Abstract
Developmental amnesia (DA) is a memory disorder due to hypoxia/ischaemia-induced damage to the hippocampus early in life. To test the hypothesis that this disorder is associated with a disproportionate impairment in recall vis-à-vis recognition, we examined a group of 10 patients with DA on the Doors and People test, which affords a quantitative comparison between measures of the two memory processes. The results supported the hypothesis in that the patients showed a sharp, though not complete, recall-recognition dissociation, exhibiting impairment on both measures relative to their matched controls, but with a far greater loss in recall than in recognition. Whether their relatively spared recognition ability is due to restriction of their medial temporal lobe damage to the hippocampus or whether it is due instead to their early age at injury is still uncertain.
Keywords: Hippocampus, Hypoxia, Familiarity, Recollection, Doors and People Test
INTRODUCTION
Patients with developmental amnesia, a syndrome caused by relatively selective damage to the hippocampus following hypoxic-ischaemic episodes sustained in childhood, are able to acquire normal levels of intelligence and general knowledge despite a severe impairment in remembering the events of daily life (Vargha-Khadem et al., 1997). This dissociation between semantic and episodic memory (Tulving, 1972), seems to be accompanied by a second dissociation, one between relatively preserved recognition ability and a marked impairment in recall (Vargha-Khadem et al., 1997; Mishkin et al., 1997). This finding, however, is open to the objection that recognition tasks are ordinarily much less demanding than recall tasks, raising the possibility that the apparent dissociation is a spurious one. In an attempt to overcome this objection, we had selected one such DA patient, Jon, who sustained hypoxia-induced hippocampal damage at birth, and examined him on the Doors and People test (Baddeley et al., 2001). This test, designed by Baddeley and colleagues (1994) to allow direct quantitative comparison between recognition and recall, equates the scores of normal controls across the different measures through the incorporation of easy recall tasks combined with difficult recognition tasks, and also through conversion of the data to scaled scores across the tasks and age groups. Compared to two controls matched for age, sex, and IQ, Jon showed severe impairment on the recall tests, scoring between the 1st and 5th percentile, but was unimpaired on the recognition tasks, falling in the normal range, with scores between the 50th and 75th percentile.
Having recently identified a sizeable group of patients with DA (Adlam et al., 2005), we had the opportunity to determine whether a dissociation between preserved recognition and impaired recall was unique to Jon or whether it could be replicated in the larger number of cases. To test this, we compared the DA group with a matched group of normal controls, and, to rule out any potential confounds due to age differences within the two groups, we scaled the scores of the individuals in both groups using age-appropriate norms (Baddeley et al., 2006).
METHODS
Participants
Ten patients (including Jon) with developmental amnesia (DA group) and twelve healthy normal individuals (NC group) took part in the study. Compared to their controls, the DA group was significantly impaired on numerous tests of episodic memory but not on measures of semantic memory, such as academic attainment, vocabulary, or comprehension (Adlam et al., 20051). Details of the patients are provided in Table 1, including age at injury and etiology. The normal participants were group-matched to the patients for sex, age, and verbal IQ (Table 2). Bilateral reduction of hippocampal volumes in the DA group relative to hippocampal volumes in the controls ranged from 33.2 to 48.6 percent (left, 34.2-56.6; right, 32.3-54.1; Adlam et al., 20051).
Table 1.
Details of patients in the DA group1.
| Case | Sex | Age at injury (year:month) | Age at test (year:month) | Aetiology |
|---|---|---|---|---|
| DA1 | M | Perinatal | 19:11 | Birth asphyxia |
| DA2 | F | Perinatal | 18:00 | Birth asphyxia |
| DA3 | M | Perinatal | 16:00 | Birth asphyxia |
| DA4 | M | Perinatal | 14:01 | Birth asphyxia |
| DA5 | F | Perinatal | 10:06 | Birth asphyxia |
| DA6 | F | 2 days | 14:07 | Hypoxia-ischaemia |
| DA7 | M | 11 weeks | 15:09 | Hypoxia-ischaemia |
| DA10 | F | 9:01 | 26:03 | Hypoxia-ischaemia |
| DA11 | F | 12:05 | 19:07 | Hypoxia-ischaemia |
| DA12 | M | 15:05 | 17:11 | Hypoglycaemia |
DA, developmental amnesia; M, male; F, female.
Table 2.
