Synopsis
Patients with acquired and inherited bone marrow failure syndromes are at risk for the development of clonal neoplasms including AML, MDS, and PNH. This chapter reviews the evidence supporting a model of clonal selection, a paradigm that provides a reasonable expectation that these often fatal complications might be prevented in the future.
Keywords: Clonal evolution, myelodysplasia, aplastic anemia, natural selection, Fanconi anemia, apoptosis, leukemogenesis
Patients with bone marrow failure syndromes are at risk for the development of clonal neoplasms including AML, MDS, and PNH (reviewed in preceding chapters in this volume and in1,2). From 10 to 20% of survivors of acquired aplastic anemia will develop a clonal disease within the decade following their diagnosis,3-6 and the relative risk of clonal neoplasms is even more significantly increased in children and adults with inherited bone marrow failure syndromes as well.7 Informed by advances in studies on evolutionary adaptation8-10 recent studies testing the adaptive nature of clonal evolution in mammalian hematopoietic stem cells are clearly positive.
The “inflammation and cancer” canon
Inflammatory disorders, infectious and non-infectious, are known to predispose patients to neoplastic diseases. While the type of inflammatory disorder can be variable, one constant is that the tissue at risk is the inflamed tissue itself. For example inflammatory bowel disease is a risk factor for colon cancer; hepatitis C infection is a risk factor for hepatocellular carcinoma and so on. A recent wave of interest in the linkage between “inflammation” and cancer has resulted in a canonical view that the neoplastic cells grow because they respond to the proliferative, antiapoptotic, and pro-angiogenic effects of the cytokines released in the inflamed tissue. Given that a large population of stem cells would be under the influence of these pro-proliferative cytokines, if this feed-forward model were sufficient to account for subsequent neoplasia the neoplasms themselves ought to be polyclonal. They aren’t. Because neoplasms are usually clonal (arising from one mutant stem cell) the model fails to explain a key step in this process, the emergence and domination of the niche by the progeny of a single mutant stem cell. An alternative model, one that applies the rules of natural selection, better reconciles the clinical course of disease in humans and mice. To compare this model with the more canonical one requires a thorough understanding of the concept of selection coefficients.
Selection coefficients and determinants of clonal evolution
Principles of natural selection were developed in reference to studies on species but are legitimately applicable to “asexual” populations as well.9 In fact, when these principles are tested in the laboratory using hematopoietic stem cells of mice with marrow failure, they have informed us about the importance of the microenvironment in processes of clonal evolution.11,12 In selection of species and subspecies, emergence and fixation of adaptive mutations depends first upon an environmentally induced stress upon a population that makes that population less fit. An apt example is found in studies on selection of dark and light colored rock pocket mice13 that differ in only one allele that influences coat color. In a neutral environment, both strains survive equally well but in the real world (in this case an environment with owls in it), the dark colored mice are easier for the predators to spot if they’re running about on sand. Conversely, the light colored ones are easy pickings sitting on lava-beds. In effect, the environment does its work to select the fit population not by influencing directly that population but by purging its unfit competitors. There is evidence that this is true not only for bacteria in which adaptation to antibiotic challenge results in resistance14,15 but for mammalian cells as well.16 This unabashedly Darwinian model is a perfectly applicable model to apply to populations of stressed hematopoietic stem cells.
To develop a clear picture of clonal evolution that occurs in the setting of bone marrow failure requires clarification of the relationships that exist between the target cells and the selective forces in the environment that determine fitness.17 Some mathematical models have even suggested that selective pressure is a more important determinant in initiation of a tumor than is an increased baseline mutation rate18 but it is intuitively more appealing to accept that variations in fitness in stem cell populations increase not only as a function of the relative fitness differences between two competing populations (a notation of which is known as the coefficient of selection) but in proportion to the population size and mutation rate as well.9
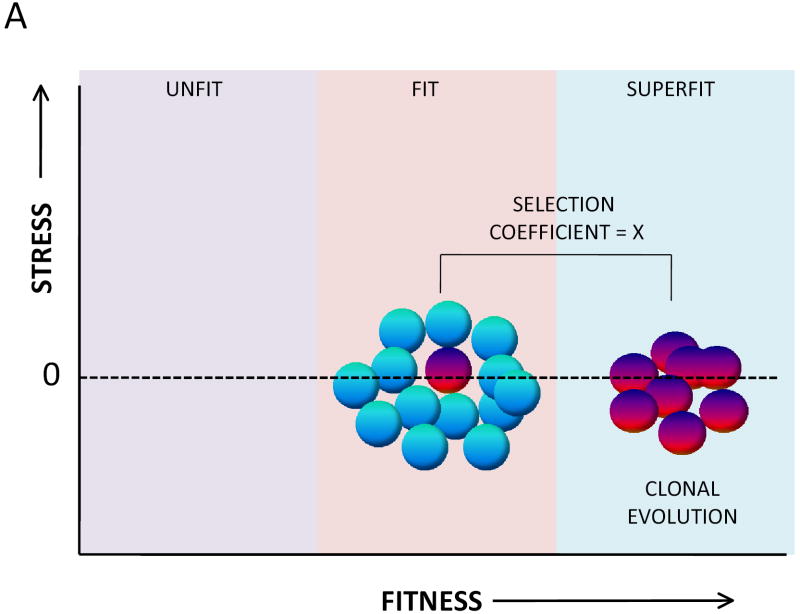
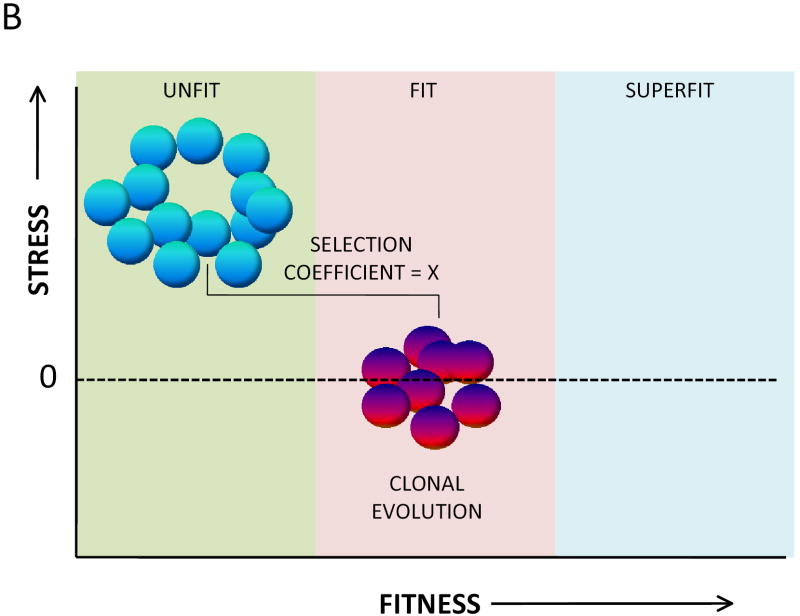
The likelihood of clonal evolution depends upon the relative fitness differences between normal stem cells and mutant (potentially adapted) stem cells. This relative difference is expressed as the “selection coefficient.” A high selection coefficient exists when a somatic mutation accords to cells a uniquely strong advantage. Two models of clonal evolution are presented in Figure 1. Note that each model results in the same coefficient of selection. Studies on clonal evolution using murine stem cells clearly support the model in Figure 1.B.11 The model in Figure 1.B. makes the argument that the selectability of a specific mutation will be highest when the reference population is disadvantaged at the outset. This model suggests that if one wants to prevent clonal evolution, one must focus on restoring the fitness of the unfit stem cells. This requires, of course, that the precise molecular mechanisms that reduce fitness in the stem cell pool are clarified. The focus needs to be both on mechanisms intrinsic to stem cells and pathways that influence the microenvironment.
