Abstract
An explanation of the principles and mechanisms involved in peaceful co-existence between animals and the huge, diverse, and ever-changing microbiota that resides on their mucosal surfaces represents a challenging puzzle that is fundamental in everyday survival. In addition to mechanical barriers and a variety of innate defense factors, mucosal immunoglobulins (Igs) provide protection by two complementary mechanisms: specific antibody activity and innate, Ig glycan-mediated binding, both of which serve to contain the mucosal microbiota in its physiological niche. Thus, the interaction of bacterial ligands with IgA glycans constitutes a discrete mechanism that is independent of antibody specificity and operates primarily in the intestinal tract. This mucosal site is by far the most heavily colonized with an enormously diverse bacterial population, as well as the most abundant production site for antibodies, predominantly of the IgA isotype, in the entire immune system. In embodying both adaptive and innate immune mechanisms within a single molecule, S-IgA maintains comprehensive protection of mucosal surfaces with economy of structure and function.
Keywords: Secretory IgA, Mucosal immunity, Glycans, Bacterial adherence
1. Role of secretory IgA (S-IgA) in mucosal immunity
Large surface areas of mucosal membranes (∼200–400 m2) are in constant contact with a highly diverse microbiota [1], [2], [3], [4], [5], [6] estimated to comprise ∼15,000–36,000 species and 1800 genera [7], [8] and exceeding the total number of nucleated cells by an order of magnitude [1], [2], [5], [9] (1013 nucleated cells vs. ∼1014 bacterial cells). More than 99.9% of all commensal bacteria are found in the gastrointestinal tract, particularly in the large intestine [5], [10]. Through evolution, the selective pressure arising from environmental antigens of microbial and food origin has resulted in a strategic, functionally advantageous distribution of cells involved in antigen uptake and processing, and the initiation of immune responses in mucosal tissues [9], [11], [12], [13]. The mucosal immune system contains this antigenic onslaught without compromising the integrity of the mucosal barrier [11] or exhausting the immune system, in part through the induction of mucosal (oral) tolerance [14], [15]. In addition to mechanical barriers and humoral effectors of innate immunity [6], [11], [16], mucosal antibodies and mucosal T cells provide antigen-specific protection [12], [17].
The characteristic distribution of antibodies in blood and external secretions, including the intestinal fluid, reflects the functional adaptation of various Ig isotypes to different immune compartments. Given that mucosal membranes are the most important site of antigen encounter, it should not be surprising that most antibody production takes place in mucosal tissues, particularly the intestine, rather than in the bone marrow, spleen, and lymph nodes [12], [18], [19], [20], [21], and that the daily synthesis of IgA far exceeds that of IgG, IgM, IgD and IgE combined [19], [20], [21], [22]. Importantly for mucosal protection, more than two-thirds of total IgA production ends up in the external secretions [19], [21]. Quantitative studies of the origin of mucosal antibodies, particularly in the intestinal tract, demonstrate that >95% is of local origin and only trace amounts are derived from the circulation [19], [22], [23].
The mucosal microbiota, epithelial cells, and the mucosal immune system constitute a stable and interdependent “tripod” that maintains mucosal homeostasis by complex mechanisms [3], [4], [6], [24], [25], [26], [27], [28]. For example, epithelial cells display surface receptors that are selectively exploited by bacteria adhering to their apical surfaces [1], [2], [28], [29], [30], and express the basolateral membrane receptor (polymeric Ig receptor; pIgR) that transports locally produced polymeric (p) IgA into the external secretions [23]. Bacteria endogenous to the intestinal tract, oral cavity, and probably also the respiratory and genital tracts, are coated in vivo with S-IgA [9], [13], [17], [31], [32], [33], [34], [35], [36], [37], [38], [39] that limits their epithelial adherence and penetration, thereby confining them to the mucosal surfaces. Numerous models have demonstrated the role of antibodies, especially S-IgA, in protecting the intestinal and other mucosal tracts. This has most convincingly been demonstrated in vivo in germ-free, colostrum-deprived newborn piglets [40], [41], [42], which, unlike humans, mice, rats, or rabbits, are born without transplacentally acquired Ig. In the absence of maternal as well as endogenous antibodies, milk-deprived piglets die of septicemia (usually E. coli) within 1–2 days after birth, whereas milk-fed animals survive [40]. Furthermore, piglets fed milk or serum, survive oral challenge with E. coli, whereas control animals deprived of Ig, irrespective of its source, succumb to the infection. In mice in which pIgR is copiously expressed on hepatocytes (not the case in humans, pigs, or dogs) and pIgA from the circulation is selectively transported into the bile and thence into the gut lumen [23], [43], pathogen-specific pIgA hybridoma antibodies derived from “backpack tumors” [44], [45], [46], [47] protect mice against oral challenge with Salmonella enterica serovar Typhimurium, Vibrio cholerae, or rotavirus [44], [45], [47], [48], [49]. In contrast IgG hybridoma antibodies of the same specificity are not protective, due to the lack of receptor-mediated transport of IgG into the intestine.