Means (and ranges) for Age at test and IQ.
| Age at test on D & P Year:month | Full Scale IQ Standard score | Verbal IQ Standard score | Performance IQ Standard score | |
|---|---|---|---|---|
| Control | 16:11 (11:03-27:04) | 97.5 (80-114) | 95.3 (82-113) | 101.4 (75-128) |
| DA | 17:03 (10:06-26:03) | 90.0 (75-114) | 92.7 (82-108) | 89.0 (70-121) |
DA, developmental amnesia; D & P, Doors & People Test
Behavioural Procedures
The Doors and People test was administered to all participants according to the instructions in the published manual (Baddeley et al., 1994), and therefore the test procedure will be described here only briefly. The test consists of four subtests: verbal recall, visual recall, verbal recognition, and visual recognition, thereby providing comparisons between the two memory processes across both modalities. The subtests are described in the order in which they were administered2.
Verbal recall (People test)
Four colour pictures were presented on separate cards for 3 seconds each. Each card depicted a photograph of a person together with a printed name and occupation. After viewing the fourth picture, participants were immediately asked to recall each name cued by the occupation (e.g. “What is the name of the doctor?”). This procedure (presentation of four pictures and cued recall) was repeated until all four names were correctly recalled or for a maximum of three presentations. Errors were not corrected.
Visual recognition (Doors test)
This subtest consisted of two study-test blocks. In the study phase of the first block, participants viewed photographs of 12 doors, each presented for 3 seconds on separate sheets accompanied by an appropriate but ultimately unhelpful label, for example, “this is a church door”. Immediately thereafter, participants viewed 12 arrays of four doors, each array on a separate sheet, and tried to identify the door from the study list. By ensuring that the items within each array all have the same label (e.g. church doors), the role of verbal labelling is minimised. This same test was repeated with a second block consisting of 12 photographs of doors presented in exactly the same way as the first study-test block, but with foils that are more similar to the doors on the study list than is the case on the first block.
Visual recall (Shapes test)
Participants copied each of four simple line drawings, resembling crosses. They then tried to draw the four shapes from memory. This procedure (presentation of four simple designs and recall from memory) was repeated until all four shapes were correctly recalled or for a maximum of three presentations. Errors were not corrected. For the second and third trials, participants viewed the shapes but did not copy them.
Verbal recognition (Names test)
This subtest consisted of two study-test blocks. In the study phase of the first block, 12 female names (both a first name and a surname) were presented on separate cards for 3 seconds each, and the experimenter read them aloud. Immediately thereafter, participants saw 12 lists of four names, each list presented on a separate card, and tried in each case to select the name from the study list. All four names in each list had the same first name and the same initial letter of the surname. The same test was repeated in a second block, this time consisting of male names, but with the foils and the names on each test list differing from the study list in only one syllable of the surname.
Scoring
Each participant’s raw score on each test was corrected for age by conversion to a z score relative to the mean score and standard deviation (SD) of the normal, age-appropriate, reference group. (For participants aged 10-16, means and SDs were provided by C. Jarrold, S. Wood, F. Vargha-Khadem and A. Baddeley [personal communication]; for participants above age 16, means and SDs were obtained from Baddeley et al., 2006, Fig. 1.) Scores were then converted to T scores (mean, 50; SD, 10) to eliminate negative z-scores. A T score of zero is reported for z <= -5.0, which occurred for 3 DA participants on the Shapes test.
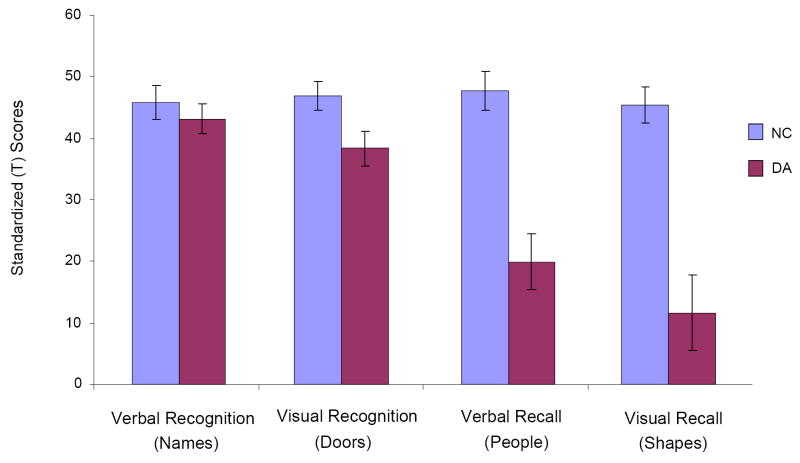
Figure 1.