Figure 1.
The fitness landscape for hematopoietic stem cells. A. A popular model of clonal evolution in hematopoietic stem cells proposes that somatic mutations occur stochastically in hematopoietic stem cells and that rare mutations of this kind confer upon the mutant stem cell (red) an inherent proliferative/survival advantage over the non mutant (blue) stem cells. A key principle of this model is that the non mutant stem cells are not unfit, at least at the time the new clone initially evolves. The fitness differences between the evolving clone and the normal stem cell pool can be described as a selection coefficient, here given a value of X. B. An alternative model, one more consistent with studies on clonal evolution in the setting of marrow failure is one in which the entire population of normal hematopoietic stem cells is unfit as a result of environmental stress. A somatic mutant (red) stem cell has developed resistance to the specific environmental stressor so remains fit while the remainder of the stem cell pool has become unfit as a result of environmental stress. In this instance the coefficient for selection of the abnormal clone is identical (X) to that of the model described in A.
Pathogenesis of Acquired Aplastic Anemia
In the past two decades experimental evidence from translational studies places acquired aplastic anemia squarely into the category of autoimmune diseases. The evidence from a number of laboratories, recently summarized by Young et al, 1 has revealed that; (1) aberrantly activated oligoclonal T-cell populations19 suppress hematopoiesis by releasing cytokines (importantly IFNγ and TNFα) (2) these cytokines and other factors induced by them cause apoptotic responses in hematopoietic stem cell and progenitor cells, (3) clinical responses to immunosuppressive therapy correlate directly with the capacity of the treatment to suppress T-cell function, and (4) immune mediated bone marrow failure can now be modeled in mice and in that model monoclonal antibodies to TNFα and IFNγ prevent fatal aplasia. Recent gene expression microarray analysis confirms predicted abnormalities in both the attacking T-cell populations20 and the progenitor cell pool.21
Clonal Evolution in Acquired Aplastic Anemia
Stem cells assaulted by cytotoxic lymphoid populations22 represent perfect models of a disadvantaged population (Figure 2). Unless the offending T-cell population is eradicated or inactivated, the selective pressure they exert on the stem cell pool would favor the evolution of somatically mutated stem cells that had acquired, by virtue of the mutation, the capacity to resist the attack of T-cells. The requirements for clonal evolution are; (a) that a sufficiently high coefficient of selection exists(ongoing selection against the non-adapted stem cells as would be the case with ongoing immune attack in acquired aplastic anemia), (b) that a stem cell population is of sufficient size (as would be the case prior to the onset of aplasia), and (c) that there is an appreciable mutation rate (as would be the case in many of the congenital aplastic states because of the additional feature of genetic instability).
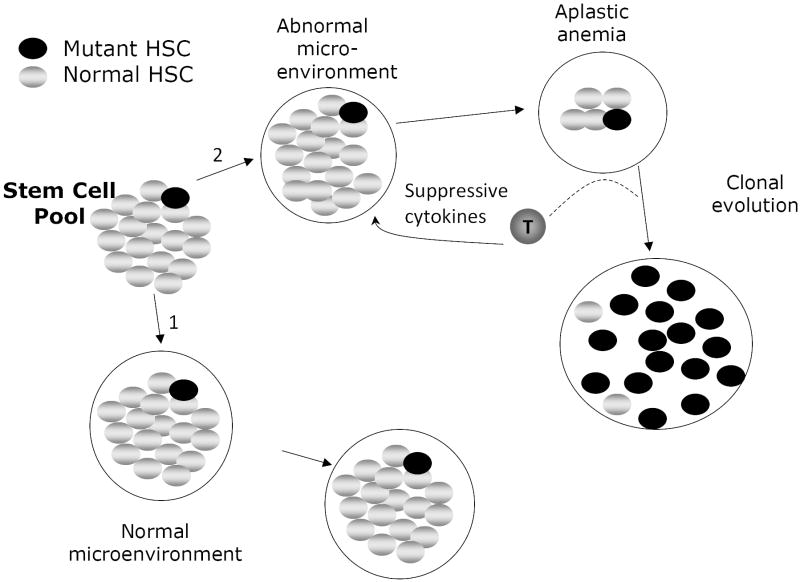
Figure 2.
