Abstract
Introduction
Adaptive growth of the intestinal mucosa in response to massive gut loss is fundamental for autonomy from parenteral nutrition. While angiogenesis is essential for cellular proliferation in other tissues, its relevance to intestinal adaptation is unknown. We tested the hypothesis that resection-induced adaptation is associated with new blood vessel growth.
Methods
Male C57B1/6 mice underwent either a 50% small bowel resection (SBR) or a sham (transection and reanastomosis) operation. After 1, 3, or 7 days, capillary density within the intestinal villi was measured using confocal microscopy. An mRNA RT-PCR array was used to determine angiogenic gene expression during adaptation.
Results
SBR mice had a significantly increased capillary density compared to sham operated mice at post operative day 7. This morphologic alteration was preceded by significant alterations in 5 candidate genes at post operative day 3.
Conclusion
New vessel blood growth is observed in the adapting intestine after massive small bowel loss. This response appears to follow, rather than initiate the adaptive alterations in mucosal morphology that are characteristic of adaptation. A better understanding of this progress and the signaling factors involved may improve therapeutic options for children with SGS.
Keywords: Short gut syndrome, small bowel resection, capillary density, angiogenesis
INTRODUCTION
Massive intestinal loss critically limits the absorptive capacity of the intestine needed for proper growth and development. This condition is termed short gut syndrome (SGS) and results in a high degree of morbidity and mortality in the pediatric population. Our lab as well as others has shown that in animal models of SGS the remnant bowel undergoes an adaptive increase in villus height, crypt depth, and length resulting in improved surface area and absorptive capacity1,2. Several mitogenic pathways and growth factors that influence adaptation have been identified and may have therapeutic implications in the management of SGS.
A large body of work exists on the interactions between epithelial cell proliferation and capillary growth. Folkman et al has made seminal contributions toward the understanding of angiogenesis and tumor cell proliferation3,4. Specifically in the intestine, angiogenic growth factor supplementation has been shown to enhance mucosal growth in transplanted intestinal grafts in rats5. A recent study by Parvadia et al demonstrated that vascular endothelial growth factor (VEGF) inhibition within the saliva resulted in decreased capillary growth and a decreased adaptive response after massive intestinal loss in a murine model6. Although the interaction between capillary growth and enterocyte proliferation during resection-induced adaptation would appear to be obvious, the temporal profile of this growth in relationship to the expression of specific genes and activity of discreet signaling cascades are presently unknown.
In the current study, we tested the hypothesis that resection-induced adaptation is associated with new blood vessel growth. The aim of this study was two-fold. First, we sought to elucidate a temporal profile of capillary growth after massive intestinal loss in a murine model of short gut syndrome. Second, we tried to delineate expression alterations of the most important angiogenesis genes during adaptation. A better understanding of the pathogenesis of capillary growth during adaptation may lead to improved therapeutic options for children with SGS.
MATERIALS AND METHODS
Experimental Design
Protocols for this study were approved by the Washington University Animal Studies Committee (#20070145), Cincinnati Children’s Hospital Research Foundation Institutional Animal Care and Use Committee (#5D04222) and were in accordance with the National Institute of Health laboratory animals care and use guidelines. Mice underwent either 50% proximal SBR or sham operation as we have previously described [1]. Mice were harvested on post-operative days 1, 3, and 7. Confocal microscopy was used to determine capillary density. Alterations in the transcriptional expression of angiogenesis-related genes were measured in epithelial-free segments of adapting ileum using a commercially-available RT-PCR kit.
Animals
C57Bl/6 male mice ages 8–13 weeks were used in this study (weight range 20–25 gm, Jackson Laboratory, Bar Harbor, ME). Mice were kept on a 12 hour light-dark cycle and were housed in a standard facility and allowed to acclimate to their environment for at least 7 days. The mice were place on a liquid rodent diet (Micro-Stabilized Rodent Liquid Diet LD101; Purina Mills, St. Louis, MO) one day prior to surgery.