1.1. Mechanisms of S-IgA-mediated protection
Numerous such experiments clearly demonstrate protection in vivo dependent on the presence of antigen-specific IgA antibodies that interfere with pathogen adherence to or penetration through the mucosal barrier, or neutralize biologically active antigens such as viruses or toxins [41], [47], [48], [50], [51], [52], [53], [54]. Likewise many in vitro studies of specific antibody-mediated inhibition of bacterial adherence to epithelial cells corroborate these findings [30], [55], [56], [57]. However, agglutination and inhibition of the adherence of E. coli with Type 1 fimbriae to colonic epithelial cells that express a corresponding receptor can be mediated by IgA independently of specific antibody [30], [58], [59]. S-IgA and IgA myeloma proteins of both subclasses agglutinate E. coli, and mannose (Man) inhibits this agglutination. Furthermore, adherence of E. coli to human epithelial colonic cells can be inhibited by S-IgA as well as by IgA2 myeloma proteins. Analysis of the carbohydrate composition and complete primary structure of the oligosaccharide side-chains reveal that the most active pIgA2 myeloma protein contain several Man-rich N-linked glycan chains [30]. Thus, Man-dependent adherence of E. coli to epithelial cell receptors mediated by Type 1 fimbriae is competitively inhibited by similar glycans on S-IgA and IgA2 myeloma proteins acting as decoy receptors. Consequently, we have proposed that IgA proteins exhibit protective functions through antibody-dependent specific immunity as well as glycan-dependent innate immunity [30]. This concept was confirmed in vitro for other microbial ligand-glycan receptors [1], [26], [29], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78]. In addition to E. coli, many other bacteria such as Helicobacter pylori, Streptococcus pneumoniae, Clostridium difficile, Shigella flexneri, Pseudomonas aeruginosa and Neisseria gonorrhoeae, and some viruses (Table 1 ) interact with epithelial receptors via their glycan moiety.
Table 1.
Examples of glycans as adhesion sites and receptors for selected bacteria and viruses that colonize, or infect, mucosal surfaces (adapted from [1], [26], [29], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [132]).
| Epithelial cell |
|||
|---|---|---|---|
| Target tissue | Glycan structure | Form | |
| Bacterium | |||
| Escherichiae with Type 1 fimbriae | Intestine | Man5GlcNAcGlcNAc | Glycoprotein |
| Urinary tract | |||
| P | Intestine | Gal(α1,4)Gal | Glycoprotein |
| S | Intestine | NeuAc(α2,3), Gal(β1,3), GalNAc-O-linked | Glycoprotein |
| Helicobacter pylori | Stomach | NeuAc(α2-3)Gal | Glycolipid |
| Pseudomonas aeruginosa | Intestine | Galβ3GlcAc | Glycoprotein |
| Fuc | Glycoprotein | ||
| Man | Glycoprotein | ||
| Respiratory tract | GalNAcβ1-4Gal | Glycoprotein | |
| Shigella dysenteriae | Intestine | AsialoGM1 ganglioside | Sialoconjugate |
| Neisseria gonorrhoeae | Genital | Gal(β1,4) GalNAc | Glycoprotein |
| Bordetella pertussis | Respiratory | Gal(β1,4)Glc ceramide | Ceramide |
| Haemophilus influenzae | Respiratory tract | GlcNAcβ3Gal | Glycoprotein |
| Streptococcus pneumoniae | Respiratory tract | NeuAc(α2-3)-GalβGlcNAc | Glycoprotein |
| Virus | |||
| Influenza A, B, C | Mucosal tissues | Neu5Ac, Neu5,9Ac2 | Sialoconjugates |
| Paramyxoviruses | Mucosal tissues | Neu5Ac | Sialoconjugates |
| Coronaviruses | Mucosal tissues | Neu5, 9Ac2 | Sialoconjugates |
| Reo- and rota-viruses | Intestinal tract | Sialic acid | Sialoconjugates |
| Respiratory syncytial virus | Respiratory | Glycosamine glycans | Glycoproteins |
| Mucosal tissues | |||
| HIV | Epithelial cells | Galactosylceramide | |
Man: mannose, Fuc: fucose, Gal: galactose, GlcNAc: N-acetyl glucosamine, GalNAc: N-acetyl galactosamine, NeuAc: sialic acid.
Thus, it has become obvious that the N- and O-glycans of S-IgA provide a link between innate glycan-mediated and adaptive specific antibody-dependent protection (Fig. 1 ). This concept, of paramount importance in IgA-mediated mucosal defense, prompts additional considerations. First, it has been shown that bacteria indigenous to the oral cavity and intestinal tract are coated in vivo with IgA [9], [17], [31], [32], [33], [34], [35], [36], [37], [38], [39], [79], [80], [81]. However, it is not known whether this coating depends on specific antibody-antigen or glycan-mediated interactions. Considering the enormous numbers of bacteria (∼1012/g of intestinal content) [10], their diversity (∼15,000–36,000 species of 1800 genera) [7], and the large number of potential antigenic determinants on many bacterial structures, it is unlikely that such coating is based exclusively on specific recognition by S-IgA antibodies. Secondly, in the large intestine IgA2-producing cells are dominant in contrast to other mucosal tissues [82], [83], and antibodies to antigens (e.g., endotoxin) of Gram-negative bacteria are associated predominantly with the S-IgA2 subclass [84], [85], [86]. Thirdly, in addition to glycans on the H chain of IgA [87], [88], [89], [90], [91], secretory component (SC), the extracellular segment of pIgR, is extremely rich in glycans comprising 7 N-linked chains [88], [92], [93], [94] that also act as highly effective inhibitors of adherence for some bacterial species (e.g., Shigellae, S. pneumoniae [64], [65], [67], [68], [69], [70]). Finally, prevention of the adherence of enormously diverse and variable mucosal microbiota is likely to be at least partially independent of specific antibody activity, reflecting the immediate need for protection against a broad spectrum of daily encountered microorganisms. Thus, in concert with the postulated Fab-mediated “polyreactivity” of S-IgA antibodies [95], [96], [97], [98], [99], [100], glycan-mediated interactions are likely to further enforce protective functions of S-IgA.