Clonal evolution in acquired aplastic anemia. In the ground state, somatically mutated stem cells do not expand because the mutation doesn’t confer upon that cell a selective advantage. The coefficient of selection for clonal expansion of that cell is low. If the microenvironment remains normal over time (pathway 1), no clonal expansion occurs because the microenvironment is highly supportive of the majority of stem cells in the pool. That is, no clonal evolution occurs in pathway 1 because the relative fitness differences between the normal and mutant cells are trivial. If, however, T-lymphocytes arise that inhibit the replication, expansion or survival of normal hematopoietic stem cells, and if the somatic mutation confers upon a stem cell the capacity to resist the effect of the T cells (by resisting, for example, the apoptotic effects of suppressive cytokines like TNFα and IFNγ), then pathway 2 is most relevant. During the development of aplasia, normal stem cells are suppressed but the mutant one is not and, because the coefficient of selection for that cell is now high (as a result of a decline in fitness of the normal stem cells) it can expand clonally. In some cases the very cytokines that suppress the normal stem cells function to enhance the expansion of the mutant clone. Under continued pressure from the aberrant T cell population, the neoplastic clone preferentially expands over time while the less fit normal HSC are selected against. Not shown in this figure is a theoretical process by which a mutant stem cell arises in a population only after it is exposed to a hostile environment. This would meet strict genetic standards for a truly “adaptive mutation” in which the hostile environment per se induces mutations some of which permit an adaptive response to the environment. In light of the capacity of TNFα to induce oxidative DNA damage,82 this process is not simply a remote possibility. The coefficient of selection idea would still be relevant here as well because relief of the environmental stress (e.g. fully effective immunotherapy of aplastic anemia) might lower the coefficient in time to prevent an outgrowth of adapted clonal progeny. Finally, in some cases the new clone is able to subordinate the signals from the suppressive population and convert them to growth and survival signals (represented by the dashed line leading from the T-cell in pathway 2). For example, we have noted that in some cases of MDS arising in the context of bone marrow failure, TNFα enhances the proliferation of clonal erythroid and myeloid progenitors, a distinctly aberrant response.
As shown in Figure 2, an identical somatic mutation could occur randomly in an otherwise normal stem cell pool but because there is a low selection coefficient (the mutant cell exists in a large pool of non-disadvantaged stem cells) clonal evolution would be less favored (Figure 2, pathway 1). This situation may explain how covert leukemic clones can be found during normal fetal development yet not raise their heads even later in life.23 That is, these clones have no advantage. However, if some degree of bone marrow failure were to develop in a way that disadvantages the greater pool without influencing the mutant stem cell, the situation changes greatly in favor of clonal evolution (Figure 2, pathway 2). This paradigm fits very nicely with observations in humans and mice with acquired and inherited aplastic anemias.
Inherited Bone Marrow Failure Syndromes: Role of Cytokines in Selection
Aberrant interactions of IFNγ and TNFα with stem cells are also features of the most well-studied of the inherited aplastic anemias, Fanconi anemia (FA). Studies on hematopoietic cells from children with Fanconi anemia and later in mice nullizygous for Fancc demonstrated not only that FA-C cells release more TNFα in the ground state24,25 but that FA-C progenitor cells are inherently hypersensitive to apoptotic cues, including IFNγ, TNFα, mip1-α and TRAIL.26-28 Some of the mechanisms by which FA cells are hypersensitive to inflammatory cytokines are being clarified. For example, for TNFα hypersensitivity in FA cells, two serine/threonine kinases are important because their activity is influenced by FA proteins. The first is the protein kinase PKR a key molecular effector of the anti-viral response and the second is the apoptosis signal regulating kinase 1 (Ask1).29,30 Additional work is required to determine whether these pathways might be reasonable therapeutic targets.
Overproduction of Cytokines
Not only are FA target cells hypersensitive to apoptosis inducing cytokines, auxiliary cells that produce them have lower thresholds for cytokine release. Pang et al have reported that Fancc-/- mice treated with lps had higher mortality rates than wild type mice, had higher serum levels of TNFα, IL-6, and MIP-2, and demonstrated hematopoietic suppression that could be directly attributed to TNFα (anti-TNF antibodies protected FA target cells).31 They also showed that transplantation of hematopoietic stem cells from wild type mice protected Fancc -/- mice from endotoxin-induced mortality, clarifying the key role played by auxiliary cells of hematopoietic origin in the microenvironment.31
In summary, stem cells in patients with both acquired and certain inherited aplastic anemias are highly apoptotic and the signaling pathways, both within and external to stem cells, which ultimately execute them, are very closely related. In acquired aplastic anemia the stress initially comes from rogue clones of T-cells overproducing factors that result in stem cell death. In Fanconi anemia the production of some of the same factors is increased because the normal FA proteins set thresholds for cytokine production responses to cytokine-inducing factors in auxiliary cells (e.g. in response to endotoxin31). In addition, the stem cell pool is inherently hypersensitive to those factors because the normal FA proteins set their response thresholds for responses to some of the very apoptotic cytokines overexpressed in auxiliary cells.2 Although the experimental evidence supporting stem cell stress in other inherited marrow failure syndromes is not as robust, evidence is beginning to emerge supporting the idea that the heterogeneous inherited mutations result in higher rates of stem and progenitor cell apoptosis.32-34 These pools of damaged stem cells are all perfect environments for the selection of somatically mutated stem cell clones that have acquired the capacity to completely ignore those apoptotic cues. These clones will have a huge competitive advantage when compared to the highly disadvantaged reference population of stem cells. Therefore it is likely that in all aplastic states the coefficient of selection is high in the stem cell pool.
The Nature of the Insult and the Adaptive Tactic Are Linked
Fortunately, the clonal adaptation/selection model can be best tested directly now that some of the mechanisms that negatively impact stem cells are defined. Deductively, if the new clone has to be more fit, the type of resistance they exhibit must be precisely to the key factor or factors that put the stem cell pool under pressure in the first place. For example, if an aplastic microenvironment packed with IFNγ-producing T-cells provides the key selective pressure for the emergence of a fit clone of stem cells, the clonal progeny ought to be IFN-resistant. Clinical and laboratory observations have confirmed the accuracy of this notion.