Operative Technique
Mice underwent 50% proximal small bowel resection (SBR) or sham (transaction and reanastomosis only) as previously reported1. Briefly, mice that underwent SBR had transection of the bowel at a point 12 cm from the ileocecal junction and also at a site 2–3 cm distal to the ligament of Trietz. The intervening bowel was removed and the two ends were anastomosed. In mice undergoing sham operations, the bowel was transaction 12 cm proximal to the ileocecal junction and a reanastomosis was done. Mice were provided free access to water only for the first 24 hours and then given a liquid rodent diet ad libitum until sacrifice.
Adaptation Measurements
Villus height and crypt depth were measured using the image analysis software MetaMorph (Version 7.1.2.0, Downingtown, PA). Twenty well-oriented crypt-villus units were measured in H&E stained sections from each animal 2 cm distal to the anastomosis in a blinded fashion. Adaptive criteria was set at a 20% increase of villus height in SBR mice compared to sham on post operative day 3 and 7 as previously determined 2.
Capillary Density Measurement
Measurement of capillary density was modified from a reported technique by Stappenbeck et al7. Briefly, mice were anesthetized with isoflourane followed by an intramuscular injection of ketamine, xylazine. and acepromazine (4:1:1). A 200 μl volume of a 2 mg/ml solution of high-molecular-weight (2,000 kDa) fluorescein isothiocyanate (FITC)-labeled dextran (Sigma, St. Louis, MO) was injected into the retro-orbital plexus. After 3 minutes, the intestine was removed and the animal was sacrificed. The first 1 cm of intestine distal to the anastomosis was discarded. The next 2 cm distally was processed for H&E staining to characterize adaptation. The lumen of the next 4 cm segment of ileum was perfused with a fixation solution containing 0.5% paraformaldehyde, 15% picric acid, and 0.1 M sodium phosphate buffer (pH 7.0) at 4°C for 12-h. Specimens were rinsed in ice-cold PBS (three washes, 5 min each), followed by a 3-h incubation in 10% sucrose/PBS (4°C) and an overnight incubation in 20% sucrose/10% glycerol/PBS (4°C). The tissue was frozen in OCT and 60 μm thick sections were cut using a cryostat in an axial orientation. Sections were air dried for 2 hours and rehydrated with cold PBS for 1 minute then stained with Syto61 (1:1,000 dilution in PBS; Molecular Probes/Invitrogen, Carlsbad, CA) for 1 h at room temperature, followed by three PBS washes (5 min per cycle, room temperature) and were mounted in 50% glycerol/PBS and stored at 4°C before viewing. A LSM 510 Meta confocal microscope (Zeiss, Gottingen, Germany) was used to scan 5-μm slices and were projected in three dimensions by taking 12–16 serial images, aligning them at 7–10° intervals, and compiling/rotating them about the y axis by using LSM 510 software (Zeiss, Gottingen, Germany).
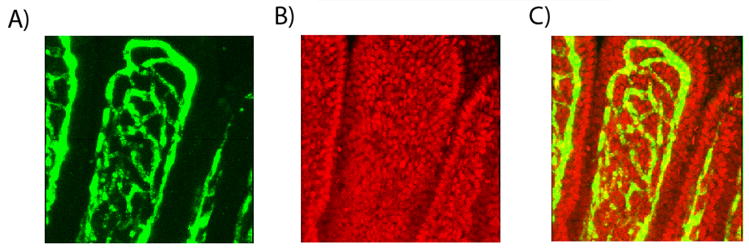
To quantify capillary density, the number of syto-61 positive nuclei was divided by the number of capillary networks or windows in the corresponding section of the upper third of the villus (Figure 1). This number was normalized to villus height. Ten to 12 villi were counted per animal.
Figure 1.
Confocal microscopic images of murine ileal villi: A) FITC-labeled dextran capillary windows, B) Syto-61 positive epithelial cells, and C) Merged confocal image from separate channels (A and B).