Fig. 1.
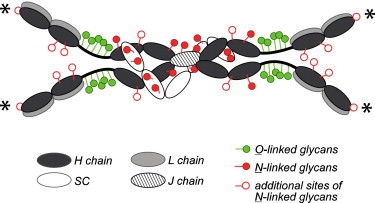
Model of a human dimeric S-IgA molecule with assigned adaptive (specific-antibody) activity and innate (glycan-dependent) activity [88]. Asterisk—possible N-glycosylation sites within the CDR3 segment of the VH region of α chains [97], [98], [128].
Skeptics of these concepts may argue that mucosal defenses in IgA-deficient individuals should be significantly compromised. Indeed, the majority of such patients display a higher incidence of respiratory and intestinal infections [101], [102], [103]. Currently, IgA deficiency is defined as <50 mg IgA/100 ml of serum, regardless of S-IgA which is not taken into account although it is usually also diminished. Because complete absence of IgA in sera or secretions of IgA-deficient patients is extremely rare, it is possible that even low levels of S-IgA may provide some level of protection. Furthermore, in most IgA-deficient individuals S-IgA is replaced by S-IgM [102], [103], [104], [105], [106], [107], [108], [109], [110]. Thus, in IgA-deficient individuals, 65–75% of total Ig-containing cells in the intestines produce IgM, in sharp contrast to normal individuals (in the large intestine, ∼90% of cells produce IgA, ∼6% IgM, and ∼4% IgG) [18], [109]. Most importantly in this context, IgM and IgA display many common structural features, including: ability to form polymers; presence of J chain [111], [112]; ability to bind pIgR (thereby forming SC-containing secretory IgM) [23], [65], [106], [113]; homologies between primary structures of Cμ3, Cμ4 and Cα2, Cα3 domains and the C-terminal “tail-piece” [111], [114], [115]; and VH gene segment representation [116], [117], indicating their close evolutionary and functional similarity [114]. This structural homology also extends to the glycan moieties: IgM and IgA molecules display a similar number and domain location of N-linked glycan side-chains, and both contain Man-rich chains [114], [118], [119], [120], [121], [122]. Therefore, despite differences in the number of Ag-binding sites (up to 10 for IgM, and 4–8 for IgA dimers and tetramers), and ability to activate complement, it is clear that IgA and IgM are structurally, functionally, and evolutionarily closely related isotypes.
2. IgA-associated glycans display remarkable heterogeneity
Structural studies of human polyclonal S-IgA and monoclonal (myeloma) IgA1 and IgA2 proteins reveal considerable heterogeneity with respect to number, sites of attachment, composition, and primary structure of their glycan side-chains [30], [71], [72], [73], [74], [75], [76], [77], [87], [88], [89], [90], [91], [92], [93], [94], [115], [122], [123], [124], [125], [126], [127], which is likely to be of enormous biological importance. Because different microorganisms interact with epithelial cells through diverse glycan receptors, heterogeneity of IgA-associated glycans affords a variety of structures that can effectively inhibit these interactions.
Glycan moieties in S-IgA molecules are associated with H chains, J chain, and SC [88], [90], [91], [92], [93], [94], [124], but Man-rich N-linked glycans that inhibit the binding of Type 1 fimbriae to epithelial receptors occur only on the H chains [30], [122]. However, other bacteria may interact with N- or O-linked glycans on H chains or SC (Table 1, section B). Although the majority of N-linked glycans are found in the Fc region of the α chains [88], [89], [114], [115], [124], there is great heterogeneity in the number and composition of individual glycan chains [30], [122] and additional N-linked glycan chains may also be present in the Fd fragment (N-terminal half of the α chain comprising VH and CH1 domains), within the third complementarity-determining region (CDR3) [97], [98], [128]. The authors of these novel and functionally important studies propose that a high rate of somatic mutation in the CDR3 taking place within intestinal IgA-producing cells [97], [98], [116], [117], [128], [129] generates a glycosylation-signaling sequence that alters the specificity of intestinal IgA antibodies. Thus, antigen-binding by Fab segments of S-IgA is determined by both specific antibody activity and glycan-dependent interactions.
The heterogeneity of N- and O-linked side-chains, with respect to their number, composition, and types of glycosidic bonds is further extended because many of them are incomplete, truncated forms [30], [78], [88], [122]. Most importantly, and in sharp contrast to the combinatorial possibilities of amino acids, glycans can generate a remarkably higher number of structures, due to the variety of glycosidic bonds. Thus, a sequence of 6 (out of 20) amino acids can theoretically generate 6.4 × 107 distinct hexapeptides, while there are potentially 1.44 × 1015 different hexasaccharides [130].
Specific antibody diversity is generated in an antigen-independent fashion during the differentiation of B lymphocytes by a number of mechanisms including recombination of multiple VJ (for L chains) and VDJ (for H chains) gene segments, combinatorial diversity of L and H chains, somatic hypermutation, gene conversion, and others [131]. The result of these genetic events is the generation of B lymphocytes with surface membrane Ig molecules that accommodate an enormous number of potential antigens, leading, after antigen-specific recognition, to B cell proliferation, differentiation, and the ultimate secretion of large amounts of antigen-specific antibodies. It is conceivable that analogous mechanisms operate in the generation of innate, glycan-mediated mechanisms of protection. Through random generation of enormously diverse glycan structures on mucosal glycoproteins, including S-IgA, S-IgM, SC, mucin, and lactoferrin, glycan configurations are generated that complement the equal heterogeneity of microbial adhesins. The protective effectiveness of these mechanisms may be further enhanced by subsequent somatic mutations within V regions of H and L chains, including the generation of glycosylation signals that lead to alterations of antibody specificities.
