Abstract
Objective
To evaluate the expression of biomarkers of implantation, Glycodelin A (GdA), Osteopontin (OPN), Lysophosphatidic acid receptor 3 (LPA3), and HOXA10, in eutopic endometrium of women with and without endometriosis.
Design
Prospective observational study.
Setting
Clinical Research Center.
Patient(s)
Twenty-four women with endometriosis and 23 healthy volunteers of similar age.
Intervention(s)
Secretory phase endometrial biopsy.
Main Outcome Measure(s)
Expression of immunohistochemical staining intensity and localization of GdA, OPN, LPA3 and HOXA10 in eutopic endometrium.
Result(s)
Endometrial GdA expression was significantly reduced in patients after cycle day 22. The endometrium from women with endometriosis also showed decreased expression of OPN in the late secretory phase and LPA3 and HOXA10 expression in the mid- and late secretory phases.
Conclusion(s)
The decreased expression of these four biomarkers of implantation may indicate impaired endometrial receptivity in endometriosis patients, providing one explanation for the subfertility observed even in women with few pelvic implants. As many of these markers are progesterone-dependent, these findings suggest the possibility of reduced endometrial progesterone action in this population.
Keywords: GdA, OPN, LPA3, HOXA10, endometrium, endometriosis, menstrual cycle, immunohistochemistry
Introduction
The rate of subfertility in women with endometriosis is two to three times that of the general population. Endometriosis may alter ovulation, cause scarring that blocks the fallopian tubes and thus prevents fertilization, or create a pelvic inflammatory environment that may harm the egg’s survival. The increased rate of subfertility in women with minimal endometriosis, and lower pregnancy rates in women undergoing IVF, however (1), suggest that the endometrium of these women may not be normally receptive to a blastocyst.
Several compounds made by the endometrium may be critical to implantation, including glycodelin A, osteopontin, lysophosphatidic acid receptor 3, and HOXA10. Differences in gene expression and translation suggest that the endometrium of women with endometriosis may appear histologically normal but, in fact, be biochemically abnormal during the implantation window (2). Microarray studies showed that patients with endometriosis aberrantly express key proteins, such as glycodelin A, within the eutopic endometrium (3). Real-time PCR has confirmed the down-regulation of glycodelin A and osteopontin (4). Immunohistochemical investigations of the endometrium in women with endometriosis demonstrated abnormal expression of putative implantation factors, such as the αvβ3 integrins, and leukemia inhibitory factor (5–7).
Glycodelin A (GdA) is secreted by endometrial glands during the secretory, but not the follicular phase of the menstrual cycle (8). The multiple progesterone response elements in the promoter region of the gene may regulate this enhanced transcription. GdA may suppress the maternal immune response and thereby support implantation of the blastocyst (8, 9).
Osteopontin (OPN) also is transcriptionally regulated by progesterone and is an acidic extracellular matrix glycoprotein (10, 11). In the endometrium, it binds to both the αvβ3 and α4β1 integrins, giving rise to speculation that it may mediate trophoblast-endometrial interactions during implantation.
Lysophosphatidic acid (LPA) is a phospholipid that acts as a G-protein-coupled receptor agonist. In the mouse, it is involved in blastocyst implantation through its ability to stimulate COX-2 induction and prostaglandin signaling. Lysophosphatidic acid receptor 3 (LPA3) null mice showed reduced litter size and altered implantation space, providing additional evidence for its role in murine implantation (12, 13).
HOXA10, a member of the homeobox family of transcription factors, is necessary for normal uterine development and implantation in the mouse (14, 15). It is expressed in human endometrium in a cyclical pattern, under estrogen and progesterone regulation, with peak expression during the window of implantation (16). Several studies have analyzed elements of HOXA10 expression in the endometrium of women with endometriosis. Northern blots as well as immunohistochemical studies show down-regulation of HOXA10 in the eutopic endometrium of women with endometriosis during the window of implantation (17, 18).
