Ca2+]ne/er elevation in undifferentiated NG108–15 cells (see Fig. 3Ab), which express NCX but low levels of GM1 in the NE (12, 18). Similarly NG-CR72 cells, which are completely devoid of GM1 (19), showed only moderate elevation of [Ca2+]ne/er (see Fig. 3Ba and Table 1). [Ca2+]ne/er rise in differentiated NG108–15 cells was inhibited to a large extent by prior application of KB-R7943 (KB) (see Fig. 3Aa), a recognized blocker of NCX (20), whereas the attenuated [Ca2+]ne/er rise in undifferentiated NG108–15 cells was enhanced by prior application of LIGA-20 (see Fig. 3Ab and Table 1). The latter is a membrane-permeable derivative of GM1 that inserts into the NE and potentiates NCX similar to GM1 (21, 22). LIGA-20 achieved similar potentiation of [Ca2+]ne/er rise in the GM1-deficient NG-CR72 cells (see Fig. 3Bb). No changes in [Ca2+]ne/er were detected when Ca2+ was applied together with the same concentration of NaCl in place of KCl (see Fig. 3Aa), indicating the Ca2+ changes did not result from hyperosmolarity.
Fig. 3.
Coordinated Ca2+ changes in NE/ER ([Ca2+]ne/er), nucleoplasm ([Ca2+]n), and cytosol ([Ca2+]cyt) in NG108–15 (A) and NG-CR72 (B) cells. NG108–15 cells were differentiated with db-cAMP/KCl, except (Ab) in which undifferentiated NG108–15 cells were used. NG-CR72 cells were differentiated with db-cAMP alone. Cells were transfected with ER- (A a and b and B a and b) or Nu- (Ac and Bc) cameleon, or loaded with fura-2 (Ad and Bd). Ca2+ imaging was started in Ca2+-free buffer and monitored as R535/480 for cameleon and R350/380 for fura-2. Reagents included Tg, NCX blocker KB-R7943 (KB), LIGA-20, and CaCl2/KCl or CaCl2/NaCl that were applied as indicated. “Control” in all panels represents CaCl2/KCl without KB or LIGA-20. LIGA-20, when used, was used for prior bath incubation of cells for 30 min. Each trace was average of >30 cells from 5 to 8 independent runs.
Table 1.
Effect of KB-R7943 and LIGA-20 on transient increase of [Ca2+]ne/er following Ca2+ application
| Control |
KB-R7943 |
LIGA-20 |
|
|---|---|---|---|
| Mean ± SEM (n) |
|||
| NG108–15 (Undifferentiated ) | 0.265 ± 0.020 (33)** | NA | 0.440 ± 0.013 (31)* |
| NG108–15 (Differentiated) | 0.452 ± 0.028 (43) | 0.155 ± 0.015 (38)* | NA |
| NG-CR72 | 0.191 ± 0.040 (30) | 0.095 ± 0.020 (36)* | 0.292 ± 0.030 (31)* |
| C6 | 0.443 ± 0.110 (32) | 0.049 ± 0.04 (32)* | NA |
| Jurkat | 0.020 ± 0.024 (31) | 0.004 ± 0.005(31) | NA |
[Ca2+]ne/er measurement was performed as in Figs. 3 and 5, and transient increase after application of extrinsic Ca2+ was calculated. Data are mean elevation of R535/480 ± SEM. Figures in parentheses indicate number of cells employed. NA, not assayed. Statistical difference was analyzed with 2-tailed Student's t-test;
*, P < 0.001 vs. control;
**, P < 0.001 vs. differentiated NG108–15 cells.
For differentiated NG108–15 cells, the changes in [Ca2+]n (see Fig. 3Ac) paralleled those in [Ca2+]cyt (see Fig. 3Ad), and these in turn approximated the changes in [Ca2+]n and [Ca2+]cyt of NG-CR72 cells (see Fig. 3B c and d). This reflected equilibration of [Ca2+]n and [Ca2+]cyt as expected on the basis of free flow of Ca2+ through the nuclear pore complexes, that aspect being independent of the Ca2+ gating function of NCX/GM1 in the NE. Elevation of [Ca2+]n and [Ca2+]cyt was prolonged by KB (see Fig. 3 A c and d, and B c and d), likely because of blocked efflux of cytosolic Ca2+ by this agent.