Parallel structural and functional exploration of the principles of adaptive (specific antibody) and innate (glycan) S-IgA-mediated immunity is likely to generate novel approaches to the design of broadly protective compounds that work by selectively interfering with the adherence and penetration of pathogens, or that contain the commensal microbiota residing at mucosal surfaces.
Acknowledgements
This work was supported in part by grants, 5 U19AI028147, the Czech Republic (VZMSM0021620812), DE06746, AI074791, and the John R. Oishei Foundation.
References
- 1.Abraham S.N., Bishop B.L., Sharon N., Ofek I. Adhesion of bacteria to mucosal surfaces. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 35–48. [Google Scholar]
- 2.Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Cash H.L., Hooper L.V. Commensal bacteria shape intestinal immune system development. ASM News. 2005;71:77–83. [Google Scholar]
- 4.Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 5.Savage D.G. Mucosal microbiota. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 19–33. [Google Scholar]
- 6.Tlaskalova-Hogenova H., Tuckova L., Mestecky J., Kolinska J., Rossmann P., Stepankova R. Interaction of mucosal microbiota with the innate immune system. Scand J Immunol. 2005;62(Suppl 1):106–113. doi: 10.1111/j.1365-3083.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- 7.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S.R., Pop M., Deboy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson A.J., Geuking M.B., McCoy K.D. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–162. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitman W.B., Coleman D.C., Wiebe W.J. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer L., Walker W.A. Development and physiology of mucosal defense: an introduction. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 5–18. [Google Scholar]
- 12.Mestecky J., Blumberg R.S., Kiyono H., McGhee J.R. The mucosal immune system. In: Paul W.E., editor. Fundamental Immunology. 5th ed. Lippincott-Raven; Philadelphia: 2003. pp. 965–1020. [Google Scholar]
- 13.Macpherson A.J., Gatto D., Sainsbury E., Harriman G.R., Hengartner H., Zinkernagel R.M. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 14.Mestecky J., Russell M.W., Elson C.O. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- 15.Mowat A.M., Faria A.M.C., Weiner H.L. Oral tolerance: physiologic basis and clinical applications. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 487–537. [Google Scholar]
- 16.Russell M.W., Bobek L.A., Brock J.H., Hajishengallis G., Tenovuo J. Innate humoral defense factors. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 73–93. [Google Scholar]
- 17.Bos N.A., Jiang H.Q., Cebra J.J. T cell control of the gut IgA response against commensal bacteria. Gut. 2001;48:762–764. doi: 10.1136/gut.48.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pabst R., Russell M.W., Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut (letter) Trends Immunol. 2008;29:206–208. doi: 10.1016/j.it.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Conley M.E., Delacroix D.L. Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense? Ann Int Med. 1987;106:892–899. doi: 10.7326/0003-4819-106-6-892. [DOI] [PubMed] [Google Scholar]
- 20.Jonard P.P., Rambaud J.C., Dive C., Vaerman J.P., Galian A., Delacroix D.L. Secretion of immunoglobulins and plasma proteins from the jejunal mucosa. Transport rate and origin of polymeric immunoglobulin A. J Clin Invest. 1984;74:525–535. doi: 10.1172/JCI111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mestecky J., Russell M.W., Jackson S., Brown T.A. The human IgA system: a reassessment. Clin Immunol Immunopathol. 1986;40:105–114. doi: 10.1016/0090-1229(86)90073-5. [DOI] [PubMed] [Google Scholar]
- 22.Woof J.M., Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaetzel C.S., Mostov K. Immunoglobulin transport and the polymeric immunoglobulin receptor. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 211–250. [Google Scholar]
- 24.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Hooper L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Hooper L.V., Gordon J.I. Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology. 2001;11:1R–10R. doi: 10.1093/glycob/11.2.1r. [DOI] [PubMed] [Google Scholar]
- 27.Ismail A.S., Hooper L.V. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;289:G779–784. doi: 10.1152/ajpgi.00203.2005. [DOI] [PubMed] [Google Scholar]
- 28.Orihuela C.J., Fogg G., DiRita V.J., Tuomanen E. Bacterial interactions with mucosal epithelial cells. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 753–767. [Google Scholar]
- 29.Adlerberth I., Ahrne S., Johansson M.L., Molin G., Hanson L.A., Wold A.E. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol. 1996;62:2244–2251. doi: 10.1128/aem.62.7.2244-2251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wold A.E., Mestecky J., Tomana M., Kobata A., Ohbayashi H., Endo T. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos N.A., Bun J.C., Popma S.H., Cebra E.R., Deenen G.J., van der Cammen M.J. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996;64:616–623. doi: 10.1128/iai.64.2.616-623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bos N.A., Cebra J.J., Kroese F.G. B-1 cells and the intestinal microflora. Curr Top Microbiol Immunol. 2000;252:211–220. doi: 10.1007/978-3-642-57284-5_22. [DOI] [PubMed] [Google Scholar]
