Abstract
Background
After small bowel resection (SBR), adaptation is initiated in intestinal crypts where stem cells reside. Prior studies revealed SBR induced enterocyte proliferation requires the expression of p21waf1/cip1. Since deficient expression of p21waf1/cip1 has been shown to result in reduced numbers of hematopoietic stem cells, we sought to test the hypothesis that p21waf1/cip1 deficiency similarly perturbs the intestinal stem cell population after SBR.
Methods
Control (n=21; C57Bl/6) and p21waf1/cip1-null mice (n=30) underwent 50% proximal SBR or sham operation. After 3 days, the ileum was harvested and the crypt stem cell population evaluated by counting crypt base columnar (CBC) cells on histological sections, determining the expression of Musashi-1 and Lgr5, and profiling the transcriptional expression of 84 known stem cell genes.
Results
There were no significant differences in CBC cells, expression of Musashi-1 or Lgr5, or in stem cell gene expression after SBR in control mice. Further, there were no differences in these markers between controls and p21waf1/cip1-null mice.
Conclusion
In contrast with bone marrow stem cells, the stem cell population of the gut is unaffected by deficient expression of p21waf1/cip1. Additional mechanisms for the role of p21waf1/cip1 in small bowel proliferation and adaptation following massive SBR must be considered.
Keywords: p21waf1/cip1, Adaptation, Proliferation, Small bowel resection, Intestinal stem cells
Introduction
Small intestine adaptation is a phenomenon that occurs after a massive amount of intestinal length is removed. Through this process the remaining intestine compensates for the loss of absorptive area, either macroscopically through increased villus height and crypt depth, and intestinal lengthening, or microscopically through increased cellular protein and DNA content per unit length 1. Patients with short bowel syndrome are in a state of malnutrition and malabsorption and are destined to remain on intravenous sources of nutrition if small bowel adaptation is incomplete 2.
Increased proliferation of the intestinal stem cells into enterocytes is critical and necessary for adaptation 3. Intestinal stem cells can give rise to any of the four types of intestinal epithelial cells (enterocytes, enteroendocrine cells, Paneth cells, Goblet cells) and also replenish their own population. Under normal conditions it is believed this occurs by asymmetric division: each stem cell divides and gives rise to one stem cell and one progenitor cell 4. Under perturbed conditions, such as chemotherapy and radiation, the intestinal stem cells undergo symmetric division and replenish their own population by dividing and giving rise to two daughter stem cells 5.
In mice that are deficient in the expression of the cell-cycle inhibitor p21waf1/cip1, we have previously demonstrated that resection-induced adaptation does not occur 6,7. In addition, baseline rates of proliferation rate are slightly less. In hematopoietic progenitor cells from p21waf1/cip1-null mice, the ability to mount a proliferative response was prevented, and there were overall decreased numbers of hematopoietic progenitor cells 8. This was postulated to be due to the requirement of p21waf1/cip1 for asymmetric stem cell division. In the absence of this protein, stem cells might divide symmetrically, leaving fewer remaining stem cells. In the small intestine, it has been suggested that intestinal stem cells undergo expansion after massive bowel resection in normal mice9. The purpose of this study therefore, was to test the hypothesis that similar to hematopoietic stem cells; the mechanism for the lack of resection-induced adaptation in p21waf1/cip1-null mice is due to diminished numbers of crypt stem cells.
Methods
Animals
The protocol for this study was approved by the Washington University Institutional Animal Care and Use Committee (Protocol 20070145; Washington University School of Medicine, St. Louis, MO). Control (C57/Blk6) and homozygous breeding pairs for p21waf1/cip1-null mice (developed on a C57/Bl6 background) were obtained from the Jackson Laboratories (Bar Harbor, ME). Male mice ages 8–13 weeks were used in this study with a weight range of 20–25g. Mice were kept on a 12 hour light-dark schedule and were housed in a standard facility and allowed to acclimate to their environment for at least 7 days.