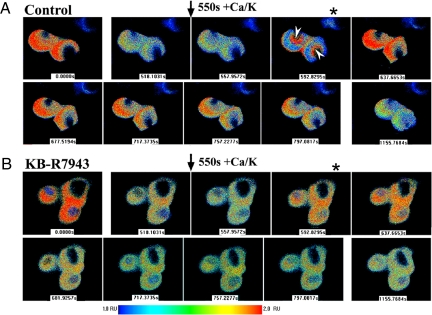
Serial analysis of [Ca2+]ne/er images in differentiated NG108–15 cells revealed initial Tg-induced depletion over 550 s, at which point CaCl2/ KCl addition caused appearance of Ca2+ in the NE region (≈593 s) (Fig. 4A); from there Ca2+ spread throughout the ER, after which it underwent Tg-induced depletion. In the presence of KB (Fig. 4B), a relatively small amount of Ca2+ entered the NE/ER, suggesting less than complete inhibition by KB (as also suggested in Fig. 3 Aa and Ba). These changes occurred in synchrony with transient (Control) or sustained (KB) elevation of [Ca2+]n and [Ca2+]cyt (see Fig. 3A c and d) and are consistent with NCX gating of Ca2+ from cytosol to ER via nucleoplasm and NE.
Fig. 4.
Ratio images of [Ca2+]ne/er in differentiated NG108–15 cells. Serial images were obtained with ER-cameleon-expressing cells as described in Fig. 3. (A) Control (no KB-R7943): Following Tg application at 50 s leading to depletion of [Ca2+]ne/er, addition of CaCl2/KCl at 550 sec caused elevation of [Ca2+]ne/er that originated in the perinuclear region (arrowheads at 592.8 s image with *) and then extended to entire NE/ER. This was followed by gradual Tg-induced depletion. (B) Repeat of above experiment with KB-R7943 showed limited uptake of Ca2+ in the perinuclear region at 592.9s (*) consistent with incomplete blockade shown in Fig. 3 Aa and Ba; modest rise in [Ca2+]ne/eroccurred briefly to 637.7 s followed by gradual Tg-induced depletion.
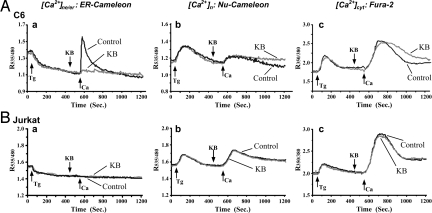
Additional support for this mechanism came from similar experiments with the other 2 used cell lines. C6 cells, as mentioned, express NCX in the NE but not the plasma membrane (see Fig. 1A and ref. 17) and accordingly resembled differentiated NG108–15 cells in regard to CaCl2/KCl-induced elevation of [Ca2+]ne/er and inhibition by KB (Fig. 5Aa; compare to Fig. 3Aa). The latter inhibitor had less effect on [Ca2+]cyt and [Ca2+]n (Fig. 5A b and c) than was the case with NG108–15 cells, likely because of the absence of NCX in the plasma membrane. Jurkat cells, which lack NCX in both NE and plasma membrane (see Fig. 1A and ref. 16), showed no elevation of [Ca2+]ne/er following Tg-induced depletion and CaCl2 addition (Fig. 5Ba), while [Ca2+]n and [Ca2+]cyt dynamics resembled those in the other cells (Fig. 5B b and c). As expected, KB was without influence in any of the trials with Jurkat cells.
Fig. 5.