- 33.Jansen G.J., Wilkinson M.H., Deddens B., van der Waaij D. Characterization of human faecal flora by means of an improved fluoro-morphometrical method. Epidemiol Infect. 1993;111:265–272. doi: 10.1017/s0950268800056971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroese F.G., de Waard R., Bos N.A. B-1 cells and their reactivity with the murine intestinal microflora. Semin Immunol. 1996;8:11–18. doi: 10.1006/smim.1996.0003. [DOI] [PubMed] [Google Scholar]
- 35.Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 36.Shroff K.E., Meslin K., Cebra J.J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Waaij L.A., Kroese F.G., Visser A., Nelis G.F., Westerveld B.D., Jansen P.L. Immunoglobulin coating of faecal bacteria in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:669–674. doi: 10.1097/01.meg.0000108346.41221.19. [DOI] [PubMed] [Google Scholar]
- 38.van der Waaij L.A., Limburg P.C., Mesander G., van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandtzaeg P., Fjellanger I., Gjeruldsen S.T. Adsorption of immunolgobulin A onto oral bacteria in vivo. J Bacteriol. 1968;96:242–249. doi: 10.1128/jb.96.1.242-249.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rejnek J., Trávnícek J., Kostka J., Sterzl J., Lanc A. Study of the effect of antibodies in the intestinal tract of germ-free baby pigs. Folia Microbiol. 1968;13:36–42. [Google Scholar]
- 41.Miller I., Cerna J., Travnicek J., Rejnek J., Kruml J. The role of immune pig colostrum, serum and immunoglobulins IgG, IgM, and IgA, in local intestinal immunity against enterotoxic strain in Escherichia coli O55 in germfree piglets. Folia Microbiol. 1975;20:433–438. doi: 10.1007/BF02877048. [DOI] [PubMed] [Google Scholar]
- 42.Tlaskalova H., Rejnek J., Travnicek J., Lanc A. The effect of antibodies present in the intestinal tract of germfree piglets on the infection caused by the intravenous administration of the pathogenic strain Escherichia coli 055. Folia Microbiol. 1970;15:372–376. doi: 10.1007/BF02880107. [DOI] [PubMed] [Google Scholar]
- 43.Peppard J.V., Kaetzel C.S., Russell M.W. Phylogeny and comparative physiology of IgA. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 195–208. [Google Scholar]
- 44.Kraehenbuhl J.P., Neutra M.R. Monoclonal secretory IgA for protection of the intestinal mucosa against viral and bacterial pathogens. In: Ogra P.L., Mestecky J., Lamm M.E., Strober W., McGhee J.R., Bienenstock J., editors. Handbook of Mucosal Immunology. Academic Press; San Diego: 1994. pp. 403–410. [Google Scholar]
- 45.Michetti P., Mahan M.J., Slauch J.M., Mekalanos J.J., Neutra M.R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renegar K.B. Passive immunization: systemic and mucosal. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 841–851. [Google Scholar]
- 47.Russell M.W., Kilian M. Biological activities of IgA. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 267–289. [Google Scholar]
- 48.Wijburg O.L., Uren T.K., Simpfendorfer K., Johansen F.E., Brandtzaeg P., Strugnell R.A. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell M.W. Biological functions of IgA. In: Kaetzel C.S., editor. Mucosal Immune Defense: Immunoglobulin A. Springer; New York: 2007. pp. 144–172. [Google Scholar]
- 50.Kilian M., Mestecky J., Russell M.W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mestecky J., Russell M.W. Intestinal immunoglobulin A: role in host defense. In: Hecht G., editor. Microbial Pathogens and the Intestinal Epithelial Cell. ASM Press; Washington, DC: 2003. pp. 95–112. [Google Scholar]
- 52.Mestecky J., Russell M.W., Elson C.O. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson D.A., McNulty N.P., Guruge J.L., Gordon J.I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Zeitlin L., Cone R.A., Whaley K.J. Using monoclonal antibodies to prevent mucosal transmission of epidemic infectious diseases. Emerg Infect Dis. 1999;5:54–64. doi: 10.3201/eid0501.990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leffler H., Svanborg Eden C. Chemical identification of a glycosphingolipid receptor for Escherichia coli attaching to human urinary tract epithelial cells and agglutinating human erythrocytes. FEMS Microbiol Lett. 1980;24:127. [Google Scholar]
- 56.Svanborg-Eden C., Carlsson B., Hanson L.A., Jann B., Jann K., Korhonen T. Anti-pili antibodies in breast milk. Lancet. 1979;2:1235. doi: 10.1016/s0140-6736(79)92348-1. [DOI] [PubMed] [Google Scholar]
- 57.Svanborg-Eden C., Svennerholm A.M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wold A., Mestecky J., Tomana M., Svanborg Eden C. Secretory IgA oligosaccharide chains as receptors for bacterial lectins. In: MacDonald T.T., Challacombe S.J., Bland P.W., Stokes C.R., Heatley R.V., Mowat M.A., editors. Advances in Mucosal Immunology. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1990. pp. 851–852. [Google Scholar]
- 59.Wold A.E., Mestecky J., Svanborg Eden C. Agglutination of E. coli by secretory IgA—a result of interaction between bacterial mannose-specific adhesins and immunoglobulin carbohydrate? Monogr Allergy. 1988;24:307–309. [PubMed] [Google Scholar]