Tissue Harvest and Isolation of Epithelial Cell-Free Ileum
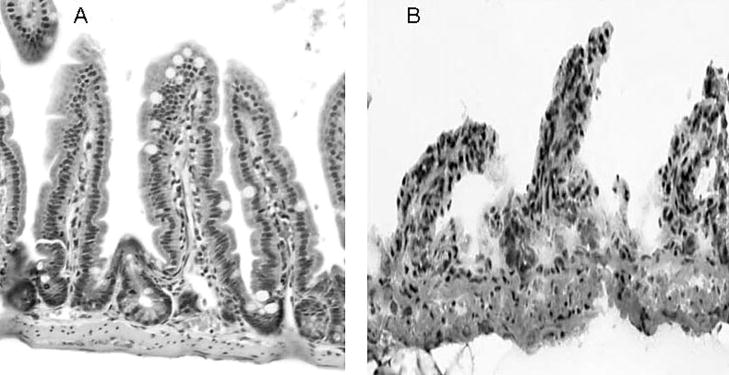
At the time of sacrifice the small bowel was excised and flushed with ice-cold phosphate buffered saline (PBS). The first centimeter distal to the anastomosis was discarded, the next 2 cm was placed in formalin for histologic examination, and the next 6 cm were used to remove the overlying epithelial cells from the muscular and vascular layers of the bowel wall. The intestine was cut longitudinally and transferred into tubes containing 5 mL of ice-cold PBS with protease inhibitors (0.2 mM PMSF, 5μg/ml Aprotinin, 1mM Benzamidine, 1mM sodium orthovanadate and 2μM Cantharidin, all are from EMD, Gibbstown, NJ). The tissue was then transferred into a solution (1.5 mM KCI, 96 mM NaCl, 27 mM Na Citrate, 8 mM KH2PO4, 5.6 mM Na2HPO4, 15 mM EDTA and 1 mM DTT) and vortexed at maximum speed for 15 minutes at 4°C to remove all villi and crypts; at this time, only the muscular layer of the intestine and the vascular layer of the lamina propria were intact as previously described 2 (Figure 2). These layers were frozen at −80°C for future analysis.
Figure 2.
Murine ileum before (A) and following (B) removal of all epithelial cells. The tissue in (B) was used for studies of angiogenesis gene expression.
Angiogenesis Gene Analysis
The muscular and vascular layer of the small intestine was isolated as described above. Tissue samples for RNA use were stored in lysis buffer (RNAqueous kit, Ambion, Austin, TX) at −80°C. The samples were homogenized (200 Series PRO Scientific Homogenizer, Oxford, CT), total RNA was isolated using a RNAqueous kit following the manufactures instructions (Ambion. Austin, TX) and the RNA was quantified using a NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington DE) then stored at −80°C. The RNA samples were evaluated for quality using a Bio-Rad Experion System with a RNA StdSens Chip and reagents (Bio-Rad Laboratories, Richmond, CA). Only RNA samples that showed good 18s and 28s ribosomal RNA peaks were used in the analysis. Complementary DNA (cDNA) was produced from quality RNA samples using a RT2 First Strand Kit (SABioscience, Fredrick, MD). The cDNA was analyzed on a mouse angiogenesis RT2 Profiler PCR Array (SABioscience, Fredrick, MD) using an Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA). The mouse angiogenesis RT2 Profiler PCR Array profiles the expression of 84 genes known to be involved in the regulation of angiogenesis. A complete list of the genes contained in the array can be viewed on the following link: http://wvw.sabiosciences.com/rt_pcr_product/HTML/PAMM-024A.html. The expression of genes in the ileum after SBR was compared with sham-operated animals using software from SABiosciences (Fredrick, MD). The genes found to be significantly different between sham and SBR animals were individually confirmed using primers and reagents from SABiosciences and the 7500 Fast Real-Time PCR system as above except that the cDNA was quantified using Quanti-iT Oligreen ssDNA Assay kit (Invitrogen, Carlsbad, CA) so that equal amounts of cDNA could be used in all reactions and beta-actin was employed as the endogenous control.