Coordinated Ca2+ changes in NE/ER, nucleoplasm, and cytosol of C6 and Jurkat cells. C6 (A) and Jurkat (B) cells were transfected with ER- (a) or Nu- (b) cameleon, or loaded with fura2 (c). Ratiometric imaging was carried out as in Fig. 3. Arrows marked with Ca at 550 s represent addition of CaCl2/KCl mixture for C6 cells or CaCl2 alone for Jurkat cells. [Ca2+]ne/er was elevated in C6 cells, which express nuclear NCX, but not in Jurkat cells, which lack nuclear NCX. Each trace was average of >30 cells from 5 to 8 independent runs.
Discussion
This study made use of whole cells transfected with genetically encoded cameleon Ca2+ fluorescent indicators to examine the role of nuclear NCX/GM1 in mediating transfer of Ca2+ from nucleoplasm to NE lumen. Such transfer provides cytoprotection to cells experiencing abnormal elevation of nuclear Ca2+ (see below). The NCX/GM1 complex, situated in the inner membrane of the NE, is seen to comprise a gating mechanism for movement of [Ca2+]cyt into the ER, a transfer result equivalent to that of the SERCA pump. Blockade of the latter with Tg revealed efficient operation of this alternate route, provided the cell possessed both NCX and GM1 in the NE (differentiated NG108–15 and C6 cells). Absence of NCX (Jurkat cells) resulted in no [Ca2+]cyt to [Ca2+]ne/er transfer, while cells deficient in NE GM1 (NG-CR72 and undifferentiated NG108–15 cells) showed impaired operation of this mechanism. Blockade was also achieved with KB, a pharmacological inhibitor of NCX originally shown to be potent in blocking reverse mode exchange (20). However, the latter study also indicated an effect on the forward mode at higher concentrations of KB, as was the case in the present study. The fact that KB is also known to affect a number of other transporters and ion channels (summarized in ref. 23) did not diminish its utility in providing confirmatory data in these experiments.
These findings also lend support to the idea of independent regulatory mechanisms for nuclear Ca2+, despite equilibration between [Ca2+]n and [Ca2+]cyt as the possible default condition. Such independence has appeared counterintuitive in view of the ubiquitous occurrence of nuclear pore complexes on the nuclear surface, which is consistent with the functional equivalence of [Ca2+]cyt and [Ca2+]n observed here and elsewhere (8). However, evidence has been presented for cytosolic-nuclear Ca2+ gradients and other indications of independent regulation of nuclear Ca2+ under specific conditions (9, 11). It was pointed out that most of our knowledge of nuclear pore function has been derived from isolated nuclei and permeabilized cells in cell lysates/extracts, and that patch-clamp study of isolated living nuclei in their native liquid environment revealed no transport of physiological ions (10). Independent Ca2+ regulatory mechanisms, if validated, would be consistent with the recently expressed concept of the nucleus as a “cell within a cell” (24).