Experimental design
Both control (n=21) and p21waf1/cip1-null (n=30) mice were randomly assigned to either 50% proximal SBR or sham operation. On postoperative day 3, the remnant small bowel was removed to measure markers for intestinal stem cells and histological parameters of adaptation (villus height and crypt depth). Protein and RNA were extracted from the isolated crypt cells to detect the expression of p21waf1/cip1 and Musashi-1 protein 10 by Western blotting. For transcriptional studies of gene expression, RT-PCR was employed to measure the expression of leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5), a putative marker of intestinal stem cells 11. We also utilized a commercially available stem cell PCR array for the simultaneous determination of expression of 84 known genes to be linked to stem cells.
Operative procedure
Specific details of this procedure have been described previously 12. Briefly, a 50% proximal SBR was performed by transecting the small bowel 12 cm proximal to the cecum, and removing approximately 12 cm of proximal small intestine. Intestinal continuity was then restored with an end-to-end, single layered, interrupted anastomosis using 9-0 monofilament suture. A sham operation was performed by transecting greater than 50 percent of the circumference of the bowel 12 cm proximal to the cecum and immediately restoring intestinal continuity with the same anastomosis. All mice were placed on a preoperative liquid diet (Micro-stabilized Rodent Liquid Diet LD101; Purina Mills, St. Louis, MO) one day prior to their operation. After their operation, the animals received water only for the first 24hours, followed by the same liquid diet until sacrifice. Animals that died, appeared ill (unkempt fur, lethargy), or had signs of intestinal obstruction at the time of sacrifice were excluded from further analyses.
Small bowel harvest and enterocyte isolation
On the third postoperative day, the mice were sacrificed with a subcutaneous injection of ketamine, xylazine, and acepromazine (4:1:1 proportion) followed by cervical dislocation. This time point was chosen as we have already established greater rates of proliferation in normal mice 12 as well as lack of proliferation in p21-null mice at this postoperative interval 6,7. The abdominal cavity was opened, the intestinal anastomosis identified and the remaining distal bowel excised from the mesentery and cecum. The intestine was immediately flushed with and placed in ice-cold phosphate-buffered saline. The first centimeter of the segment distal to the anastomosis was discarded, the next 2 cm was fixed for histology in 10% neutral-buffered formalin, and the subsequent 5 cm was cut open and transferred into tubes containing 5 mL of ice cold PBS with protease inhibitors (0.2 mM PMSF, 5μg/ml Aprotinin, 1mM Benzamidine, 1mM sodium orthovanadate and 2μM Cantharidin (EMD, Gibbstown, NJ). The tissue was then transferred into a solution containing (1.5 mM KCl, 96 mM NaCl, 27 mM Na Citrate, 8 mM KH2PO4, 5.6 mM Na2HPO4, 15 mM EDTA and 1 mM DTT). The tissue was vortexed at maximum speed in 4°C for a total of 15 minutes, and then strained over a 70μm cell strainer to separate crypts from villi. The remaining muscle tissue and villi were removed, and the crypt aliquot was centrifuged at 1000 g for 7 minutes at 4°C. The pellet was then resuspended in 1 mL of Tris buffer (50 mmol/L Tris-HCL, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA), and 200 uL removed for RNA extraction. The remaining 800 uL was centrifuged again, frozen, and stored in pellet form in a −80°C freezer.
RNA extraction and real-time PCR
RNA was extracted from the above mentioned aliquot of isolated intestinal crypts. The sample was first centrifuged and all supernatant removed and the pellet stored in lysis buffer (RNAqueous kit, Ambion, Austin, TX) at −80°C. RNA was extracted from the samples by following the instructions for the RNAqueous Kit. The concentration of total RNA was determined spectrophotometrically at A260 using a NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, DE) then stored at −80°C. The RNA samples were evaluated for quality using a Bio-Rad Experion Sysetem with a RNA StdSens Chip and reagents (Bio-Rad Laboratories, Richmond, CA). Only RNA samples that revealed adequate 18s and 28s ribosomal RNA peaks were used in the analysis. The isolated mRNA was used to produce complementary DNA (cDNA) using a RT2 First Strand Kit (SABioscience, Fredrick, MD). The cDNA was analyzed on a mouse stem cell RT2 Profiler PCR Array (SABioscience, Fredrick, MD) using an Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA). The mouse stem cell array profiles the expression of 84 genes involved in the identification, growth, and differentiation of mouse stem cells. The entire set of genes contained on this array is listed in the following link: http://www.sabiosciences.com/rt_pcr_product/HTML/PAMM-405A.html. The Lgr5 gene expression was examined using primers and reagents from SABiosciences and the 7500 Fast Real-Time PCR system as above except that the cDNA was quantified using Quanti-iT Oligreen ssDNA Assay kit (Invitrogen, Carlsbad, CA) so that equal amounts of cDNA could be used in all reactions and beta-actin was employed as the endogenous control.