Statistical Analysis
Results are presented as mean values ± standard error of the mean (SEM). Statistical differences were identified using a one-way analysis of variance (ANOVA). The Sigma Stat statistical package (SPSS, Chicago, IL) was utilized for all statistical analyses. A P value of less than 0.05 was considered significant unless otherwise stated.
RESULTS
All mice were healthy at the time of harvest and none revealed evidence for intestinal obstruction. Mice were only included for analysis that demonstrated adaptive villus growth in order of magnitude greater than 20% following SBR. We have previously characterized a normal spectrum of adaptation ranging from absent to amplified adaptation responses within populations of mice 2. Further, since the purpose of this study was to determine angiogenesis gene alterations involved in resection-induced villus growth, inclusion of mice who did not have villus growth would have significantly confounded our search for critical angiogenesis gene expression alterations. Using these rigid criteria, 8 of 12 (67%), 10 of 14 (71%), and 17 of 35 (49%) SBR mice were included at the postoperative day 1,3, and 7 time points, respectively. We are unclear at the present time why fewer mice in this study demonstrated adaptive villus growth at the 7th postoperative day.
Villus Capillary Density is Increased Following SBR
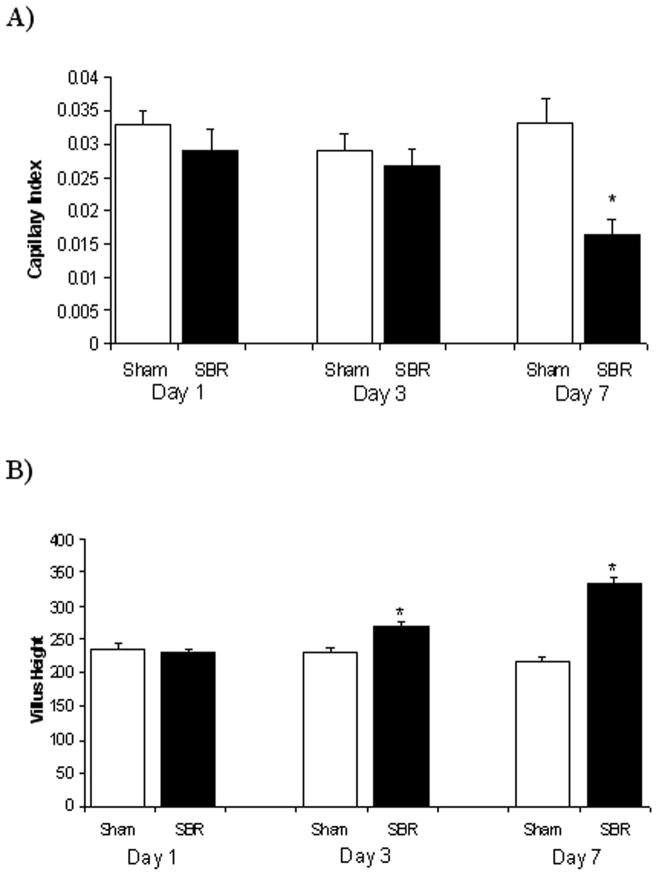
As shown in Figure 3A, lamina propria demonstrated increased capillary density at post operative day 7 when compared with mice undergoing sham operation. The capillary index (average ± SEM) was 0.033 ± 0.004 for sham operated mice and 0.017 ± 0.002 for SBR (P value = 0.01). The smaller capillary index represents increased capillary growth as there are fewer cells present within blood vessel networks and are therefore denser. This capillary growth was not identified at earlier time points (postoperative days 1 and 3). By comparison, statistically significant increases in villus growth were observed earlier (by postoperative day 3; Figure 3B).
Figure 3.