The mammalian NCX forms a multigene family with various isoforms corresponding to splice variants (25). The NCX1 subtype predominates in many neural systems and was the form observed in the cells used here. This subtype contains 6 exons (A–F) that code for a portion of the large inner loop and give rise to the alternatively spliced isoforms of NCX1 (26). Exons A and B are believed to be expressed in a mutually exclusive manner in combination with one or more of the remaining (cassette) exons (27). Three exon B-containing isoforms were shown to be the predominant transcripts in rat cortical astrocytes and C6 cells (26) and that, together with our finding that C6 cells contain only nuclear NCX (17), suggests that isoforms containing the B exon are specifically expressed in the NE. Na+/Ca2+ exchangers have been studied primarily as components of the plasma membrane where, in the forward-exchange mode, they mediate countertransport of 3 Na+ for 1 Ca2+ while promoting uphill extrusion of [Ca2+]cyt. They appear to function similarly in the NE, mediating flow of [Ca2+]n into the NE against its gradient. Exchanger activity is driven by a Na+ gradient, which was proposed to occur by means of a Na+/K+-ATPase in the inner membrane of the NE that creates a high intralumenal Na+ concentration (28). Exchanger-mediated transfer of Ca2+ to the NE can presumably be reversed, as with plasma membrane NCX (25), thus providing a potential route for Ca2+ flux from ER/NE to nucleoplasm and cytosol. We have postulated that in addition to the presence of different isoforms, NCX assumes different topological orientations in these 2 membranes that would help explain differences in affinity toward GM1 (13, 29). Evidence for charge-charge interaction between NCX and GM1 in the NE has been reported (16). Those and other studies have further suggested a cytoprotective role for nuclear NCX/GM1, as seen in the higher susceptibility to kainate-induced seizures in knockout mice lacking GM1; rescue by LIGA-20 in the latter study correlated with the ability of this membrane permeable analog of GM1 to insert into the NE and potentiate nuclear NCX (22). Cultured neurons from such knockout mice were rescued from glutamate-induced apoptosis by GM1 and, more effectively, by LIGA-20 (21). It is well known that Ca2+ is crucial for gene transcription, DNA synthesis, and other nuclear functions, while a rise in [Ca2+]n can invoke apoptosis (30). Protection against the latter is postulated as one mechanism by which NCX/GM1 asserts its cytoprotective function.
Gangliosides are now recognized as ubiquitous components of virtually all vertebrate cells and some invertebrate tissues as well (31, 32). Gangliosides belonging to the gangliotetraose family are the primary sialoglycoconjugates of vertebrate neurons and are localized primarily in the plasma membrane (33). There are, however, several indications of intracellular pools as well, as in the detection of a-series gangliosides in the NE of neuroblastoma cells and primary neurons (34). Many examples are known of Ca2+ modulation by gangliosides in neural and other cells, several of which involve GM1 (35). Regulation of nuclear Ca2+ by GM1 in association with NCX can be contrasted with a different Ca2+ regulatory function of GM1 in the plasma membrane involving TRPC5 channel activation (36). The fact that some cells possess the NCX/GM1 complex with its cytoprotective function while others do not (16) could serve to differentiate those cells with a key survival mechanism from those (e.g., certain immune cells) destined for elimination at an appropriate time for the benefit of the organism.
Materials and Methods
Cell Culture and Cameleon Transfection.
Rat/mouse NG108–15 neuroblastom × glioma cells were obtained as a kind gift from M. Nirenberg (National Institutes of Health, Bethesda MD) and were maintained in DMEM-5% FBS. Rat C6 glioma cells and Jurkat clone E6–1 acute human leukemia T cells were from the American Type Culture Collection; the former were maintained in DMEM-10% FBS and the latter in RPMI-1640–15% FBS. NG-CR72 cells were prepared in this laboratory as a GM1-deficient subclone of NG108–15 cells (19) and were maintained in DMEM-15% FBS. pcDNA plasmids encoding cameleon D1ER (ER-cameleon) (14) and Nu2 cameleon (Nu-cameleon) targeted to nucleoplasm (15) were kindly provided by R. Tsien (University of California San Diego). These were transfected into NG108–15, NG-CR72, and C6 cells with Lipofectamine and Plus Reagents (Invitrogen) in OptiMEM medium according to the manufacturer's instructions. They were maintained in culture medium containing 450 μg/ml G418 (Invitrogen). Jurkat cells were transfected using Amaxa Electroporation system with Solution V and protocol T according to the manufacturer's instructions. For Ca2+ imaging, NG108–15, NG-CR72, and C6 cells were seeded onto coverslips coated with polyL-lysine (1 mg/ml) for 24 to 48 h before use. In some trials after attachment onto coverslips, the cells were differentiated in DMEM-1% FBS-N2 supplement with db-cAMP (1 mM)/KCl (30 mM) for NG108–15, or db-cAMP (1 mM) alone for NG-CR72 and C6 cells (17, 19). Jurkat cells were grown in suspension for 24 to 48 h, then attached to polyL-lysine-coated coverslips by centrifuging at 1,000 × g for 5 min before analysis. To verify cameleon expression, cells on coverslips were fixed in cold 4% paraformaldeyde for 2 h and treated with 0.5% Triton X-100 in PBS. Nu-cameleon-expressing cells were stained with Hoechst 33342 (10 μg/ml in PBS) for 30 min at room temperature, and ER-cameleon-expressing cells were incubated with mouse anti-nuclear pore protein mAb plus goat anti-mouse IgG linked to Texas red.