Histology
All histological measurements were performed by one investigator who was blinded regarding mouse strain or operative procedure. Five-micrometer longitudinal sections of paraffin-embedded tissues sections were mounted on glass slides and used for morphology. H&E-stained sections were used to measure villus height and crypt depth with a video-assisted computer program (Metamorph, UIC, Dowington, PA). Twenty crypts and villi were counted per slide. Only intact crypts which extended from the crypt-villus junction to the basement membrane and villi in which the central lymphatic channel was apparent were measured. The columnar type cells interspersed between Paneth cells 13 were counted as crypt base columnar (CBC) cells. A minimum of twenty crypts were analyzed per slide.
Western blotting
Frozen isolated crypt samples were thawed, reconstructed with Tris buffer as above, and sonicated for 10 seconds. The samples were then lysed with sodium dodecyl sulfate (SDS) sample buffer (50 mmol/L Tris-HCL, pH 6.8, 2% SDS, 10% glycerol, and 5% mercaptoethanol). The lysate was then heated for 5 minutes at 100° C and the protein concentration was determined by using the RC DC kit (Bio-Rad, Hercules, CA) following the manufacturer’s protocol. The proteins were separated on 4–20% gels and transferred to nitrocellulose membranes. The following antibodies were used to detect protein bands: Actin, Musashi-1 (Cell Signaling Technology, Danvers, MA) and p21waf1/cip1 (BD Biosciences Pharmigen, San Diego, CA).
Statistics
All results are presented as a mean +/− standard error of measure. Statistical differences were determined by using SigmaStat software (SPSS, Chicago, IL). Statistical significance was established at P<0.05.
Results
The overall survival of the control and p21waf1/cip1-null mice after sham and SBR procedures was similar. In controls, 10 of 10 sham and 10 of 11 SBR survived, whereas in p21waf1/cip1-null 10 of 10 sham and 19 of 20 SBR survived. Of the 19 p21waf1/cip1-null mice, 8 were excluded due to anastomotic obstruction. It is unclear at this time as to the etiology of this since the operator was blinded as to the mouse strain at the time of operation.
Ileal morphology
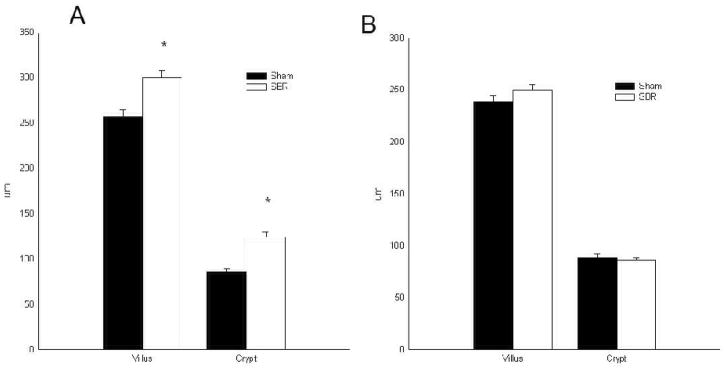
Villus height and crypt depth were significantly (P<0.05) higher after SBR in control animals compared to sham control animals (Fig. 1A). Similar to our prior studies 14, there was no significant change in villus height or crypt depth following SBR in the p21waf1/cip1-null mice (Fig. 1B). The villus height and crypt depth of sham control mice and both sham and SBR p21waf1/cip1-null were similar. This data confirms that p21waf1/cip1-null mice failed to adapt.
Figure 1.