Group means and standard errors of the mean (SEMs) on each of the four memory tests. DA, developmental amnesia; NC, normal control.
RESULTS
Mean raw scores for each of the four memory tests are shown in Table 3, and mean age-corrected scores are illustrated in Figure 1. A multivariate analysis of variance (ANOVA) on the age-corrected scores showed that the effect of Group was significant for three of them: Shapes (F (1,20) = 27.40, p = .000), People (F (1,20) = 26.30, p = .000), and Doors (F (1,20) = 5.48, p = .030), with the patient group scoring significantly below the control group in each case. Group differences on the Names test (verbal recognition) failed to reach significance (F (1,20) = 0.52, p = .479).
Table 3.
Raw score means (and standard deviations, SD) for each of the two groups (Control, n = 12; DA, n = 10) on each of the four tests
| Task | Group | Mean | SD |
|---|---|---|---|
| Visual Recall | Control | 45.42 | 10.12 |
| DA | 11.70 | 19.44 | |
| Visual Recognition | Control | 46.92 | 7.94 |
| DA | 38.40 | 9.13 | |
| Verbal Recall | Control | 47.67 | 11.04 |
| DA | 20.00 | 14.42 | |
| Verbal Recognition | Control | 45.83 | 9.28 |
| DA | 43.20 | 7.51 | |
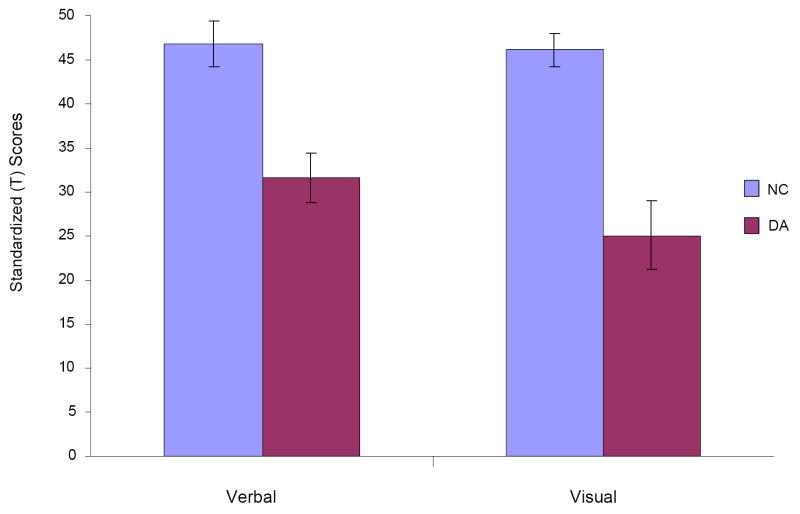
To examine the data for group differences in memory process and modality, we performed a repeated-measure, mixed-model ANOVA, with a between-subjects factor of Group (DA, NC) and within-subjects factors of Process (recall, recognition) and Modality (verbal, visual). Group mean recall scores were derived from the Shapes and People subtests, and recognition scores, from the Doors and Names subtests; similarly, group mean verbal scores were derived from the People and Names subtests, and visual scores, from the Shapes and Doors subtests. Besides a significant main effect of Group (F (1,20) = 25.78, p = .000), the analysis yielded a significant interaction between Group and Process (F (1,20) = 28.79, p = .000), indicating a greater group difference in recall than in recognition (see Fig. 2). In contrast, the interaction of Group and Modality (see Fig. 3) and of Group, Process, and Modality were not significant (F (1,20) = 3.13, p = .092 and F (1,20) = .000, p = .993, respectively).
Figure 2.
Group means and SEMs on the two tests of recognition and on the two tests of recall. Abbreviations as in Figure 1.
Figure 3.
Group means and SEMs on the two verbal tests and on the two visual tests. Abbreviations as in Figure 1.
To examine whether the Group by Process interaction was present not only across modalities but within each one separately, we performed separate ANOVAs for each, and found that both analyses yielded significant effects (verbal, i.e. People vs. Names): F (1,20) = 22.84, p = .000; visual, i.e. Shapes vs. Doors: F (1,20) = 14.97, p = .001).
DISCUSSION
The results confirm that patients with DA show dissociation between recognition and recall on a test that provides a direct quantitative comparison between these two memory processes. The dissociation is not complete, in that the patients showed a mild but significant visual recognition impairment on the Doors test; but this deficit was not nearly as large as their deficit on each of the two recall measures. That the dissociation is valid - not an artifact due to differential sensitivity of the tests for normal subjects - is indicated by the equivalent scores obtained by the control group on all four tests (see Fig. 1).