A) Capillary index (# nuclei/window/villus height) and B) villus height measurements on postoperative days 1, 3, 7 following either sham operation (bowel transaction with reanastomosis alone) or 50% proximal small bowel resection (SBR), * p=0.01 SBR versus sham.
Angiogenesis Gene Expression is Altered After SBR
The capillary growth seen after massive SBR led us to question whether there were corresponding alterations in the transcriptional expression of angiogenesis-related genes. In order to test this, we performed an RT-PCR array looking simultaneously at 84 genes known to be involved in murine angiogenesis. These changes were measured in RNA extracted from the isolated intestinal muscular and vascular layer, devoid of contamination by epithelial cells. At each time-point (1-day, 3-day and 7-day), 6 different animals (n = 3 SBR; n = 3 sham) were evaluated. There were no significant differences in the expression of any gene observed when comparing sham versus SBR animals at the 1- or 7-day postoperative times. However, at the 3-day postoperative time (preceding the measured increase in capillary density at day 7), the expression of five genes was found to be significantly different (2-fold or greater difference and p-value of 0.01 or less; Table 1) following SBR. The expression differences of these 5 genes were individually verified by RT-PCR using new cDNA and run in triplicate for each of the 3 SBR and sham samples.
Table 1.
The major angiogenesis genes found to have significant expression differences (2-fold or greater difference and p-value of 0.01 or less) between mice undergoing a 50% proximal small bowel resection versus sham operation (bowel transaction with reanastomosis alone) at post-operative day 3 by RT2 Profiler PCR Array. These differences were verified by standard RT-PCR in separate reactions. Brain-specific angiogenesis inhibitor 1 (Bail), Chemokine (C-X-C Motif) ligand 5 (Cxcl5), Frizzled Homolog 5 (Drosophila) (Fxd5), Interleukin-lβ (Il 1b) and Placental growth factor (Pgf).
| RT2 Profiler PCR Array | RT-PCR | ||
|---|---|---|---|
| Fold Difference | p-value | Fold Difference ± SEM | |
| Bai1 | −2.81 | 0.002 | −2.3 ± 0.34 |
| Cxcl5 | 14.21 | 0.005 | 18.67 ± 3.17 |
| Fzd5 | −5.67 | 0.01 | −7.01 ±2.62 |
| ll1b | 3.72 | 0.006 | 4.05 ± 0.19 |
| Pgf | 3.14 | 0.01 | 4.00 ± 1.18 |
DISCUSSION
Capillary growth and epithelial cell proliferation are vitally linked in many organ systems. In the present study, we demonstrate for the first time a significant increase in villus capillary density during the critical process of resection-induced adaptation. Furthermore we have identified expression differences in several novel genes which temporally herald the observed increases in villus capillary growth. These observations provide fundamental new information needed to pave the way for a more thorough understanding of the contribution of angiogenesis to the process of intestinal adaptation
Significant adaptive changes have been characterized in our murine SBR model as early as post operative day 3 1,2. In the current study, a significant difference in capillary growth after SBR was not observed until later. This observation would support the notion that enterocyte proliferation and mucosal growth are major stimuli for angiogenesis. Alternatively, this observation would refute the notion that angiogenesis plays a role in the initiation of adaptive mucosal growth. These data therefore provide important mechanistic insight into the pathogenesis of resection-induced intestinal adaptation. On the other hand, it must be considered that the threshold for detecting significant changes in capillary density by confocal microscopy may not reached until post operative day 7. Although the use of this technique is well established to measure capillary density in the intestine 7, it may not be the most sensitive marker to detect significant changes at earlier time points.