The goal of this study was to use immunohistochemical techniques to evaluate the expression of these substances implicated in implantation (glycodelin A, osteopontin, Lysophosphatidic acid receptor 3, and HOXA10) in women with and without endometriosis.
MATERIAL AND METHODS
Study Design
The protocols were approved by the National Institute of Child Health and Human Development Institutional Review Board. Women with and without endometriosis were recruited and informed consent was obtained prior to study entry.
Patients with histologically documented (n = 19) or visually diagnosed (n = 5) endometriosis were age 29.9 + 6.7 years (range 20–44); 18 were Caucasian, 4 were African-American, 1 was Asian, and 1 was Hispanic. Seven had stage I endometriosis, 8 had stage II, 5 had stage III, and 4 had stage IV as judged by r-AFS scoring system (19). Seventeen of the 24 women had never been pregnant and were not attempting pregnancy; two gravida 0 women became pregnant at the time of the study; two gravida 1 para 0 women had a therapeutic abortion, one gravida 2 para 1 became pregnant during the study (after the surgery), one gravida 2 woman had no children, and one gravida 3 woman had one miscarriage and two live births. These women underwent laparoscopy for evaluation and possible treatment of endometriosis, at which time an endometrial biopsy was obtained. Women were included only if their endometrial sample was histologically dated as in the secretory phase.
Similarly age-matched healthy volunteers were chosen as controls from those participating in an endometrium tissue-collection protocol. They included 23 women aged 30.0 + 5.6 years (range 21–43); 12 were Caucasian, 8 were African-American, 1 was Asian, and 2 did not designate an ethnic background. None of these healthy women had a history suggestive of endocrinological or gynecological disorders or subfertility. Each woman recorded her menstrual cycles for at least 3 months and performed in-home evaluation of the urine luteinizing hormone (LH) surge using a commercial kit (OvuQUICK™; Quidel, San Diego, CA) to determine whether she had normal menstrual cycle dynamics, defined as a cycle length of 24 to 35 days, with a secretory phase of at least 12 days.
Healthy volunteers who met these criteria for three months had a timed endometrial biopsy obtained using the Pipelle™ curette (Unimar, Wilton CT) in the secretory phase of the cycle (categorized by days since onset of the LH surge).
Endometrial Samples
Endometrial samples were placed in 4% paraformaldehyde prior to paraffin embedding. Paraffin blocks were sectioned, representative slides stained with hematoxylin and eosin, and samples dated according to the criteria of Noyes et al.(20). In healthy volunteers, the histologic date was within two days of the chronologic date from the LH surge. These secretory phase samples were further characterized histologically as early (cycle day 15–18), mid (cycle day 19–23) or late (cycle day 24–28) according to established criteria (21).
Immunohistochemistry
All immunohistochemical experiments were performed using Vectastain EliteR ABC kits (Vector Laboratories, Burlingame, CA, USA) and a standard protocol: the sections were deparaffinized in xylene solutions at room temperature, hydrated in serial diluted alcohols, and subjected to antigen retrieval as needed. No antigen retrieval was used for OPN slides. Citric acid antigen retrieval, used for LPA3 and HoxA10 slides, involved boiling in 0.01M, PH6.0, citric acid buffer (0.0M, PH 6.0) for 20 minutes. Trypsin antigen retrieval was used on GdA slides (0.05% solution at 37°C for 30 min). After antigen retrieval, slides were incubated for 10 minutes in a 5% peroxide solution to inactivate endogenous peroxidases, and then incubated with 2.5% normal horse or goat serum to reduce non-specific staining. Slides were then incubated with primary antibody for 16 hours at 4C (for GdA 1:200 dilution: ab 17247, Abcam Inc, Cambridge MA; for OPN 1:400 dilution: AB1870, Chemicon international, Temecula, CA; for LPA4 1:300 dilution LS-A1014:4177/78AP3-2, LifeSpan, Inc; and for HoxA10 1:400 dilution: sc-17159, Santa Cruz Biotechnology, Inc). The secondary antibody was Vector Universal, except for HoxA10, which was incubated with 1:400 horse antigoat antibody. Slides were then incubated with ABC reagent for 30 minutes at room temperature and stained using 3, 3–diaminobenzidine (DAB) horseradish peroxidase staining solution. Slides were then counterstained with Harris hematoxylin, dehydrated in serial diluted alcohol and xylene solutions, and mounted. Primary antibody was omitted for negative control slides.