Villus height and crypt depth measurements taken from the ileum of male control (A; C57/Blk6) and p21-null (B) mice at 3 days following either sham (transection and reanastomosis) or 50% proximal small bowel resection (SBR): *P< 0.05 SBR versus Sham.
Stem cell mouse array
RNA extracted from intestinal crypt isolates was used to profile the expression of 84 genes identified to be involved in the identification, growth, and differentiation of mouse stem cells. There were no significant differences in expression of these mouse stem cell markers after SBR in the crypts of control mice (Fig. 2A). A similar result was observed when comparing sham and SBR in the p21waf1/cip1-null mice (Fig. 2B). Finally, there were no significant differences between control and p21waf1/cip1-null mice following SBR (Fig. 2C).
Figure 2.
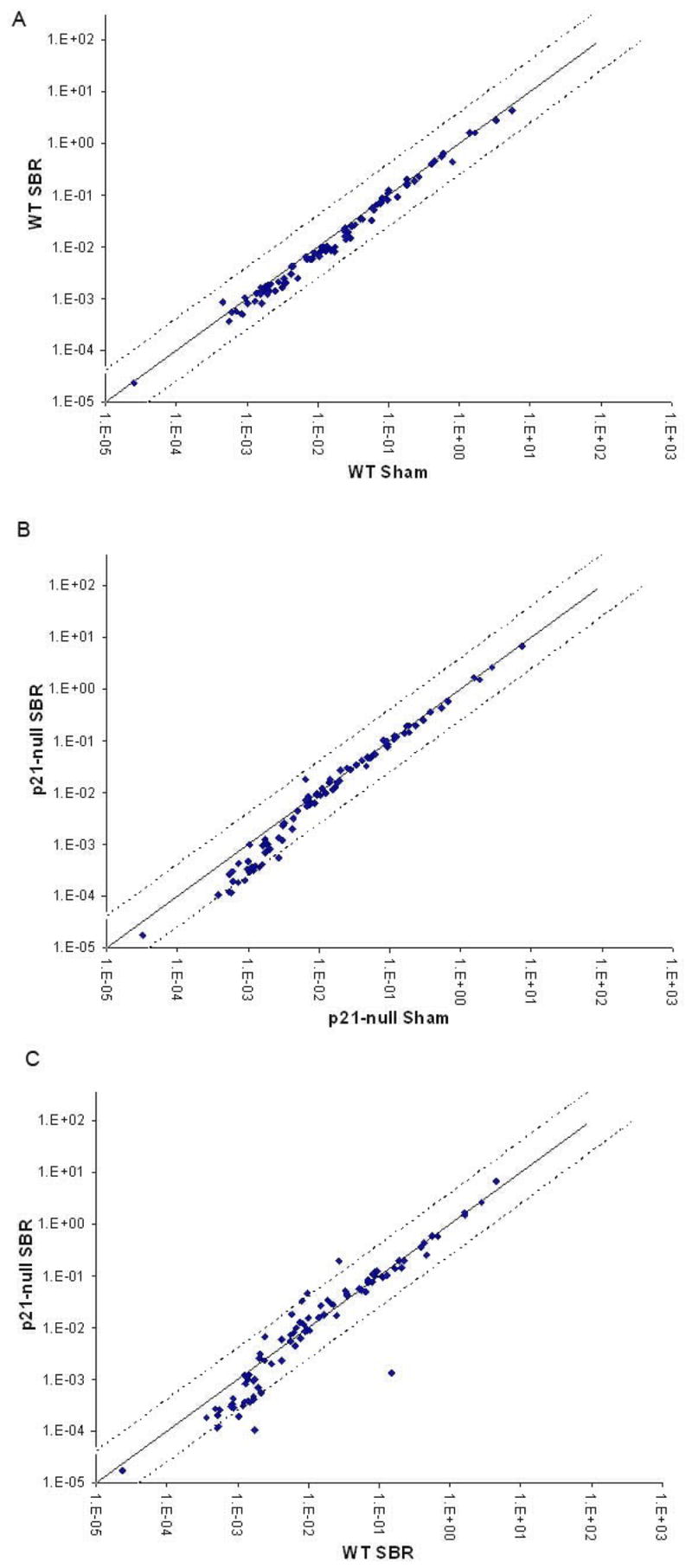
Differences in mRNA expression of mouse stem cell genes in (A) control (wild-type; WT; C57/Blk6) mice sham (transection and reanastomosis) vs. 50% proximal small bowel resection (SBR), (B) p21-null mice sham vs. SBR, and (C) WT SBR vs. p21-null SBR. Black dotted lines represent 4 fold differences.