Calcium Determination in Subcellular Compartments.
Calcium concentrations in 3 subcellular compartments were determine with a Nikon Diaphot microscope equipped with UltraView image system employing SpectroMaster (II) (Perkin–Elmer) as illuminating source. [Ca2+]ne/er and [Ca2+]n were determined with ER-cameleon and Nu-cameleon, respectively, using single-emission wavelength at 437 nm and dual excitation wavelengths of 535 and 480 nm. Changes in the 535/480 ratio (R535/480) monitored Ca2+ dynamics in those compartments. [Ca2+]cyt was determined with fura-2 fluorescent indicator, which was loaded into cells by incubation for 30 min with 5 μM fura-2(AM) in medium containing 250-μM sulfinpyrazone. Fura-2 fluorescence was determined at an emission wavelength of 525 nm with dual excitation wavelengths of 350- and 380 nm. The 350/380 ratio (R350/380) was taken to represent [Ca2+]cyt. To avoid possible spectral overlap, fura-2 and the cameleon indicators were used independently. In some experiments, undifferentiated NG108–15 and differentiated NG-CR72 cells were incubated with 1 μM LIGA-20 in culture for 30 min, followed by removal of the latter. All measurements were initiated in Ca2+-free Hank's balanced salt solution supplemented with 10 mM Hepes (pH 7.2), 1 mM MgCl2, and 10% glucose. Tg (2 μM) was applied at 50 s followed in some cases by NCX inhibitor KB (10 μM) at 450 s and subsequently a mixture of CaCl2 (0.5 mM)/KCl (50 mM) for NG108–15, NG-CR72, and C6 cells, or CaCl2 (2 mM) alone for Jurkat cells at 550 s. When KCl was used, the same concentration of NaCl (50 mM) was used in parallel runs as the control for hyperosmolarity. Measurements were continued for another 650 s. Statistical analysis with Student's 2-tailed t-test was applied to 5 to 8 independent runs with 30 to 40 cells in total, based on peak and duration levels of Ca2+ in indicated subcellular compartment.
Detection of NCX and GM1.
Nuclei, NE, and non-nuclear membrane mixture (which included plasma membrane) were obtained as previously described (12, 17). Briefly, cells grown as above were rinsed twice with PBS and frozen at –80° for 2 h. After thawing on ice, they were collected by scraping in a minimum volume of TM buffer consisting of 20 mM Tris-HCl (pH 7.2) and 1 mM MgCl2 and then gently homogenized. Crude nuclei were pelleted from homogenate by centrifugation at 1,000 × g for 10 min. The resultant supernatant was centrifuged at 100,000 × g for 1 h to pellet non-nuclear membrane mixture, and the crude nuclear pellet was further purified with 2 high density sucrose gradients (2.0 and 2.2 mM). Part of the purified nuclei was treated with DNase/RNase plus NaCl followed by centrifugation at 100,000 × g for NE isolation, and the remainder was fixed in 4% paraformaldehyde in PBS for immunocytochemistry analysis. The latter was incubated overnight with a mixture of anti-NCX mAb (clone C2C12, IgM, Sigma) and CtxB-FITC in PBS containing 2% BSA at 4 °C; to this was added goat anti-mouse IgM Ab linked to Texas red at room temp for 2 h and staining patterns observed with a Nikon Diaphot fluorescent microscope. For IP/IB assay, the above isolated NE and the non-nuclear membrane mixture were dispersed in 1% Triton X-100 in TM buffer, subjected to IP using the above anti-NCX mAb and protein-L-linked agarose beads. The resultant precipitate was subjected to SDS/PAGE on a 7% polyacrylamide gel under nonreducing conditions. The proteins were electrophoretically transferred to a PVDF membrane and blotted with polyclonal rabbit anti-NCX Ab (Swant Swiss Antibodies) plus goat anti-rabbit IgG Ab linked to HRP; a parallel gel was blotted with cholera toxin B-HRP for GM1. Both blots were developed on film using ECL reagent (Amersham Pharmacia Biotech).