Statistical Analysis
Except for the HOXA10 glandular score, two independent observers scored the glandular epithelial and stromal cells staining intensity (i) from 0 (none) to 3 (strong) for each slide. An HSCORE=ΣPi(i+1) was calculated for each biopsy, where Pi is the percentage of cells for each intensity, varying from 0 to 100%. The two observers were very consistent in their scores, with p-value of 0.7 –0.97 by paired t-test, except for glandular assessment of OPN late secretory volunteers. In the latter instances, if the scores furthest from the endometriosis group were not used, the significance of the findings did not change. Thus, the average scores for each slide were used for statistical analysis. The Wilcoxon test was used to compare HSCOREs between the endometriosis and volunteer groups grouped according to the time of the secretory phase. Exact p-values were computed. All statistical analyses were two-tailed, with statistical significance defined as a p-value of ≤0.05. Results are presented as mean ± standard deviation (SD), unless otherwise specified. Data were analyzed using SAS system software, release 9.1 (SAS Institute, Inc., Cary, North Carolina).
Results
The HSCORE and statistical comparison of biomarker expression from mid- and late secretory endometrium in both cohorts are summarized in Table 2. Compared to that of healthy volunteers, the late secretory phase epithelial cells of patients with endometriosis showed decreased expression of all four markers. Mid-secretory LPA3 and HOXA10 expression also were decreased (Figures 1 and 2). Except for HOXA10, staining was absent in the stroma.
Fig 1.
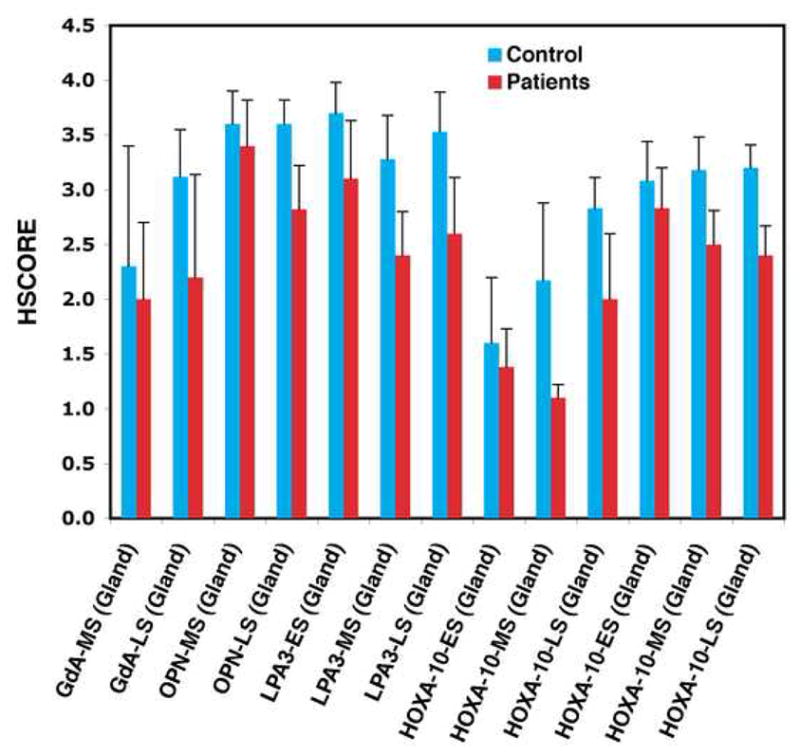
HSCOREs of biomarker expression in glandular epithelium or stroma (S, HOXA10 only) of healthy volunteers (blue bars) and women with endometriosis (red bars) during the early, mid- and late secretory phase (ES, MS, LS, respectively). * indicates p < 0.05
Fig 2.