Ileal protein expression
Western blotting of protein derived from intestinal crypt isolates from control and p21waf1/cip1-null mice were done to confirm absence of p21waf1/cip1 expression in the null mouse (not shown). Musashi-1, a putative marker of intestinal stem cells was also examined (Fig. 3). There were no differences in expression of Musashi-1 between control sham, control SBR, p21waf1/cip1-null sham, and p21waf1/cip1-null SBR mice.
Figure 3.
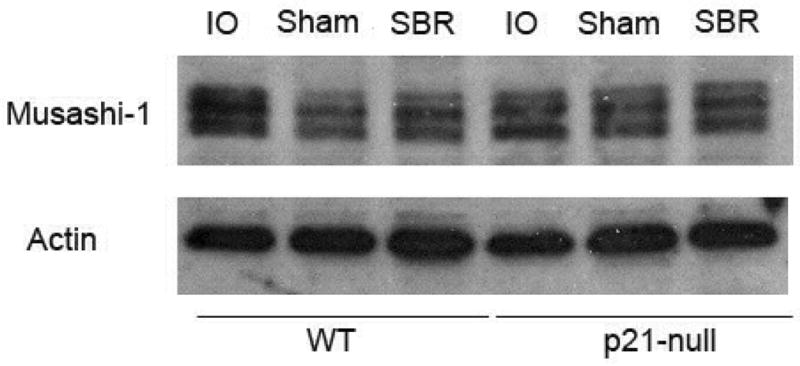
Western blot for the expression of Musashi-1 protein in the ileal crypts of unoperated (intraoperative bowel; IO), sham-operated (transection and reanastomosis), and 50% proximal small bowel resection (SBR) in control (C57/Blk6) and p21-null mice. Actin loading was used as the control.
Crypt base columnar cells and Lgr5
The average numbers of crypt base columnar cells per crypt were the same after sham and SBR in the control mice (Fig. 4A). The p21waf1/cip1-null mice also had similar averages of crypt base columnar cells per crypt when comparing sham and SBR (Fig. 4A). Lgr5 is a putative marker of intestinal stem cells and specifically for crypt base columnar cells 15. The expression of Lgr5 was then examined on RNA isolated from intestinal crypt enterocytes by real-time PCR. As shown in Fig. 4B expression of Lgr5 was not statistically significant after SBR in either control (P= 0.7), or p21waf1/cip1-null mice (P= 0.5). While there was a trend of decreased Lgr5 expression in p21waf1/cip1-null mice overall when compared with control mice, this was not statistically significant (P=0.4).
Figure 4.