Mosaicism; the Best Adaptive Response in Inherited Marrow Failure Syndromes
Some patients with Fanconi anemia exhibit a phenomenon (“mosaicism”) of genetic reversion in which one of the mutant FA alleles has been corrected in an hematopoietic stem cell. This cell and all its progeny have gained fitness in a perfect (not maladaptive) way.35,36 That is, the growth of clonal progenitors can be inhibited by cytokines but only at high doses (in the way normal progenitors respond). In cases in which the entire hematopoietic organ includes progeny of a stem cell corrected in this way, the occurrence of AML/MDS has not been reported. Indeed, in a patient with incomplete mosaicism (a mixture of unfit and fit [reverted] stem cell pools), clonal evolution has occurred in the uncorrected stem cell pool, not the reverted one.37 These clinical observations are consistent with the importance of the selection coefficient in stem cells. Even stronger evidence exists from systematic experimental studies.
PNH
The most common somatic mutation in the context of acquired bone marrow failure is somatic inactivation of the PIG-A gene and while the precise mechanisms involved are not completely known, genome wide transcriptomal surveys of the PIG-A mutant progenitor cells indicate that they are less apoptotic than the non-mutant cells (reviewed in1).
MDS/AML
MDS or AML clones that evolve in the context of aplastic anemia are characterized by non-random chromosomal abnormalities, the most common of which are trisomy 8 and monosomy 7.38 Cells bearing these abnormalities have a demonstrable advantage over the cells not bearing these rearrangements.39,40 We have observed cytokine hypersensitivity in progenitor cells of patients with Fanconi anemia but have found that progenitor cells from their affected siblings who have clonal evolution41 are resistant. That is, FA patients in the aplastic phase have hypersensitive progenitors but clonal progenitors from the same patients studied later during the MDS phase are resistant to precisely the same cytokines (TNFα and IFNγ). In murine models of FA, while stem cells and progenitors are hypersensitive to a variety of cytokines (reviewed in Bagby and Alter2) neoplastic clones are resistant. 11,12,42
Variations on the Selection Theme
The adaptation/selection model of clonal evolution demands that the environmental factor damaging the cells is the one to which the new clone is resistant but it doesn’t mean that the change is sufficient to initiate the entire leukemogenic process. The new clone may also be resistant to other factors as well,43 a phenomenon that would only make the neoplastic clone even more capable of competing against the disadvantaged stem cells. Secondly, somatic changes that lead to clonal evolution may not always be genetic because epigenetic events have been described as factors in the evolution of hematologic neoplasms as well. In fact, genetic loss and epigenetic silencing may cooperate in some instances. For example, in myeloid leukemic clones with allelic losses due to chromosomal deletions, the retained allele can be suppressed epigenetically44 resulting in a functional loss of heterozygosity.
Relevance of the clonal selection/adaptation model to clinical management
Choosing proper therapy
A number of advances in transplantation technology and supportive care have improved the success rates of stem cell transplantation in primary therapy of aplastic anemia.45-47 In addition, the incidence of clonal evolution after immunosuppressive therapy is high5,48 but is lower in patients treated only with matched related donor bone marrow transplantation.5 In addition, in patients treated only with immunosuppressive therapy, more instances of clonal evolution have been found among those who had incomplete remissions and an ongoing requirement for immunosuppression. 49-52 While loss of immune surveillance may play a causal role in clonal evolution in such instances, there is to date no direct experimental evidence in support of this. We believe that the physiological basis for the superiority of stem cell transplantation (at least for patients under 41 years of age53) over immunosuppressive therapy for acquired aplastic anemia is accounted for by the clonal selection model presented in Figures 1.B. and 2. Specifically, stem cell transplantation results not only in the replacement of stressed and depleted stem cells but replaces the offending auxiliary cells (the stem cell suppressors) as well. In patients treated with immunosuppressive therapy this model is also applicable because the seminal difference between a responder and non-responder is that the coefficient of selection has been altered in the former but not the latter. Clearly, for patients with a suitable related donor the evidence-based path is bone marrow transplantation and in such cases it is undesirable to use immunosuppressive therapy first.45
Therapeutic decisions in older patients are more difficult because complications of transplantation are substantial. Such patients should be treated using clinical trials focusing on improving complete response rates for immunotherapy or improving survival in recipients of bone marrow transplantation. For all severe aplastic anemia patients ineligible for stem cell transplantation the goal must be to terminate the immune attack altogether and thereby; (a) normalize hematopoiesis and (b) lower the coefficient of selection for stem cells bearing polymorphisms or mutations that make them much “more fit” than the pressured wild type stem cell population. The cocktail and doses of immunosuppressive agents should be evidence-based and the therapeutic goals should be aggressive. For example, if a patient treated with ATG and cyclosporine A has an improvement in peripheral blood counts sufficient to reduce their risk of intercurrent infections or bleeding but has not demonstrated normalization of blood counts, ongoing or alternative immunosuppressive therapy should be considered. While it is also essential to take care in balancing the risks of ongoing immunosuppression, this more aggressive path has the potential advantages of lowering the incidence of relapse 49-52 and reducing the coefficient of selection for new clones. Therefore, we propose that in patients whose remission is incomplete, hematologists should resist temptations to reduce the conventional doses of immunosuppressive agents used or to terminate immunsuppressive therapy early based on strictly theoretical concerns about potential adverse events associated with immunosuppression.
Conducting surveillance
For some of the inherited aplastic states, general surveillance guidelines are available (e.g. at www.fanconi.org) and while they are helpful, the levels of certainty are not particularly high because they are such rare conditions. For some cytogenetic rearrangements, the best action is to do nothing because they are associated with a phenotype that rarely evolves to hematologic neoplasia (e.g. isochromosome 7q in patients with Shwachman-Diamond syndrome). Other chromosomal abnormalities, like duplications of chromosome 3q in FA patients, are more ominous and predict leukemic transformations so ought to raise considerations of higher risk stem cell transplantation options (reviewed in Guinan 54).