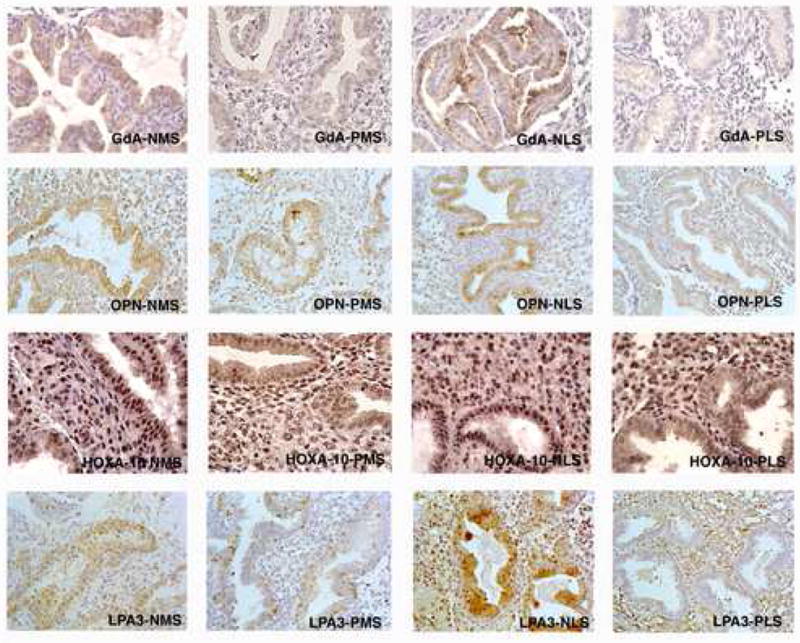
IHC localization of GdA, OPN, LPA3 and HOXA10 in the endometrium of healthy women (denoted N) and women with endometriosis (denoted P), during the early, mid- and late secretory phase (denoted ES, MS and LS, respectively). Expression was significantly reduced in endometriosis endometrium of all paired phase samples, except for mid-secretory OPN and GdA.
Glycodelin A
Compared to healthy volunteers, endometriosis patients had similar glycodelin A HSCOREs in mid-secretory glandular epithelium (p=0.761), but reduced staining in the late luteal phase (p=0.048). Apart from this difference in staining intensity, glycodelin A showed similar localization patterns in the cytoplasm, nuclei, and secretions of the epithelial cells of the endometrial glands, beginning in the mid-secretory phase (Figures 1 and 2). There was very little staining in stromal cells.
Osteopontin
Like glycodelin A, osteopontin HSCOREs in the glandular epithelial cells were similar in both subject groups in the mid-secretory phase (p=0.127), but decreased in the endometriosis group in the late secretory phase (p=0.0001). Qualitatively, patient and control staining were similar in the localization to the cytoplasm, nuclei, and secretions of the epithelial cells of the endometrial glands, beginning in the mid-secretory phase (Figures 1 and 2). There was very little staining in the stromal cells in either group.
LPA3
There was reduced LPA3 expression in the mid- and late secretory phase of endometriosis patients’ glandular epithelial cells compared to healthy volunteers (early, p=0.06; middle, p=0.005; late, p=0.0038; Figures 1 and 2). Immunohistochemical localization of LPA3 was similar in both groups, occurring primarily in glandular cytoplasm, nuclei and lumen. Staining was often localized towards the apical and basal membranes of the epithelial cells, although during the mid- and late secretory phase, cytoplasmic staining was more consistent within each cell. There was inconsistent and faint staining in the cytoplasm of stromal cells.