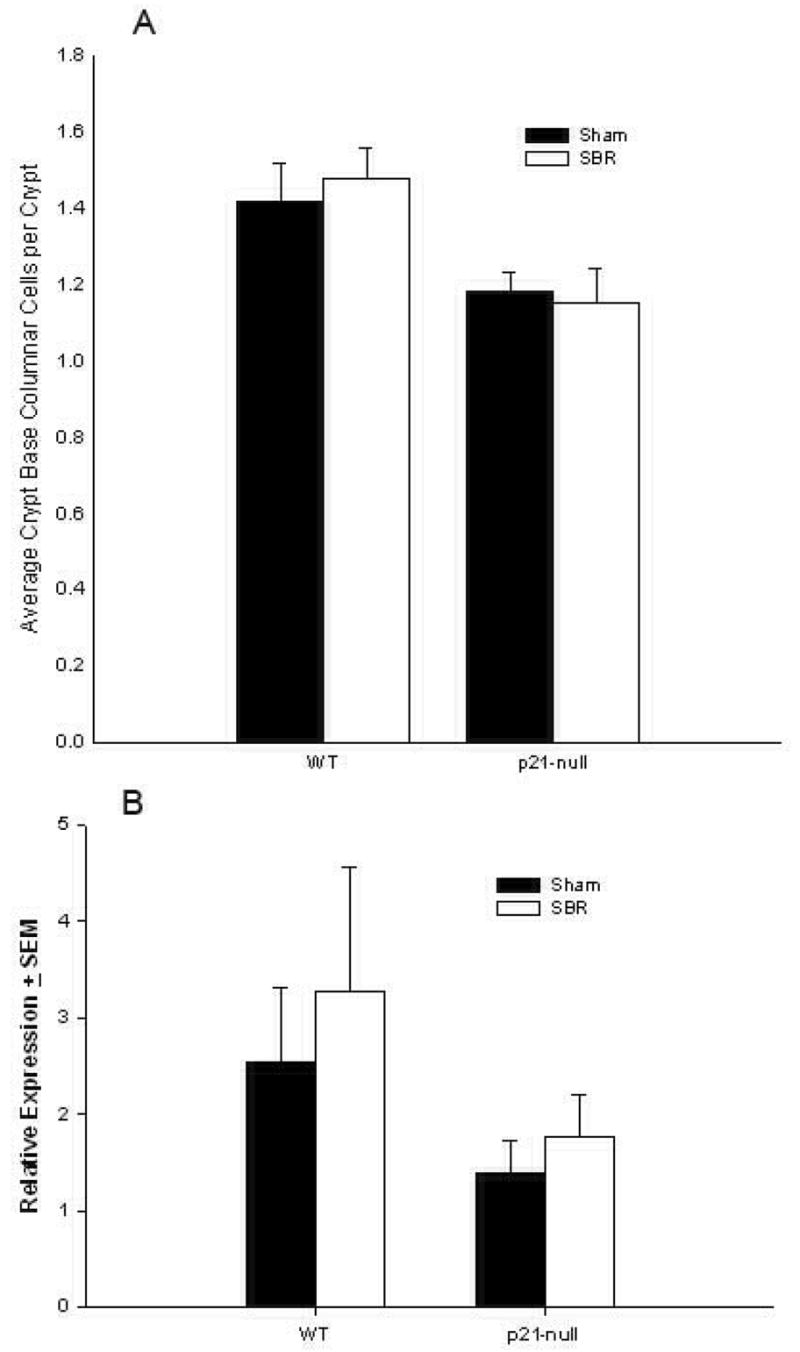
A) Crypt base columnar cell numbers per crypt of wild type control (C57/Blk6) and p21-null mice at 3 days following either sham operation (transection and reanastomosis) or 50% proximal small bowel resection (SBR). (B) Expression of Lgr5 mRNA in the ileal crypts of wild type and p21-null mice at 3 days following sham operation or SBR.
Discussion
In the present study, we sought to determine a mechanism for why p21waf1/cip1-null mice fail to demonstrate an intestinal adaptation response to massive SBR. We specifically tested the hypothesis that deficient expression of this protein would result in an attenuated population of stem cells within the intestinal crypt. We found no evidence that the intestinal stem cell pool was affected by either massive SBR or p21waf1/cip1 deficiency. These results suggest that expansion of stem cells does not occur after SBR and the lack of resection-induced adaptation in p21waf1/cip1-null mice is due to another mechanism.
Identification of specific stem cells within the intestinal crypt has been difficult, primarily due to the lack of specific, universally agreed-upon stem cell markers. Indeed, the stem cell genes that were screened in our array kit contained gene constructs associated with stem cells in other locations and tissues. As such, one limitation of this study is that we could have overlooked expression differences in intestine-specific stem cell genes that are either presently unknown, or not present on the array. This latter consideration is the rationale for performing a separate analysis of Musashi-1 and Lgr5. These are two markers that have been most directly associated with stem cells of the intestine. An additional possibility is to consider that we have overlooked alterations in genes that might have occurred at earlier or later time points. The 3rd postoperative day time point was chosen as we have already demonstrated measurable changes in proliferation by 3 days under normal conditions 12.