For patients with acquired aplastic anemia, there exist few formal surveillance guidelines. Although transplanted patients and complete responders to immunosuppressive therapy have fewer clonal events49 there is no certain way to identify all patients at risk for clonal disease.48 Therefore, it is our opinion that even stable transfusion-independent patients should be followed at least annually. In the population under surveillance, whether annual bone marrow biopsies provide sufficient information to warrant their use is debatable. Clearly because of technical considerations, bone marrow hypocellularity alone is not a meaningful data point. However, marrow aspirates should be obtained not only as a tool to seek morphological evidence of trilineage dysplasia but as a source of cells for the application of colony forming unit or flow cytometric assays that can be used to identify ongoing evidence of hematopoietic inhibitory T cell activation22,55 and for cytogenetic analysis using conventional metaphase methods and interphase FISH.
If there are signs that clones of potential significance are evolving (e.g. monosomy 7), medical management and the pathways of surveillance must be altered in ways that best fit the medical evidence. For example if a patient with an evolving monosomy 7 clone is being treated with G-CSF along with immunosuppressive therapy, G-CSF should be discontinued and the clone followed with more frequent surveillance.40,56 If this approach doesn’t work, a novel transplantation trial should be considered. On the other hand, patients with trisomy 8 can have a more stable clinical course and high risk procedures may not be warranted on the grounds of the cytogenetic defect per se.48
The future of leukemia prevention research
Basic research
Much scientific and financial energy today focuses, for good reason, on developing targeted therapies for leukemic disorders. Taking into account the recent evidence, reviewed above that new clones arise in the context of an unfit cohort of stem cells, it is a perfect time to apply the same systems, molecular, genetic and chemical biology approaches to the problem of clonal evolution with the goal of preventing MDS and AML in patients at risk. Novel agents could, by reducing apoptotic stresses on hematopoietic stem cell pool, lower the selection coefficient and thereby lower the risk of clonal evolution. While this would require matching the screening assay with the particular type of bone marrow failure syndrome, there is sufficient scientific evidence that a stressed stem cell population is a major factor in the evolution of preleukemic clones to warrant such an approach.
Use of new preclinical models
Reliable murine models now exist and will permit the assessment of new agents. For acquired (autoimmune) aplastic anemia, the infusion of F1 mice with cells from parental lymph nodes results in marrow failure and increases in serum IFNγ. The F1 mice respond favorably to both immunosuppressive therapy and neutralizing antibodies to IFNγ and TNFα. 57 For Fanconi anemia at least two tractable models of clonal evolution exist now. The first is one in which murine Fanconi cells cultured ex vivo then transplanted into radiated recipients are at risk for clonal evolution12 a complication prevented by correcting the FA defect.42 The second model (Figure 3) is one in which clonal evolution can be forced in vitro by exposing Fancc -/- cells to TNFα.11 These models will allow identification of small molecules that normalize responses to stress factors like TNFα. Indeed, candidate molecular targets (e.g. TNFα, IFNγ, reactive oxygen species, p38, and JNK) have already been identified in preclinical models for acquired aplastic anemia57 and Fanconi anemia31. The ultimate goal would be to reduce the impact of unusual environmental stress factors on stem cells without reducing the responses of those cells to normal regulatory factors.
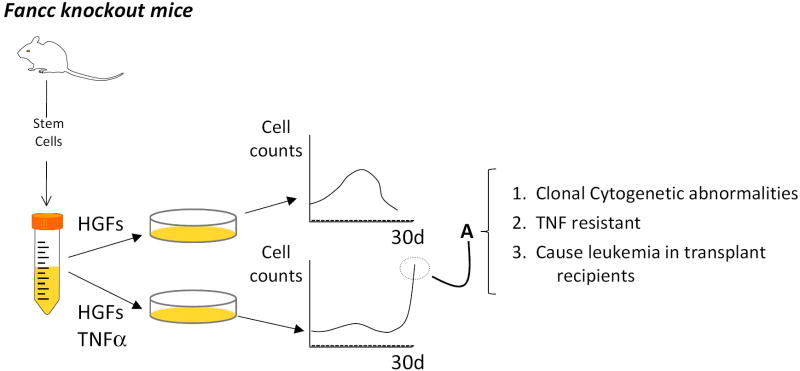
Figure 3.
Clonal evolution is adaptive. Fanconi anemia group C mutant cells are intrinsically hypersensitive to the apoptotic effects of TNFα yet clonally evolved cells are resistant.41,42 Li et al11 tested the hypothesis that TNF exposure would change the fitness landscape significantly enough to permit clonal evolution ex vivo. When mutant Fancc stem cells (Kit+, Lin-, Sca1+) were exposed to a combination of hematopoietic growth factors (HGFs) in suspension culture they expanded logarithmically, then, as differentiated cells accumulated cell counts declined. This proliferative capacity of stem cell progeny in vitro was slightly suppressed in Fancc knockout mice, but the slope of the expansion curve did not differ from that of stem cells from wild type mice. However, when the Fancc mutant cells were exposed to both growth factors and TNFα (to which they are intrinsically hypersensitive), there was little expansion at all until late in culture. At this late time point (A) cells that began to proliferate in the face of ongoing TNFα exposure exhibited clonal cytogenetic abnormalities, were TNF resistant, and, when injected into sublethally radiated mice, gave rise to acute myelogenous leukemia.
Design of clinical prevention trials
Clinical trials designed to increase the number of completely responsive patients are warranted because if the goal is achieved the relative risks of clonal evolution will likely decline. However, it is equally important that long-term interventional trials be developed for patients who have already received immunosuppressive therapy for aplastic anemia. The goal of these studies must focus on the goal of reducing late morbidity and mortality including the relative risk of clonal evolution. In light of the limited sensitivity of FISH and Giemsa banded cytogenetic analyses, more sensitive methods now available for quantifying genetic losses and gains ought to be exploited.58 Identification of potential molecular targets for prevention can also be addressed using systems biology approaches. They would necessarily include attempts to define the emergent phenotypes associated with discrete cytogenetic rearrangements on a genome-wide scale (as has been done with 5q- cells44) and to establish robust target validation analyses for interventional studies using new targeted agents.