Our analysis of angiogenesis genes revealed the most robust alterations at 3 days following massive SBR. Three genes were significantly increased: Chemokine (C-X-C) ligand 5 (Cxcl5), interleukin-1 beta (IL-1β), and placental growth factor (Pgf). These genes are renowned as promoters of new blood vessel growth in the context of colon cancer and inflammatory bowel disease8–12. All three of these genes produce proteins which are excreted by the cell and none have been studied exclusively in the small intestine in the perspective of adaptation. Two genes were significantly decreased in this study: Brain-specific angiogenesis inhibitor 1 (Bai-1) and Frizzled Homology 5 (Drosophilia) (Fzd5). Both of these genes produce cell membrane receptor proteins, Bai-1 is an angiogenesis suppressor gene that was first identified in relation to human glioblastomas 13. Bai-1 has been identified in other tissues and has shown to be downregulated in colorectal and gastric cancer 14, 15 allowing for rapid tumor cell proliferation. The specific role for Bai-1 in the small intestine is not well understood. The reduced expression of Bai-1 in our model may promote new blood vessel growth; however the full contribution of this gene toward angiogenesis during adaptation remains to be elucidated.
The expression of Fzd5 is also reduced in our model of small bowel adaptation, Fzd5 is transmembrane receptor involved in the Wnt signaling pathway 16, 17. It has already been established that Fzd5 plays an important role in yolk sac angiogenesis in developing mice 18, 19. The interaction between Wnt and Fzd5 is poorly understood but the Wnt signaling pathway has clearly been associated with proliferation in the gastrointestinal tract 20, 21, More research into the specific role of Fzd5 will need to be completed in the future to fully understand this gene’s effects on small bowel adaptation and angiogenesis.
Although this study provides important insight into the angiogenic response involved in adaptation, it does have several limitations. First, one of the goals for this study was to evaluate those genetic factors involved in the angiogenic process within the isolated muscular and vascular layers of intestine – devoid of epithelial cells. It is possible that epithelial-derived factors may drive the angiogenic response that was not detected within the tissues studied. Studies are presently underway in our laboratory to evaluate alterations in angiogenesis genes within the epithelial cell compartment at various time points. Further, the actual endothelial cells probably comprise a rather small fraction of the total mRNA isolated from the tissue studied. As such, confounding mRNA from multiple other cell types could have prevented detection of significant changes within genes of the blood vessel network. Finally, it should be considered that the source of the proangiogenic peptides, hormones, or relevant genetic alterations to drive angiogenesis may reside outside of the intestine. This would be suggested by the finding that removal of the submandibular glands results in an impaired adaptation response to massive SBR 6. Since the saliva is rich in multiple proangiogenic growth factors, this would be one candidate source to consider.
New blood vessel growth in the adapting intestine may play several important functions. Dubin et al has revealed that villi hypoperfusion is associated with decreased mucosal integrity and a propensity for bacterial translocation and sepsis 22. Since sepsis is a major cause of mortality in children with short gut syndrome 23, 24, targeted therapy intended to promote angiogenesis during adaptation might possibly enhance mucosal integrity, thereby attenuating bacterial translocation. Further, since post-prandial absorption of normal digestive loads cannot be sustained without concomitant increases in villus capillary blood flow 25, it must be considered that increased microvascular flow may enhance absorptive capacity thus contributing toward an important adaptive mechanism in patients with short gut syndrome. Finally, our findings would suggest that new blood vessel growth appears to be needed to sustain the taller and more cellular villi that develop In response to massive SBR. A more comprehensive understanding the factors that regulate angiogenesis could therefore lead to new clinical strategies to address mucosal integrity, absorptive capacity, and intestinal regrowth in patients suffering from short gut syndrome.