In addition to expression differences, stem cells have been classically considered to reside in roughly the fourth cell position from the crypt base 16. Another cell nested between Paneth cells in the crypt termed crypt-based columnar cells (CBC) has more recently been targeted as the crypt stem cell 17,18. By virtue of relative ease of identification on histologic sections, we were able to determine that there were no differences in either the CBC number or expression of the CBC-associated protein Lgr5 as a consequence of p21waf1/cip1 deficiency or SBR.
Our findings of lack of changes in stem cell populations (as determined by CBC numbers and expression of various stem cell markers) is at variance with the study by Dekaney, et al in which an expansion of the intestinal stem cell population was observed after ileocecal resection in mice 19. In that report, increased crypt fission and Musashi-1 immunohistochemistry staining was observed after 7 days. It is possible that changes in stem cell numbers and/or expression of various markers might have occurred in our model had we extended our observations to later postoperative time points. Another reason for the disparate findings between this study and ours is that they analyzed the jejunum following a distal ileal and cecal resection while we evaluated the ileum following a proximal jejunal resection. It is therefore possible that intestinal stem cell responses to SBR may be dependent upon the intestinal segment removed. Finally, the expression of Musashi-1 in their study was gauged by immunohistochemistry versus direct Western blotting in the present study. We would argue that the method of explicit enterocyte isolation, protein, quantification, and Western blotting would be more objective and accurate when compared with simple immunohistochemistry.
The involvement of p21waf1/cip1 on stem cells has been mostly studied in bone marrow-derived hematopoietic stem cells. Some investigators have concluded that p21waf1/cip1 is solely a cell cycle inhibitor 20, while others believe it is involved in proliferation 21,22, and yet others like ourselves have recognized p21waf1/cip1 to have a dual role in the cell cycle: necessary for proliferation at low levels, but inhibitory at high levels 23,24.
In neural stem cells, loss of p21waf1/cip1 leads to long term loss of stem cells, thought to be a consequence of hyper-proliferation and subsequent exhaustion of the stem cell population 25. Hematopoietic stem cells underwent normal proliferation in p21waf1/cip1 deficient mice under normal conditions, but when perturbed, their stem cell population was depleted resulting in death 26,27. This protein has also been found to be necessary for maintenance of umbilical cord blood stem cells 28. This group concluded that p21waf1/cip1 was necessary for the maintenance and self-renewal of the stem cell population and is the rationale for the experiments in this report.
Mantel’s group found p21waf1/cip1 to be necessary for proliferation of the hematopoietic stem cell population 29. They found absolute numbers of bone marrow progenitors to be significantly decreased in p21waf1/cip1 deficient mice. This same group also described an antiapoptotic role for p21waf1/cip1 in the hematopoietic progenitor cell pathway 30, further supporting the importance of p21waf1/cip1 in the kinetics of cell turnover.
A dual role for p21waf1/cip1 has been described by several groups. An inverse relationship was found between p21waf1/cip1 levels in megakaryocytes and the cycling state of the cells 31. p21waf1/cip1 was necessary for megakaryocyte differentiation, but arrested the cell cycle at high levels 32. More recently another role for p21waf1/cip1 was suggested in that p21waf1/cip1 can activate retinoblastoma protein (Rb) via dephosphorylation and yet inactivate Rb via degradation, suggesting it can regulate cell cycle progression and/or arrest 33. Work from our laboratory has revealed p21waf1/cip1 was required for stabilization of the Cyclin D/Cdk 4 complexes and therefore necessary for intestinal cell proliferation 34.