Marrow Transplantation
In both acquired and inherited types of aplastic anemia, novel approaches to transplantation ought to be developed because of the clear superiority of that approach in preventing clonal evolution. Key limiting factors for transplantation are lack of perfectly matched donors, age over 40 years, and the high transplant related mortality for recipients of mismatched marrows, so increasing the safety of transplantation in the mismatch setting is an important objective. To date there has been some improvement in outcomes using alternative donors for aplastic anemia. The improvement has evolved for a variety of reasons including: (1) the use of non-TBI or low dose TBI-based conditioning regimens,59-63 (2) the development of high-resolution histocompatibility typing 64,65 and (3) improvements in supportive care.66,67
In the future, for patients ineligible for transplantation three strategic research paths ought to be considered. First, new immunosuppressive agents and combinations should be developed; ones that induce a higher fraction of complete responses. The second investigative opportunity is to identify or develop agents that suppress discrete apoptotic pathways in stem cells ignited by the immune system (or by inherited mutations that reduce their fitness). The third opportunity is to do both things; combine new immunosuppressive agents with agents that lower the coefficient of selection by raising thresholds of adverse stem cell responses to the activated immune system. This approach is no longer simply theoretically appealing. Robust preclinical models now exist11 that could facilitate this kind of approach and will clearly point the way to agents that ought to be tested first.
Modulating responses to TNF
TNFα is a key mediator not only in aplastic anemia but in clonal evolution as described above. This cytokine also plays a seminal role in the pathogenesis of other autoimmune diseases and a number of agents targeting the TNFα pathway have been effective in controlling some of them, including rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease.68-72 The extensive use of these agents in humans to treat multiple disease states and relatively good tolerance of these drugs makes them attractive options for management of the pro-inflammatory state characteristic of aplastic anemia.
While there are little preclinical and clinical data for the use of these agents in the treatment of marrow failure, the evidence supports consideration of these or like agents in the treatment of this disease. In their mouse model of aplastic anemia, Bloom et al describe significant improvement in survival in aplastic mice treated with TNFα blocking antibodies compared to control mice.57 In addition, Dufour et al have recently reported the successful use of etanercept in a child with refractory aplastic anemia.73
More importantly, because TNF is a kind of final common pathway, more work needs to be done to identify the factors that underlie TNF overproduction and, in Fanconi anemia stem cells, the characteristic TNF-hypersensitivity of stem cells. Natural modulators of the TNF-response are being identified regularly and some of them not only function as nodal points that determine whether the TNF-response will be cell survival or death74 but play key roles in stem cell maintenance.75 If dysfunction of such molecules underlies the pathogenesis of some marrow failure states, they may prove to be ideal targets. Therefore, future approaches do need to focus precisely on molecular pathogenesis in individual patients.
Blocking TNF-induced oxidative stress
TNFα stimulation leads to a rapid increase in intracellular levels of ROS, with multiple downstream implications ending in activation of cell death (apoptosis and necrosis) pathways.76-79 and in some instances, antioxidants can protect against TNFα induced cytotoxicity,80 including in hematopoietic cells of Fanconi anemia knockout mice.31 Some anti-oxidants, like N-acetyl cysteine, are safe to use in humans so use of this and other ROS scavengers may find a place in the future management of TNFα-induced marrow injury and subsequent clonal adaptation.
Other cytokine pathways of potential relevance
Additional biologic agents targeting other inflammatory cytokines, in particular IL-1, IL-6, IL-15 and TNF superfamily members, in particular LIGHT, BlyS, APRIL, and RANKL, are currently in clinical trials for the treatment of rheumatoid arthritis and other inflammatory diseases.81 It is fortunate for the bone marrow failure field that the search for additional agents targeting these pathways is brisk.
Summary
From 10 to 20% of acquired aplastic anemia survivors will develop a clonal disease within the decade following their diagnosis as will up to 40% of children and young adults with some of the congenital marrow failure syndromes. A good amount of recent evidence from the disparate fields of genetics, adaptation, stem cell biology, and hematopoiesis leads inescapably to the conclusion that clonal evolution in aplastic states arises in the context of ongoing stem cell damage through a process of clonal selection and adaptation. In the past two years this theoretical paradigm has been validated in clinical and preclinical models robust enough to inform surveillance strategies and reconsider therapeutic objectives in patients with aplastic states and to support the planning and development of rationally designed leukemia prevention trials in patients with bone marrow failure syndromes.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health; 1P01 HL48546 (GB), R01 CA138237-01 (GB) and K23 RR 020043 (GM), the Veterans Affairs Merit Review Program (GB), the Aplastic Anemia and MDS International Foundation (GM), the Children’s Leukemia Research Association Inc.(GM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43:147–156. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leuk Lymphoma. 2004;45:433–440. doi: 10.1080/10428190310001602363. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Bruno B, Saracco P, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. Blood. 2000;95:1931–1934. [PubMed] [Google Scholar]
- 5.Socie G, Henry-Amar M, Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N Engl J Med. 1993;329:1152–1157. doi: 10.1056/NEJM199310143291603. [DOI] [PubMed] [Google Scholar]