We had proposed earlier (Mishkin et al., 1997) that the relatively preserved recognition ability in cases with DA is due to sparing of the rhinal cortices, a proposal based on evidence from animal studies that recognition ability is critically dependent on this medial temporal cortical region rather than on the hippocampus. Since that proposal was advanced, however, a qualification must now be added, which stems from the current view that recognition itself depends on two different memory processes, familiarity and recollection (Jacoby, 1991); and, further, though this is still debated (e.g. Wais et al., 2006; Squire et al., 2007), that the familiarity process is mediated by the rhinal cortices, whereas the recollection process is served by the hippocampus (Aggleton and Brown, 1999; Yonelinas, 2002; Bowles et al., 2007; Yonelinas et al., 2007; Turriziani et al., 2008. For recent reviews on the topic, see Diana et al., 2007; Eichenbaum et al., 2007; Mayes et al., 2007). Studies in the DA patient, Jon, provides support for this position by showing that he has little or no recollection-dependent recognition (Gardiner et al., 2006) and, further, that he lacks the event-related potential that appears to be a selective neural correlate of this form of recognition (Düzel et al., 2001). The familiarity process, which may well predominate in animals, may often be sufficient for recognition in humans as well, particularly on tests of item (as opposed to associative) recognition, which is the type measured in the Doors and People test. Yet the notion that the relative preservation of the familiarity process in DA is due to sparing of the rhinal cortices cannot be advanced with any confidence.
Indeed, difficulty in identifying the exact locus and extent of damage may be responsible for the conflicting results that have been reported in studies comparing recognition and recall in adult-onset amnesia. In their comprehensive review relating memory impairment to the underlying neuropathology, Aggleton and Brown (1999) also concluded that recognition memory might be preserved when damage is limited to the hippocampus and is likely to be impaired only when the medial temporal pathology involves the rhinal cortices. To test for dissociation between recognition and recall in patients with limited pathology, Aggleton and colleagues (2000) examined three patients with damage to the fornix using the Doors and People test, and found greater impairment in recall than in recognition. Holdstock and colleagues (2000) observed an even stronger dissociation on the Doors and People test in a patient with apparently selective hippocampal damage. Other investigators, however, who also examined patients with selective hippocampal pathology on this test, have reported a different outcome; thus, both Cipolotti and colleagues (2001; 1 patient) and Manns et al. (2003; 6 patients) found equivalent deficits on the two memory process measures.
How to reconcile these contradictory findings in cases of ‘hippocampal’ amnesia is still unclear. The possibility remains that differences in neuropathology are responsible, since brain areas, and in particular the rhinal cortices, can appear intact based on structural imaging of grey matter3, yet show abnormality when white matter is examined with diffusion tensor imaging (e.g. Papanicolaou et al., 2007) or show reduced activity when neural tissue is assessed with functional imaging (e.g. Nestor et al., 2003). Perhaps resolution of the above discrepancies must await the application of these more comprehensive structural and functional analyses.
The other factor, of course, that may play a role in whether or not recognition memory is preserved after selective damage to the hippocampus is the age at which the pathology occurs. The greater neural plasticity in children than in adults could lead to greater functional sparing in DA than in adult-onset amnesia (Nelson, 2000), even when the locus and extent of damage at these two life stages is the same. It should be noted, however, that among patients with DA, there seems to be little difference in memory function between children with perinatal onset of pathology and those with onset in later childhood (Vargha-Khadem et al., 2003).
Whatever the explanation for the relative sparing of recognition memory in DA, it is clear that the fairly large group of cases with developmental amnesia described here, who show dissociation between semantic and episodic memory, also show a sharp dissociation between recognition and recall, with recall exhibiting a markedly greater loss. Why recall was more severely affected than recognition is open to many interpretations, but a possibility related to the two-process theory of memory is that although recall of new information, like recognition of new information, may be served by both familiarity and recollection, it is probably disproportionately dependent on the recollection process, which we and others have proposed is largely dependent, in turn, on the hippocampus.
Acknowledgments
We are grateful to the participants and their families for their continued support of our research, and we thank Kling Chong for reviewing the MRI. Support for A-L.R.A. was provided by a Medical Research Council Ph.D. Studentship. The Wellcome Trust and the National Institute of Mental Health Intramural Research Program/National Institutes of Health/Department of Health and Human Services provided additional support.