- 60.Anthony B.F., Concepcion N.F., Puentes S.M., Payne N.R. Nonimmune binding of human immunoglobulin A to type II group B streptococci. Infect Immun. 1990;58:1789–1795. doi: 10.1128/iai.58.6.1789-1795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davin J.C., Senterre J., Mahieu P.R. The high lectin-binding capacity of human secretory IgA protects nonspecifically mucosae against environmental antigens. Biol Neonate. 1991;59:121–125. doi: 10.1159/000243333. [DOI] [PubMed] [Google Scholar]
- 62.Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- 63.Giugliano L.G., Ribeiro S.T., Vainstein M.H., Ulhoa C.J. Free secretory component and lactoferrin of human milk inhibit the adhesion of enterotoxigenic Escherichia coli. J Med Microbiol. 1995;42:3–9. doi: 10.1099/00222615-42-1-3. [DOI] [PubMed] [Google Scholar]
- 64.Hanisch F.G., Hacker J., Schroten H. Specificity of S fimbriae on recombinant Escherichia coli: preferential binding to gangliosides expressing NeuAc α (2-3)Gal and NeuAc α (2-8)NeuAc. Infect Immun. 1993;61:2108–2115. doi: 10.1128/iai.61.5.2108-2115.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaetzel C.S. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 66.Korhonen T.K., Vaisanen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984;159:762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu L., Lamm M.E., Li H., Corthesy B., Zhang J.R. The human polymeric immunoglobulin receptor binds to Streptococcus pneumoniae via domains 3 and 4. J Biol Chem. 2003;278:48178–48187. doi: 10.1074/jbc.M306906200. [DOI] [PubMed] [Google Scholar]
- 68.Perrier C., Sprenger N., Corthesy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem. 2006;281:14280–14287. doi: 10.1074/jbc.M512958200. [DOI] [PubMed] [Google Scholar]
- 69.Phalipon A., Cardona A., Kraehenbuhl J.P., Edelman L., Sansonetti P.J., Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 70.Phalipon A., Corthesy B. Novel functions for mucosal SIgA. In: Kaetzel C.S., editor. Mucosal Immune Defense: Immunoglobulin A. Springer; New York: 2007. pp. 183–202. [Google Scholar]
- 71.Schroten H., Stapper C., Plogmann R., Kohler H., Hacker J., Hanisch F.G. Fab-independent antiadhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infect Immun. 1998;66:3971–3973. doi: 10.1128/iai.66.8.3971-3973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walz A., Odenbreit S., Mahdavi J., Boren T., Ruhl S. Identification and characterization of binding properties of Helicobacter pylori by glycoconjugate arrays. Glycobiology. 2005;15:700–708. doi: 10.1093/glycob/cwi049. [DOI] [PubMed] [Google Scholar]
- 73.Ruhl S., Sandberg A.L., Cisar J.O. Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J Dent Res. 2004;83:505–510. doi: 10.1177/154405910408300614. [DOI] [PubMed] [Google Scholar]
- 74.Palmer R.J., Jr., Gordon S.M., Cisar J.O., Kolenbrander P.E. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J Bacteriol. 2003;185:3400–3409. doi: 10.1128/JB.185.11.3400-3409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi Y., Ruhl S., Yoon J.W., Sandberg A.L., Cisar J.O. Adhesion of viridans group streptococci to sialic acid-, galactose- and N-acetylgalactosamine-containing receptors. Oral Microbiol Immunol. 2002;17:257–262. doi: 10.1034/j.1399-302x.2002.170409.x. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi Y., Sandberg A.L., Ruhl S., Muller J., Cisar J.O. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to alpha2-3-linked sialic acid-containing receptors. Infect Immun. 1997;65:5042–5051. doi: 10.1128/iai.65.12.5042-5051.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cisar J.O., Takahashi Y., Ruhl S., Donkersloot J.A., Sandberg A.L. Specific inhibitors of bacterial adhesion: observations from the study of gram-positive bacteria that initiate biofilm formation on the tooth surface. Adv Dent Res. 1997;11:168–175. doi: 10.1177/08959374970110010801. [DOI] [PubMed] [Google Scholar]
- 78.Ruhl S., Sandberg A.L., Cole M.F., Cisar J.O. Recognition of immunoglobulin A1 by oral actinomyces and streptococcal lectins. Infect Immun. 1996;64:5421–5424. doi: 10.1128/iai.64.12.5421-5424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apperloo-Renkema H.Z., Wilkinson M.H., van der Waaij D. Circulating antibodies against faecal bacteria assessed by immunomorphometry: combining quantitative immunofluorescence and image analysis. Epidemiol Infect. 1992;109:497–506. doi: 10.1017/s0950268800050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kroese F.G.M., Kantor A.B., Herzenberg L.A. The role of B-1 cells in mucosal immune responses. In: Ogra P.L., Mestecky J., Lamm M.E., Strober W., McGhee J.R., Bienenstock J., editors. Handbook of Mucosal Immunology. Academic Press; San Diego: 1994. pp. 217–224. [Google Scholar]
- 81.van der Waaij L.A., Mesander G., Limburg P.C., van der Waaij D. Direct flow cytometry of anaerobic bacteria in human feces. Cytometry. 1994;16:270–279. doi: 10.1002/cyto.990160312. [DOI] [PubMed] [Google Scholar]
- 82.Crago S.S., Kutteh W.H., Moro I., Allansmith M.R., Radl J., Haaijman J.J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J Immunol. 1984;132:16–18. [PubMed] [Google Scholar]
- 83.Brandtzaeg P., Carlsen H.S., Farstad I.N. The human mucosal B-cell system. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 617–654. [Google Scholar]