Our data would suggest p21waf1/cip1 deficiency does not decrease or deplete the intestinal stem cell population after massive small bowel resection. Additional mechanisms for the requirement of p21waf1/cip1 in the adaptation story must be explored. A more thorough understanding of p21waf1/cip1 role in intestinal proliferation and adaptation will undoubtedly contribute to optimization of future therapies for patients suffering from the short bowel syndrome.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 DK53234 (Dr. Warner), T32 CA009621 (Dr. Longshore) and P30DK52574 - Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.O’Brien DP, Nelson LA, Huang FS, et al. Intestinal adaptation: structure, function, and regulation. Semin Pediatr Surg. 2001;10:56–64. doi: 10.1053/spsu.2001.22383. [DOI] [PubMed] [Google Scholar]
- 2.Warner BW, Vanderhoof JA, Reyes JD. What’s new in the management of short gut syndrome in children. J Am Coll Surg. 2000;190:725–736. doi: 10.1016/s1072-7515(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 3.Loran MR, Althausen TL. Cellular proliferation of intestinal epithelia in the rat two months after partial resection of the ileum. J Biophys Biochem Cytol. 1960;7:667–672. doi: 10.1083/jcb.7.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 5.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stehr W, Mercer TI, Bernal NP, et al. Opposing roles for p21(waf1/cip1) and p27(kip1) in enterocyte differentiation, proliferation, and migration. Surgery. 2005;138:187–194. doi: 10.1016/j.surg.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Stern LE, Falcone RA, Jr, Kemp CJ, et al. p21 (WAF1/CIP1) is required for the mitogenic response to intestinal resection. J Surg Res. 2000;90:45–50. doi: 10.1006/jsre.2000.5834. [DOI] [PubMed] [Google Scholar]
- 8.Mantel C, Luo Z, Canfield J, et al. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 9.Dekaney CM, Fong JJ, Rigby RJ, et al. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1013–G1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 10.Kayahara T, Sawada M, Takaishi S, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 11.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 12.Helmrath MA, VanderKolk WE, Can G, et al. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 13.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I Columnar cell. Am J Anat. 1974;141:461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 14.Stehr W, Mercer TI, Bernal NP, et al. Opposing roles for p21(waf1/cip1) and p27(kip1) in enterocyte differentiation, proliferation, and migration. Surgery. 2005;138:187–194. doi: 10.1016/j.surg.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 16.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 17.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 18.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 19.Dekaney CM, Fong JJ, Rigby RJ, et al. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1013–G1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kippin TE, Martens DJ, van der KD. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantel C, Luo Z, Canfield J, et al. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 22.Fukuda S, Mantel CR, Pelus LM. Survivin regulates hematopoietic progenitor cell proliferation through p21WAF1/Cip1-dependent and -independent pathways. Blood. 2004;103:120–127. doi: 10.1182/blood-2003-05-1756. [DOI] [PubMed] [Google Scholar]
- 23.Cheng M, Olivier P, Diehl JA, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper JW, Adami GR, Wei N, et al. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 25.Kippin TE, Martens DJ, van der KD. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 27.Van OR, Kamminga LM, Ausema A, et al. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- 28.Kadereit S, Deeds LS, Haynesworth SE, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20:573–582. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 29.Mantel C, Luo Z, Canfield J, et al. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 30.Fukuda S, Mantel CR, Pelus LM. Survivin regulates hematopoietic progenitor cell proliferation through p21WAF1/Cip1-dependent and -independent pathways. Blood. 2004;103:120–127. doi: 10.1182/blood-2003-05-1756. [DOI] [PubMed] [Google Scholar]
- 31.Baccini V, Roy L, Vitrat N, et al. Role of p21(Cip1/Waf1) in cell-cycle exit of endomitotic megakaryocytes. Blood. 2001;98:3274–3282. doi: 10.1182/blood.v98.12.3274. [DOI] [PubMed] [Google Scholar]
- 32.Baccini V, Roy L, Vitrat N, et al. Role of p21(Cip1/Waf1) in cell-cycle exit of endomitotic megakaryocytes. Blood. 2001;98:3274–3282. doi: 10.1182/blood.v98.12.3274. [DOI] [PubMed] [Google Scholar]
- 33.Broude EV, Swift ME, Vivo C, et al. p21(Waf1/Cip1/Sdi1) mediates retinoblastoma protein degradation. Oncogene. 2007 doi: 10.1038/sj.onc.1210516. [DOI] [PubMed] [Google Scholar]
- 34.Sheng G, Bernabe KQ, Guo J, et al. Epidermal growth factor receptor-mediated proliferation of enterocytes requires p21waf1/cip1 expression. Gastroenterology. 2006;131:153–164. doi: 10.1053/j.gastro.2006.05.007. [DOI] [PubMed] [Google Scholar]