Acknowledgments.
This study was supported by National Institutes of Health Grant NS033912.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Petersen OH, Gerasimenko OV, Gerasimenko JV, Mogami H, Tepikin AV. The calcium store in the nuclear envelope. Cell Calcium. 1998;23:87–90. doi: 10.1016/s0143-4160(98)90106-3. [DOI] [PubMed] [Google Scholar]
- 2.Strubing C, Clapham DE. Active nuclear import and export is independent of lumenal Ca2+ stores in intact mammalian cells. J General Physiol. 1999;113:239–248. doi: 10.1085/jgp.113.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP- ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 4.Koppler P, Matter N, Malviya AN. Evidence for stereospecific inositol 1,3,4,5-[3H]tetrakisphosphate binding sites on rat liver nuclei. J Biol Chem. 1993;268:26248–26252. [PubMed] [Google Scholar]
- 5.Stehno-Bittel L, Lückhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 6.Humbert J-P, Matter N, Artault JC, Köppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. J Biol Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- 7.Gerasimenko JV, et al. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signaling. J Cell Sci. 2004;117:3087–3094. doi: 10.1242/jcs.01295. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mohanna FA, Caddy KWT, Bolsover SR. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994;367:745–750. doi: 10.1038/367745a0. [DOI] [PubMed] [Google Scholar]
- 10.Bustamante JO. Nuclear pore ion channel activity in live syncytial nuclei. Pflugers Arch – Eur J Physiol. 2002;444:286–290. doi: 10.1007/s00424-002-0813-1. [DOI] [PubMed] [Google Scholar]
- 11.Badminton MN, Kendall JM, Rembold CM, Campbell AK. Current evidence suggests independent regulation of nuclear calcium. Cell Calcium. 1998;23:79–86. doi: 10.1016/s0143-4160(98)90105-1. [DOI] [PubMed] [Google Scholar]
- 12.Xie X, Wu G, Lu Z-H, Ledeen RW. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem. 2002;81:1185–1195. doi: 10.1046/j.1471-4159.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- 13.Ledeen RW, Wu G. Gangliosides of the nuclear membrane: a crucial locus of cytoprotective modulation. J Cellul Biochem. 2006;97:893–903. doi: 10.1002/jcb.20731. [DOI] [PubMed] [Google Scholar]
- 14.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci USA. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X, Wu G, Lu Z-H, Rohowsky-Kochan C, Ledeen RW. Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger-GM1 interaction. Neurochem Res. 2004;29:2135–2146. doi: 10.1007/s11064-004-6887-8. [DOI] [PubMed] [Google Scholar]
- 17.Xie X, Wu G, Ledeen RW. C6 cells express a sodium-calcium exchanger/GM1 complex in the nuclear envelope but have no exchanger in the plasma membrane: comparison to astrocytes. J Neurosci Res. 2004;76:363–375. doi: 10.1002/jnr.20068. [DOI] [PubMed] [Google Scholar]
- 18.Kozireski-Chuback DF, Wu G, Ledeen RW. Upregulation of nuclear GM1 accompanies axon-like but not dendrite-like outgrowth in NG108–15 cells. J Neurosci Res. 1999;55:107–118. doi: 10.1002/(SICI)1097-4547(19990101)55:1<107::AID-JNR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Wu G, Lu Z-H, Li L, Ledeen RW. Mutant NG108–15 cells (NG-CR72) deficient in GM1 synthase respond aberrantly to axonogenic stimuli and are vulnerable to calcium-induced apoptosis; they are rescued with LIGA-20. J Neurochem. 2001;76:690–702. doi: 10.1046/j.1471-4159.2001.00036.x. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of the Na+/Ca2+ exchanger in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Lu Z-H, Xie X, Ledeen RW. Susceptibility of cerebellar granule neurons from GM2/GD2 synthase-null mice to apoptosis induced by glutamate excitotoxicity and elevated KCl: rescue by GM1 and LIGA20. Glycoconj J. 2004;21:305–313. doi: 10.1023/B:GLYC.0000046273.68493.f7. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, et al. Enhanced susceptibility to kainite-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides. Protection with LIGA 20, a membrane-permeant analog of GM1. J Neurosci. 2005;25:11014–11022. doi: 10.1523/JNEUROSCI.3635-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezier A, Bobkov YV, Ache BW. The Na+/Ca2+ exchanger inhibitor, KB-R7943, blocks a nonselective cation channel implicated in chemosensory transduction. J Neurophysiol. 2009;101:1151–1159. doi: 10.1152/jn.90903.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bkaily G, et al. G-protein-coupled receptors, channels, and Na+-H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can J Physiol Pharmacol. 2006;84:431–441. doi: 10.1139/y06-002. [DOI] [PubMed] [Google Scholar]
- 25.Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annu Rev Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 26.He S, Ruknudin A, Bambrick LL, Lederer WJ, Schulze DH. Isoform-specific regulation of the Na+/Ca2+ exchanger in rat astrocytes and neurons by PKA. J Neurosci. 1998;18:4833–4841. doi: 10.1523/JNEUROSCI.18-13-04833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kofuji P, Lederer WJ, Schulze DH. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na+/Ca2+ exchanger. J Biol Chem. 1994;269:5145–5149. [PubMed] [Google Scholar]
- 28.Garner MH. Na,K-ATPase in the nuclear envelope regulates Na+: K+ gradients in hepatocyte nuclei. J Membr Biol. 2002;187:97–115. doi: 10.1007/s00232-001-0155-5. [DOI] [PubMed] [Google Scholar]
- 29.Ledeen R, Wu G. GM1 in the nuclear envelope regulates nuclear calcium through association with a nuclear sodium-calcium exchanger. J Neurochem. 2007;103(Suppl. 1):126–134. doi: 10.1111/j.1471-4159.2007.04722.x. [DOI] [PubMed] [Google Scholar]
- 30.Nicotera P, Bellomo G, Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Ann Rev Pharmacol Toxicol. 1994;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- 31.Yu R, Saito M. Structure and localization of gangliosides. In: Margolis RU, Margolis RK, editors. Neurobiology of Glycoconjugates. New York: Plenum; 1989. pp. 1–42. [Google Scholar]
- 32.Hakomori S-I. Traveling for the glycosphingolipid path. Glycoconj J. 2001;17:627–647. doi: 10.1023/a:1011086929064. [DOI] [PubMed] [Google Scholar]
- 33.Ledeen RW. Biosynthesis, metabolism, and biological effects of gangliosides. In: Margolis RU, Margolis RK, editors. Neurobiology of Glycoconjugates. New York: Plenum; 1989. pp. 43–83. [Google Scholar]
- 34.Wu G, Lu Z-H, Ledeen RW. Induced and spontaneous neuritogenesis are associated with enhanced expression of ganglioside GM1 in the nuclear membrane. J Neurosci. 1995;15:3739–3746. doi: 10.1523/JNEUROSCI.15-05-03739.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ledeen RW, Wu G. Ganglioside function in calcium homeostasis and signaling. Neurochem Res. 2002;27:637–647. doi: 10.1023/a:1020224016830. [DOI] [PubMed] [Google Scholar]
- 36.Wu G, Lu Z-H, Obukhov AG, Nowycky MC, Ledeen RW. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with alpha5beta1 integrin initiates neurite growth. J Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]