Acknowledgments
Supported by the National Institutes of Health Digestive Disease Research Development Core Center of Cincinnati -P30DK078392, Digestive Disease Research Development Core Center of St. Louis - P30DK052574, and T32DK064581 (Dr. Martin)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helmrath MA, VanderKolk WE, Can G, et al. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 2.Taylor JA, Martin CA, Nair R, et al. Lessons learned: optimization of a murine small bowel resection model. J Pediatr Surg. 2008;43:1018–1024. doi: 10.1016/j.jpedsurg.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Is angiogenesis an organizing principle in biology and medicine? J Pediatr Surg. 2007;42:1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 5.Lee KD, Yamataka A, Kato Y, et al. Basic fibroblast growth factor and granulocyte colony-stimulating factor enhance mucosal surface expansion after adult small bowel transplantation without vascular reconstruction in rats. J Pediatr Surg. 2006;41:737–741. doi: 10.1016/j.jpedsurg.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Parvadia JK, Keswani SG, Vaikunth S, et al. Role of VEGF in small bowel adaptation after resection: the adaptive response is angiogenesis dependent. Am J Physiol Gastrointest Liver Physiol. 2007;293:G591–598. doi: 10.1152/ajpgi.00572.2006. [DOI] [PubMed] [Google Scholar]
- 7.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimberg J, Dienus O, Lofgren S, et al. Expression and gene polymorphisms of the chemokine CXCL5 in colorectal cancer patients. Int J Oncol. 2007;31:97–102. [PubMed] [Google Scholar]
- 9.Etoh T, Shibuta K, Barnard GF, et al. Angiogenin expression in human colorectal cancer; the role of focal macrophage infiltration. Clin Cancer Res. 2006;6:3545–3551. [PubMed] [Google Scholar]
- 10.Pousa ID, Mate J, Salcedo-Mora X, et al. Role of vascular endothelial growth factor and angiopoietin systems In serum of Crohn’s disease patients. Inflamm Bowel Dis. 2008;14:61–67. doi: 10.1002/ibd.20269. [DOI] [PubMed] [Google Scholar]
- 11.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis. 2008;11:215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 12.Rubie C, Frick VO, Wagner M, et al. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. doi: 10.1186/1471-2407-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimori H, Shiratsuchi T, Urano T, et al. A novel brain-specific p53-target gene, BAH, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene. 1997;15:2145–2150. doi: 10.1038/sj.onc.1201542. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Koh JT, Shin BA, et al. Comparative study of angiostatic and anti-invasive gene expressions as prognostic factors in gastric cancer. Int J Oncol. 2001;18:355–361. [PubMed] [Google Scholar]
- 15.Yoshida Y, Oshika Y, Fukushima Y, et al. Expression of angiostatic factors in colorectal cancer. Int J Oncol. 1999;15:1221–1225. doi: 10.3892/ijo.15.6.1221. [DOI] [PubMed] [Google Scholar]
- 16.Bhanot P, Brink M, Samos CH, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 17.Yang-Snyder J, Miller JR, Brown JD, et al. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 18.Burns CJ, Zhang J, Brown EC, et al. Investigation of Frizzled-5 during embryonic neural development In mouse. Dev Dyn. 2008;237:1614–1626. doi: 10.1002/dvdy.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa T, Tamai Y, Zorn AM, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 20.MacLeod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403–411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- 21.You J, Nguyen AV, Albers CG, et al. Wnt pathway-related gene expression in inflammatory bowel disease. Dig Dis Sci. 2008;53:1013–1019. doi: 10.1007/s10620-007-9973-3. [DOI] [PubMed] [Google Scholar]
- 22.Dubin A, Kdul VS, Pozo MO, et al. Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med. 2008;36:535–542. doi: 10.1097/01.CCM.0000300083.74726.43. [DOI] [PubMed] [Google Scholar]
- 23.Aprahamian CJ, Chen M, Yang Y, et al. Two-hit rat model of short bowel syndrome and sepsis: independent of total parenteral nutrition, short bowel syndrome is proinflammatory and injurious to the liver. J Pediatr Surg. 2007;42:992–997. doi: 10.1016/j.jpedsurg.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 24.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47(Suppl 1):S33–36. doi: 10.1097/MPG.0b013e3181819007. [DOI] [PubMed] [Google Scholar]
- 25.Pappenheimer JR, Michel CC. Role of villus microcirculation in intestinal absorption of glucose: coupling of epithelial with endothelial transport. J Physiol. 2003;553:561–574. doi: 10.1113/jphysiol.2003.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]