HOXA10
HOXA10 HSCOREs were significantly decreased in both the mid- and late secretory phase in patients compared to healthy controls, in both stroma (early, p=0.225; middle, p=0.0006; late, p=0.0001) and epithelial cells (early, p=0.69, middle, p=0.006, late p=0.002) (Figures 1 and 2). In both healthy volunteers and patient endometrium, HOXA10 staining was localized to the nuclei of stromal and glandular cells.
Overlap between the Groups
Individual data were compared when the overall groups showed a statistical difference. We noted the number of patients with endometriosis whose score was equal to or above that of the lowest healthy volunteer. There was no overlap in HOXA10 late secretory stroma scores. One patient overlapped the control range in the HOXA10 and LPA mid-secretory scores, and the OPN late secretory scores. Two patients overlapped the control range in the HOXA10 mid-and late secretory glandular scores and the LPA late secretory scores. In all of these cases the overlap was with one or two values in the control range. By contrast, four of the endometriosis patients overlapped the healthy volunteer range for late secretory GdA, with one individual achieving the highest score seen in the control group.
Discussion
A receptive endometrium is characterized by abundant secretory activity (22). Many genes are expressed during the window of implantation, but their functional roles remain unclear (2). We evaluated the expression of four proteins known or suspected to be involved in the embryonic implantation process, in women with endometriosis and healthy volunteers.
In women, OPN has been associated with glandular epithelial cells during the secretory phase and in increasing concentrations in uterine secretions during the mid-to-late secretory phase during which implantation would be expected to occur (11, 23). We found a dramatic decrease of OPN expression in the mid-late secretory phase endometrium from patients with endometriosis. The current report extends previous findings indicating that osteopontin mRNA is reduced in the early secretory phase of women with moderate-to-severe endometriosis (4). An earlier report of patients with endometriosis suggested reduced OPN binding to the surface epithelium, but only in cases where αvβ3 was decreased (24). Taken together, these data support the hypothesis that reduced osteopontin production or binding may be involved in subfertility of endometriosis patients.
This study is the first to show decreased expression of LPA3 in the mid- and late secretory phase endometrial epithelium from patients with endometriosis. Low expression of Cox2 in LPA3 null mice results in low production of prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2), leading to low vascular permeability and endometrial adhesiveness and delayed implantation. By extrapolation, decreased expression of the receptor in women may reduce fertility.
We found decreased expression of HOXA10 in the mid- and late secretory phase glands and stroma from endometriosis patients. The current study also confirmed previous reports localizing the expression of HOXA10 to both stromal and glandular cells, and also reports showing reduced HOXA10 expression in the mid-secretory phase endometrium of women with endometriosis (16, 18, 25). In contrast to one previous report, we found decreased HOXA10 expression in the stromal cells as well as the glands. Findings that baboons with endometriosis and decreased HOXA10 expression also have reduced expression of beta3 integrin suggests a possible mechanism by which HOXA10 deficiency might impair fertility (26). While mice lacking HOXA10 showed decreased litter size due to failure of implantation (14), decreased HOXA10 expression has been described in women with other disorders associated with infertility, suggesting a role in human receptivity defects (27)
Our finding of reduced glycodelin expression in the epithelial endometrium of women with endometriosis confirms previous findings that mRNA expression is reduced in these women (4, 14). As the major secretory protein of the endometrium, glycodelin has been suspected to have a role in implantation. A previous study of women with unexplained infertility supported this hypothesis by demonstrating that infertile women had a marked decrease in immunohistochemical staining of endometrial glycodelin during the implantation window compared to fertile controls (28). Others have also noted decreased glycodelin levels in uterine flushings of women with endometriosis (29).
It is important to emphasize that the women in these studies were not chosen based on a history of infertility. Thus further studies in women attempting pregnancy or with infertility are need to establish a more causal association of abnormal markers with abnormal endometrial function.