- 6.Tichelli A, Gratwohl A, Wursch A, Nissen C, Speck B. Late haematological complications in severe aplastic anaemia. Br J Haematol. 1988;69:413–418. doi: 10.1111/j.1365-2141.1988.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 8.Orr HA. Theories of adaptation: what they do and don’t say. Genetica. 2005;123:3–13. doi: 10.1007/s10709-004-2702-3. [DOI] [PubMed] [Google Scholar]
- 9.Desai MM, Fisher DS, Murray AW. The speed of evolution and maintenance of variation in asexual populations. Curr Biol. 2007;17:385–394. doi: 10.1016/j.cub.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orr HA. The genetic theory of adaptation: a brief history. Nat Rev Genet. 2005;6:119–127. doi: 10.1038/nrg1523. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Sejas DP, Zhang X, et al. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest. 2007;117:3283–3295. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XX, Le Beau MM, Ciccone S, et al. Ex vivo culture of Fancc-/- stem/progenitor cells predisposes cells to undergo apoptosis, and surviving stem/progenitor cells display cytogenetic abnormalities and an increased risk of malignancy. Blood. 2005;105:3465–3471. doi: 10.1182/blood-2004-06-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachman MW, Hoekstra HE, D’Agostino SL. The genetic basis of adaptive melanism in pocket mice. Proc Natl Acad Sci U S A. 2003;100:5268–5273. doi: 10.1073/pnas.0431157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjedov I, Tenaillon O, Gerard B, et al. Stress-induced mutagenesis in bacteria. Science. 2003;300:1404–1409. doi: 10.1126/science.1082240. [DOI] [PubMed] [Google Scholar]
- 15.Foster PL. Stress responses and genetic variation in bacteria. Mutat Res. 2005;569:3–11. doi: 10.1016/j.mrfmmm.2004.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–915. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Gatenby RA. Commentary: carcinogenesis as Darwinian evolution? Do the math! Int J Epidemiol. 2006;35:1165–1167. doi: 10.1093/ije/dyl192. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson IP, Novelli MR, Bodmer WF. The mutation rate and cancer. Proc Natl Acad Sci USA. 1996;93:14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. LANCET. 2004;364:355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 20.Zeng W, Kajigaya S, Chen G, Risitano AM, Nunez O, Young NS. Transcript profile of CD4+ and CD8+ T cells from the bone marrow of acquired aplastic anemia patients. Exp Hematol. 2004;32:806–814. doi: 10.1016/j.exphem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Zeng W, Chen G, Kajigaya S, et al. Gene expression profiling in CD34 cells to identify differences between aplastic anemia patients and healthy volunteers. Blood. 2004;103:325–332. doi: 10.1182/blood-2003-02-0490. [DOI] [PubMed] [Google Scholar]
- 22.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100:1185–1191. doi: 10.1182/blood-2002-01-0035. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Colman SM, Xiao Z, et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci USA. 2002;99:8242–8247. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dufour C, Corcione A, Svahn J, Haupt R, Battilana N, Pistoia V. Interferon gamma and tumour necrosis factor α are overexpressed in bone marrow T lymphocytes from paediatric patients with aplastic anaemia. Br J Haematol. 2001;115:1023–1031. doi: 10.1046/j.1365-2141.2001.03212.x. [DOI] [PubMed] [Google Scholar]
- 25.Dufour C, Corcione A, Svahn J, et al. TNF-{alpha} and IFN-{gamma} are over expressed in the bone marrow of Fanconi anemia patients and TNF-{alpha} suppresses erythropoiesis in vitro. Blood. 2003 doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- 26.Whitney MA, Royle G, Low MJ, et al. Germ cell defects and hematopoietic hypersensitivity to γ-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood. 1996;88:49–58. [PubMed] [Google Scholar]
- 27.Pigullo S, Ferretti E, Lanciotti M, et al. Human Fanconi A cells are susceptible to TRAIL-induced apoptosis. Br J Haematol. 2007;136:315–318. doi: 10.1111/j.1365-2141.2006.06432.x. [DOI] [PubMed] [Google Scholar]
- 28.Haneline LS, Broxmeyer HE, Cooper S, et al. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from FAC -/- mice. Blood. 1998;91:4092–4098. [PubMed] [Google Scholar]
- 29.Zhang XL, Li J, Sejas DP, Rathbun KR, Bagby GC, Pang QS. The Fanconi anemia proteins functionally interact with the protein kinase regulated by RNA (PKR) J Biol Chem. 2004;279:43910–43919. doi: 10.1074/jbc.M403884200. [DOI] [PubMed] [Google Scholar]
- 30.Bijangi-Vishehsaraei K, Saadatzadeh MR, Werne A, et al. Enhanced TNF-α-induced apoptosis in Fanconi anemia type C-deficient cells is dependent on apoptosis signal-regulating kinase 1. Blood. 2005;106:4124–4130. doi: 10.1182/blood-2005-05-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sejas DP, Rani R, Qiu Y, et al. Inflammatory reactive oxygen species-mediated hemopoietic suppression in Fancc-deficient mice. J Immunol. 2007;178:5277–5287. doi: 10.4049/jimmunol.178.8.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dror Y, Freedman MH. Shwachman-Diamond syndrome marrow cells show abnormally increased apoptosis mediated through the Fas pathway. Blood. 2001;97:3011–3016. doi: 10.1182/blood.v97.10.3011. [DOI] [PubMed] [Google Scholar]
- 33.Kollner I, Sodeik B, Schreek S, et al. Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood. 2006;108:493–500. doi: 10.1182/blood-2005-11-4689. [DOI] [PubMed] [Google Scholar]
- 34.Yoon A, Peng G, Brandenburg Y, et al. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 35.Lo Ten Foe JR, Kwee ML, Rooimans MA, et al. Somatic mosaicism in Fanconi anemia: Molecular basis and clinical significance. Eur J Hum Genet. 1997;5:137–148. [PubMed] [Google Scholar]
- 36.Mankad A, Taniguchi T, Cox B, et al. Natural gene therapy in monozygotic twins with Fanconi anemia. Blood. 2006;107:3084–3090. doi: 10.1182/blood-2005-07-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregory JJ, Jr, Wagner JE, Verlander PC, et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc Natl Acad Sci U S A. 2001;98:2532–2537. doi: 10.1073/pnas.051609898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kearns WG, Sutton JF, Maciejewski JP, Young NS, Liu JM. Genomic instability in bone marrow failure syndromes. Am J Hematol. 2004;76:220–224. doi: 10.1002/ajh.20101. [DOI] [PubMed] [Google Scholar]