- 84.Brown T.A., Mestecky J. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect Immun. 1985;49:459–462. doi: 10.1128/iai.49.2.459-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown T.A., Mestecky J. Subclass distribution of IgA antibodies to microbial and viral antigens. In: Strober W., Lamm M.E., McGhee J.R., James S.P., editors. Mucosal Immunity and Infections at Mucosal Surfaces. Oxford University Press; New York: 1988. pp. 340–345. [Google Scholar]
- 86.Ladjeva I., Peterman J.H., Mestecky J. IgA subclasses of human colostral antibodies specific for microbial and food antigens. Clin Exp Immunol. 1989;78:85–90. [PMC free article] [PubMed] [Google Scholar]
- 87.Novak J., Tomana M., Kilian M., Coward L., Kulhavy R., Barnes S. Heterogeneity of O-glycosylation in the hinge region of human IgA. Molec Immunol. 2001;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 88.Royle L., Roos A., Harvey D.J., Wormald M.R., van Gijlswijk-Janssen D., Redwan el R.M. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 89.Torano A., Tsuzukida Y., Liu Y.S., Putnam F.W. Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc Natl Acad Sci USA. 1977;74:2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomana M., Niedermeier W., Mestecky J., Hammack W.J. The moieties of IgA, J chain, secretory piece and IgD. In: Sox H.C., editor. Carbohydrate Moieties of Immunoglobulin. MSS Information Corporation; New York: 1974. pp. 161–185. [Google Scholar]
- 91.Tomana M., Mestecky J., Niedermeier W. Studies on human secretory immunoglobulin A. IV. Carbohydrate composition. J Immunol. 1972;108:1631–1636. [PubMed] [Google Scholar]
- 92.Hughes G.J., Reason A.J., Savoy L., Jaton J., Frutiger-Hughes S. Carbohydrate moieties in human secretory component. Biochim Biophys Acta. 1999;1434:86–93. doi: 10.1016/s0167-4838(99)00168-5. [DOI] [PubMed] [Google Scholar]
- 93.Mizoguchi A., Mizuochi T., Kobata A. Structures of the carbohydrate moieties of secretory component purified from human milk. J Biol Chem. 1982;257:9612–9621. [PubMed] [Google Scholar]
- 94.Tomana M., Niedermeier W., Spivey C. Microdetermination of monosaccharides in glycoproteins by gas-liquid chromatography. Anal Biochem. 1978;89:110–118. doi: 10.1016/0003-2697(78)90731-5. [DOI] [PubMed] [Google Scholar]
- 95.Bouvet J.P., Dighiero G. From natural polyreactive autoantibodies to a la carte monoreactive antibodies to infectious agents: is it a small world after all? Infect Immun. 1998;66:1–4. doi: 10.1128/iai.66.1.1-4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouvet J.P., Fischetti V.A. Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun. 1999;67:2687–2691. doi: 10.1128/iai.67.6.2687-2691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernandez C., Alarcon-Riquelme M.E., Abedi-Valugerdi M., Sverremark E., Cortes V. Polyreactive binding of antibodies generated by polyclonal B cell activation. I. Polyreactivity could be caused by differential glycosylation of immunoglobulins. Scand J Immunol. 1997;45:231–239. doi: 10.1046/j.1365-3083.1997.d01-397.x. [DOI] [PubMed] [Google Scholar]
- 98.Fernandez C., Alarcon-Riquelme M.E., Sverremark E. Polyreactive binding of antibodies generated by polyclonal B cell activation. II. Crossreactive and monospecific antibodies can be generated from an identical Ig rearrangement by differential glycosylation. Scand J Immunol. 1997;45:240–247. doi: 10.1046/j.1365-3083.1997.d01-398.x. [DOI] [PubMed] [Google Scholar]
- 99.Quan C.P., Berneman A., Pires R., Avrameas S., Bouvet J.P. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65:3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimoda M., Inoue Y., Azuma N., Kanno C. Natural polyreactive immunoglobulin A antibodies produced in mouse Peyer's patches. Immunology. 1999;97:9–17. doi: 10.1046/j.1365-2567.1999.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burrows P.D., Cooper M.D. IgA deficiency. Adv Immunol. 1997;65:245–276. [PubMed] [Google Scholar]
- 102.Cunningham-Rundles C. Immunodeficiency and mucosal immunity. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 1145–1157. [Google Scholar]
- 103.Mestecky J., Hammarström L. IgA-associated diseases. In: Kaetzel C.S., editor. Mucosal Immune Defense: Immunoglobulin A. Springer; New York: 2007. pp. 321–344. [Google Scholar]
- 104.Arnold R.R., Cole M.F., Prince S., McGhee J.R., Secretory IgM antibodies to Streptococcus mutans in subjects with selective IgA deficiency. Clin Immunol Immunopathol. 1977;8:475–486. doi: 10.1016/0090-1229(77)90011-3. [DOI] [PubMed] [Google Scholar]
- 105.Barros M.D., Porto M.H., Leser P.G., Grumach A.S., Carneiro-Sampaio M.M. Study of colostrum of a patient with selective IgA deficiency. Allergol Immunopathol. 1985;13:331–334. [PubMed] [Google Scholar]
- 106.Brandtzaeg P. Human secretory immunoglobulin M. An immunochemical and immunohistochemical study. Immunology. 1975;29:559–570. [PMC free article] [PubMed] [Google Scholar]
- 107.Brandtzaeg P., Fjellanger I., Gjeruldsen S.T. Immunoglobulin M: local synthesis and selective secretion in patients with immunoglobulin A deficiency. Science. 1968;160:789–791. doi: 10.1126/science.160.3829.789. [DOI] [PubMed] [Google Scholar]
- 108.Brandtzaeg P., Karlsson G., Hansson G., Petruson B., Bjorkander J., Hanson L.A. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin Exp Immunol. 1987;67:626–636. [PMC free article] [PubMed] [Google Scholar]