Footnotes
All of the participants in the previous study (12 patients and 12 controls) also participated in the present study, but two of the patients (DA8 and DA9) were later eliminated from this one because they differed from the other patients in several critical features, including: Aetiology (unknown as opposed to known aetiology of the neuropathology); hippocampal pathology (bilateral volume reduction of less than 30 percent below control volumes as opposed to more than 30 percent reduction); and episodic memory (least impaired among the 12 patients) [A. Adlam, University College London Unpublished Ph.D. Thesis, 2003]. Although inclusion of the scores of patients DA8 and DA9 did not significantly alter the statistical results, their scores were nevertheless excluded from the analyses reported here for the reasons stated.
In addition to the procedures described below, delayed (cued) verbal recall was tested after completion of the visual recognition test, and, similarly, delayed (cued) visual recall was tested after completion of the verbal recognition test. Because norms for the delayed recall scores are not available for children, those scores are not considered here.
Although ’hippocampal’ amnesia implies that radiological examination of MRI scans failed to reveal any other medial temporal pathology, the possibility cannot be excluded that varying amounts of reduction in the volumes of the rhinal cortices accompanies the hippocampal atrophy. The attempt to measure rhinal cortical volumes, however, faces the obstacle that significant hippocampal shrinkage shifts the position of hippocampal landmarks used to measure this subjacent tissue in the MRI scans of normal subjects, rendering quantitative comparison with measurements in patients unreliable.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlam AL, Vargha-Khadem F, Mishkin M, de Haan M. Deferred imitation of action sequences in developmental amnesia. Journal of Cognitive Neuroscience. 2005;17(2):240–248. doi: 10.1162/0898929053124901. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral Brain Science. 1999;22(3):425–444. [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, Hornak J, Kapur N, Halpin S, Wiles CM, Kamel H, Brennan P, Carton S, Gaffan D. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain. 2000;123(4):800–815. doi: 10.1093/brain/123.4.800. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Emslie H, Nimmo-Smith I. The Doors and People Test. Thames Valley Test Company; Bury St Edmunds, England: 2006. [Google Scholar]
- Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: Implications for the acquisition of semantic memory? Journal of Cognitive Neuroscience. 2001;13(3):357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Kohler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci USA. 2005;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L, Shallice T, Chan D, Fox N, Scahill R, Harrison G, Stevens J, Rudge P. Long-term retrograde amnesia: the crucial role of the hippocampus. Neuropsychologia. 2001;39:151–172. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas RA, Ranganath C. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Düzel E, Vargha-Khadem F, Heinze HJ, Mishkin M. Brain activity evidence for recognition without recollection after early hippocampal damage. Proc Natl Acad Sci USA. 2001;98(14):8101–8106. doi: 10.1073/pnas.131205798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JM, Brandt KR, Vargha-Khadem F, Baddeley A, Mishkin M. Effects of level of processing but not of task enactment on recognition memory in a case of developmental amnesia. Cognitive Neuropsychology. 2006;23(6):930–948. doi: 10.1080/02643290600588442. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Cezayirli E, Isaac CL, Aggleton JP, Roberts N. A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia. 2000;38:410–425. doi: 10.1016/s0028-3932(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Suzuki W, Gadian DG, Vargha-Khadem F. Hierarchical organisation of cognitive memory. Phil Trans R Soc Lond B. 1997;352:1461–1467. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA. Neural plasticity and human development: the role of early experience in sculpting memory systems. Developmental Science. 2000;3(2):115–136. [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Annals of Neurology. 2003;54(3):343–51. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Hasan KM, Boake C, Eluvathingal TJ, Kramer L. Disruption of limbic pathways in a case of profound amnesia. Neurocase. 2007;13(4):226–8. doi: 10.1080/13554790701594854. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8(11):872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Plenum; 1972. pp. 381–403. [Google Scholar]
- Turriziani P, Serra L, Fadda L, Caltagirone C, Carlesimo GA. Recollection and Familiarity in Hippocampal Amnesia. Hippocampus. 2008 doi: 10.1002/hipo.20412. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential Effects of Early Hippocampal Pathology on Episodic and Semantic Memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Salmond CH, Watkins KE, Friston KJ, Gadian DG, Mishkin M. Developmental amnesia: Effect of age at injury. Proc Natl Acad Sci USA. 2003;100:10055–10060. doi: 10.1073/pnas.1233756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49(3):459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]