The reduced expression of these putative biomarkers of implantation provides additional evidence for an abnormal endometrium, and in particular, abnormal epithelial function, in women with endometriosis. Most of these women had mild endometriosis, suggesting that endometrial abnormalities are intrinsic to the disorder and are not a late manifestation. The underlying mechanisms for these differences in peptide expression are not clear and the design of this descriptive study did not examine whether message is reduced or whether there is a post-transcriptional abnormality.
Although the markers studied all have a progesterone-responsive element(s) in their promoter region, and the blood progesterone level is reportedly normal in women with endometriosis, we found reduced expression of these genes. This supports previous speculation of progesterone resistance in eutopic endometrium from women with endometriosis (2–4). In aggregate, these studies demonstrate an altered mRNA “fingerprint” in the secretory phase endometrium of women with endometriosis, characterized by persistence of proliferative phase transcripts and decreases in progesterone-dependent genes. Thus the expression of many genes is altered in the endometrium of women with endometriosis. Further studies are needed to understand the mechanism of this apparent progesterone resistance, which may be effected by alterations in the progesterone receptors, transcription factors or downstream messengers.
One intriguing mechanism for this was suggested by demonstration of increased methylation in the HOXA10 gene promoter in the endometrium of endometriosis patients (30). Hypermethylation also has been documented in the progesterone receptor B gene, whose endometrial expression is reduced in these patients (31, 32). The recent demonstration by the same group of increased expression in women with endometriosis of deoxyribonucleic acid methyltransferases responsible for methylation suggests a plausible mechanism by which multiple genes might be down-regulated in this disorder (26).
Table 1.
Summary of biomarker expression and statistical comparison of biomarker expression in endometrium from healthy control women and patients with endometriosis. GdA = Glycodelin A; OPN = Osteopontin, LPA3 = Lysophosphatidic acid receptor 3, HOXA10
| Biomarker | Histologic secretory phase | Mean HSCORE + SD (n = no. of subjects) | p-value a | |
|---|---|---|---|---|
| Normal | Endometriosis | |||
| GdA | Mid | 2.3 ± 1.1 (n=9) | 2.0 ± 0.7 (n=6) | .761 |
| Late | 3.1 ± 0.4 (n=8) | 2.2 ± 0.9 (n=9) | .049 | |
| OPN | Mid | 3.6 ± 0.3 (n=9) | 3.4 ± 0.4 (n=6) | .127 |
| Late | 3.6 ± 0.2 (n=8) | 2.8 ± 0.4 (n=9) | .0001 | |
| LPA3 | Early | 3.7 ± 0.3 (n=7) | 3.1 ± 0.5 (n=7) | .061 |
| Mid | 3.3 ± 0.4 (n=9) | 2.4 ± 0.4 (n=6) | .006 | |
| Late | 3.5 ± 0.4 (n=8) | 2.6 ± 0.5 (n=9) | .002 | |
| HOXA10 Stroma | Early | 3.1 ± 0.4 (n=7) | 2.8 ± 0.4 (n=7) | .225 |
| Mid | 3.2 ± 0.3 (n=9) | 2.5 ± 0.3 (n=6) | .0006 | |
| Late | 3.2 ± 0.2 (n=8) | 2.4 ± 03 (n=9) | .0001 | |
| HOXA10 Gland | Early | 1.6 ± 0.6 (n=7) | 1.4 ± 0.4 (n=7) | 0.691 |
| Mid | 2.2 ± 0.7 (n=9) | 1.1 ± 0.1 (n=6) | 0.006 | |
| Late | 2.8 ± 0.3 (n=8) | 2.0 ± 0.6 (n=9) | 0.002 | |
p-value determined by Wilcoxon test comparing HSCORES values between endometriosis and healthy control subjects and computing exact p-values
Acknowledgments
We thank Dr. Rhonda Hearns-Stokes, who obtained some of these endometrial biopsies in healthy women.
Financial Support: This research was supported, in part, by the Reproductive Biology and Medicine Branch, NICHD, of the intramural NIH, Bethesda, MD.