- 109.Savilahti E. IgA deficiency in children. Immunoglobulin-containing cells in the intestinal mucosa, immunoglobulins in secretions and serum IgA levels. Clin Exp Immunol. 1973;13:395–406. [PMC free article] [PubMed] [Google Scholar]
- 110.Savilahti E., Pelkonen P. Clinical findings and intestinal immunoglobulins in children with partial IgA deficiency. Acta Paediatr Scand. 1979;68:513–519. doi: 10.1111/j.1651-2227.1979.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 111.Mestecky J., Moro I., Kerr M.A., Woof J.M. Mucosal immunoglobulins. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 153–181. [Google Scholar]
- 112.Mestecky J., Zikan J., Butler W.T. Immunoglobulin M and secretory immunoglobulin A: presence of a common polypeptide chain different from light chains. Science. 1971;171:1163–1165. doi: 10.1126/science.171.3976.1163. [DOI] [PubMed] [Google Scholar]
- 113.Crago S.S., Kulhavy R., Prince S.J., Mestecky J. Secretory component of epithelial cells is a surface receptor for polymeric immunoglobulins. J Exp Med. 1978;147:1832–1837. doi: 10.1084/jem.147.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Low T.L., Liu Y.S., Putnam F.W. Structure, function, and evolutionary relationships of Fc domains of human immunoglobulins A, G, M, and E. Science. 1976;191:390–392. doi: 10.1126/science.1246619. [DOI] [PubMed] [Google Scholar]
- 115.Putnam F.W. Structure of the human IgA subclasses and allotypes. Protides Biol Fluids. 1989;36:27–37. [Google Scholar]
- 116.Boursier L., Dunn-Walters D.K., Spencer J. Characteristics of IgVH genes used by human intestinal plasma cells from childhood. Immunology. 1999;97:558–564. doi: 10.1046/j.1365-2567.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fischer M., Kuppers R. Human IgA- and IgM-secreting intestinal plasma cells carry heavily mutated VH region genes. Eur J Immunol. 1998;28:2971–2977. doi: 10.1002/(SICI)1521-4141(199809)28:09<2971::AID-IMMU2971>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 118.Chapman A., Kornfeld R. Structure of the high mannose oligosaccharides of a human IgM myeloma protein. I. The major oligosaccharides of the two high mannose glycopeptides. J Biol Chem. 1979;254:816–823. [PubMed] [Google Scholar]
- 119.Cohen R.E., Ballou C.E. Linkage and sequence analysis of mannose-rich glycoprotein core oligosaccharides by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980;19:4345–4358. doi: 10.1021/bi00559a031. [DOI] [PubMed] [Google Scholar]
- 120.Jouanneau J., Fournet B., Bourrillon R. Localization and overall structure of a mannose-rich glycopeptide from a pathologic immunoglobulin. Biochim Biophys Acta. 1981;667:277–284. doi: 10.1016/0005-2795(81)90193-8. [DOI] [PubMed] [Google Scholar]
- 121.Monica T.J., Williams S.B., Goochee C.F., Maiorella B.L. Characterization of the glycosylation of a human IgM produced by a human-mouse hybridoma. Glycobiology. 1995;5:175–185. doi: 10.1093/glycob/5.2.175. [DOI] [PubMed] [Google Scholar]
- 122.Endo T., Mestecky J., Kulhavy R., Kobata A. Carbohydrate heterogeneity of human myeloma proteins of the IgA1 and IgA2 subclasses. Mol Immunol. 1994;31:1415–1422. doi: 10.1016/0161-5890(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 123.Field M.C., Amatayakul-Chantler S., Rademacher T.W., Rudd P.M., Dwek R.A. Structural analysis of the N-glycans from human immunoglobulin A1: comparison of normal human serum immunoglobulin A1 with that isolated from patients with rheumatoid arthritis. Biochem J. 1994;299(Pt 1):261–275. doi: 10.1042/bj2990261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattu T.S., Pleass R.J., Willis A.C., Kilian M., Wormald M.R., Lellouch A.C. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 125.Renfrow M.B., Cooper H.J., Tomana M., Kulhavy R., Hiki Y., Toma K. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem. 2005;280:19136–19145. doi: 10.1074/jbc.M411368200. [DOI] [PubMed] [Google Scholar]
- 126.Renfrow M.B., Mackay C.L., Chalmers M.J., Julian B.A., Mestecky J., Kilian M. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal Bioanal Chem. 2007;389:1397–1407. doi: 10.1007/s00216-007-1500-z. [DOI] [PubMed] [Google Scholar]
- 127.Tarelli E., Smith A.C., Hendry B.M., Challacombe S.J., Pouria S. Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-of-flight mass spectrometry. Carbohydr Res. 2004;339:2329–2335. doi: 10.1016/j.carres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 128.Dunn-Walters D., Boursier L., Spencer J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol Immunol. 2000;37:107–113. doi: 10.1016/s0161-5890(00)00038-9. [DOI] [PubMed] [Google Scholar]
- 129.Dunn-Walters D.K., Spencer J. Strong intrinsic biases towards mutation and conservation of bases in human IgVH genes during somatic hypermutation prevent statistical analysis of antigen selection. Immunology. 1998;95:339–345. doi: 10.1046/j.1365-2567.1998.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weiss A.A., Lyer S.S. Glycomics aims to interpret the third molecular language of cells. Microbe. 2007;2:489–497. [Google Scholar]
- 131.Max E.E. Immunoglobulins: molecular genetics. In: Paul W.E., editor. Fundamental Immunology. 6th ed. Lippincott, Williams & Wilkins; Philadelphia: 2008. pp. 192–236. [Google Scholar]
- 132.Compans R.W., Herrler G. Virus infection of epithelial cells. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 769–782. [Google Scholar]