Footnotes
Where the work was done: The Reproductive Biology and Medicine Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD
Conflict of interest: None
Presented at a meeting: Presented in part at the 88th Annual meeting of the Endocrine Society, Boston, MA 2006
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–55. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002;955:252–64. doi: 10.1111/j.1749-6632.2002.tb02786.x. discussion 93-5, 396–406. [DOI] [PubMed] [Google Scholar]
- 3.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 4.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 5.Lessey BA. The use of integrins for the assessment of uterine receptivity. Fertil Steril. 1994;61:812–4. doi: 10.1016/s0015-0282(16)56688-6. [DOI] [PubMed] [Google Scholar]
- 6.Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994;62:497–506. [PubMed] [Google Scholar]
- 7.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 8.Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23:401–30. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]
- 9.Brown SE, Mandelin E, Oehninger S, Toner JP, Seppala M, Jones HW., Jr Endometrial glycodelin-A expression in the luteal phase of stimulated ovarian cycles. Fertil Steril. 2000;74:130–3. doi: 10.1016/s0015-0282(00)00586-0. [DOI] [PubMed] [Google Scholar]
- 10.Johnson GA, Burghardt RC, Bazer FW, Spencer TE. Osteopontin: roles in implantation and placentation. Biol Reprod. 2003;69:1458–71. doi: 10.1095/biolreprod.103.020651. [DOI] [PubMed] [Google Scholar]
- 11.Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, et al. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab. 2001;86:4991–5000. doi: 10.1210/jcem.86.10.7906. [DOI] [PubMed] [Google Scholar]
- 12.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah BH, Catt KJ. Roles of LPA3 and COX-2 in implantation. Trends Endocrinol Metab. 2005;16:397–9. doi: 10.1016/j.tem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–3. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 15.Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7:1378–84. doi: 10.1038/sj.gt.3301245. [DOI] [PubMed] [Google Scholar]
- 16.Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–73. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- 18.Browne H, Taylor H. HOXA10 expression in ectopic endometrial tissue. Fertil Steril. 2006;85:1386–90. doi: 10.1016/j.fertnstert.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 19.Weitzman GA, Buttram VC., Jr Classification of endometriosis. Obstet Gynecol Clin North Am. 1989;16:61–77. [PubMed] [Google Scholar]
- 20.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 21.Noyes RWHATRJ. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. [Google Scholar]
- 22.Noyes RW. Endometrial development and fertility. J Miss State Med Assoc. 1963;4:5–7. [PubMed] [Google Scholar]
- 23.von Wolff M, Bohlmann MK, Fiedler C, Ursel S, Strowitzki T. Osteopontin is up-regulated in human decidual stromal cells. Fertil Steril. 2004;81 (Suppl 1):741–8. doi: 10.1016/j.fertnstert.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Lessey BA. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002;955:265–80. doi: 10.1111/j.1749-6632.2002.tb02787.x. discussion 93-5, 396–406. [DOI] [PubMed] [Google Scholar]
- 25.Sarno JL, Kliman HJ, Taylor HS. HOXA-10, Pbx2, and Meis1 Protein Expression in the Human Endometrium: Formation of Multimeric Complexes on HOXA-10 Target Genes. JCEM. 2005;90:522–28. doi: 10.1210/jc.2004-0817. [DOI] [PubMed] [Google Scholar]
- 26.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13:323–32. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- 27.Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–55. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 28.Mylonas I, Jeschke U, Kunert-Keil C, Shabani N, Dian D, Bauerfeind I, et al. Glycodelin A is expressed differentially in normal human endometrial tissue throughout the menstrual cycle as assessed by immunohistochemistry and in situ hybridization. Fertil Steril. 2006;86:1488–97. doi: 10.1016/j.fertnstert.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 29.Stavreus-Evers A, Mandelin E, Koistinen R, Aghajanova L, Hovatta O, Seppala M. Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil Steril. 2006;85:1803–11. doi: 10.1016/j.fertnstert.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193:371–80. doi: 10.1016/j.ajog.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–11. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]