- 39.Sloand EM, Pfannes L, Chen G, et al. CD34 cells from patients with trisomy 8 myelodysplastic syndrome (MDS) express early apoptotic markers but avoid programmed cell death by up-regulation of antiapoptotic proteins. Blood. 2007;109:2399–2405. doi: 10.1182/blood-2006-01-030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloand EM, Yong AS, Ramkissoon S, et al. Granulocyte colony-stimulating factor preferentially stimulates proliferation of monosomy 7 cells bearing the isoform IV receptor. Proc Natl Acad Sci U S A. 2006;103:14483–14488. doi: 10.1073/pnas.0605245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lensch MW, Rathbun RK, Olson SB, Jones GR, Bagby GC., Jr Selective pressure as an essential force in molecular evolution of myeloid leukemic clones: a view from the window of Fanconi anemia. Leukemia. 1999;13:1784–1789. doi: 10.1038/sj.leu.2401586. [DOI] [PubMed] [Google Scholar]
- 42.Haneline LS, Li X, Ciccone SL, et al. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc -/- hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101:1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- 43.Kamarajan P, Sun NK, Chao CC. Up-regulation of FLIP in cisplatin-selected HeLa cells causes cross-resistance to CD95/Fas death signalling. Biochem J. 2003;376:253–260. doi: 10.1042/BJ20030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu TX, Becker MW, Jelinek J, et al. Chromosome 5q deletion and epigenetic suppression of the gene encoding alpha-catenin (CTNNA1) in myeloid cell transformation. Nat Med. 2007;13:78–83. doi: 10.1038/nm1512. [DOI] [PubMed] [Google Scholar]
- 45.Ades L, Mary JY, Robin M, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103:2490–2497. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 46.Kahl C, Leisenring W, Deeg HJ, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long-term follow-up. Br J Haematol. 2005;130:747–751. doi: 10.1111/j.1365-2141.2005.05667.x. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy-Nasser AA, Leung KS, Mahajan A, et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant. 2006;12:1277–1284. doi: 10.1016/j.bbmt.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129–3135. doi: 10.1182/blood.v99.9.3129. [DOI] [PubMed] [Google Scholar]
- 49.Viollier R, Tichelli A. Predictive factors for cure after immunosuppressive therapy of aplastic anemia. Acta Haematol. 2000;103:55–62. doi: 10.1159/000041005. [DOI] [PubMed] [Google Scholar]
- 50.Kojima S, Ohara A, Tsuchida M, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100:786–790. doi: 10.1182/blood.v100.3.786. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 52.Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long-term observation follow-up. Br J Haematol. 2008;140:197–205. doi: 10.1111/j.1365-2141.2007.06903.x. [DOI] [PubMed] [Google Scholar]
- 53.Marsh J. Making therapeutic decisions in adults with aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:78–85. doi: 10.1182/asheducation-2006.1.78. [DOI] [PubMed] [Google Scholar]
- 54.Guinan EC. Aplastic Anemia: Management of Pediatric Patients. Hematology. 2005;2005:104–109. doi: 10.1182/asheducation-2005.1.104. [DOI] [PubMed] [Google Scholar]
- 55.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamazaki E, Kanamori H, Taguchi J, Harano H, Mohri H, Okubo T. The evidence of clonal evolution with monosomy 7 in aplastic anemia following granulocyte colony-stimulating factor using the polymerase chain reaction. Blood Cells Mol Dis. 1997;23:213–218. doi: 10.1006/bcmd.1997.0138. [DOI] [PubMed] [Google Scholar]
- 57.Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp Hematol. 2004;32:1163–1172. doi: 10.1016/j.exphem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Gondek LP, Tiu R, O’Keefe CL, Sekeres MA, Theil KS, Maciejewski JP. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–1542. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bacigalupo A, Locatelli F, Lanino E, et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36:947–950. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 60.Deeg HJ, Amylon ID, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208–215. doi: 10.1053/bbmt.2001.v7.pm11349807. [DOI] [PubMed] [Google Scholar]
- 61.Deeg HJ, O’Donnell M, Tolar J, et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006;108:1485–1491. doi: 10.1182/blood-2006-03-005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta V, Ball SE, Sage D, et al. Marrow transplants from matched unrelated donors for aplastic anaemia using alemtuzumab, fludarabine and cyclophosphamide based conditioning. Bone Marrow Transplant. 2005;35:467–471. doi: 10.1038/sj.bmt.1704799. [DOI] [PubMed] [Google Scholar]
- 63.Kang HJ, Shin HY, Choi HS, Ahn HS. Fludarabine, cyclophosphamide plus thymoglobulin conditioning regimen for unrelated bone marrow transplantation in severe aplastic anemia. Bone Marrow Transplant. 2004;34:939–943. doi: 10.1038/sj.bmt.1704720. [DOI] [PubMed] [Google Scholar]
- 64.Maury S, Balere-Appert ML, Chir Z, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007;92:589–596. doi: 10.3324/haematol.10899. [DOI] [PubMed] [Google Scholar]
- 65.Viollier R, Socie G, Tichelli A, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41:45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 66.Yagasaki H, Takahashi Y, Kudo K, et al. Feasibility and results of bone marrow transplantation from an HLA-mismatched unrelated donor for children and young adults with acquired severe aplastic anemia. Int J Hematol. 2007;85:437–442. doi: 10.1532/IJH97.06229. [DOI] [PubMed] [Google Scholar]
- 67.Yoshimi A, Kojima S, Taniguchi S, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant. 2008;14:1057–1063. doi: 10.1016/j.bbmt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. LANCET. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 69.Furst DE, Schiff MH, Fleischmann RM, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- 70.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 71.Peyrin-Biroulet L, Deltenre P, de SN, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Saad AA, Symmons DP, Noyce PR, Ashcroft DM. Risks and benefits of tumor necrosis factor-alpha inhibitors in the management of psoriatic arthritis: systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2008;35:883–890. [PubMed] [Google Scholar]
- 73.Dufour C, Giacchino R, Ghezzi P, et al. Etanercept as a salvage treatment for refractory aplastic anemia. Pediatr Blood Cancer. 2008 doi: 10.1002/pbc.21886. [DOI] [PubMed] [Google Scholar]
- 74.Lee HY, Youn SW, Kim JY, et al. FOXO3a turns the tumor necrosis factor receptor signaling towards apoptosis through reciprocal regulation of c-Jun N-terminal kinase and NF-kappaB. Arterioscler Thromb Vasc Biol. 2008;28:112–120. doi: 10.1161/ATVBAHA.107.153304. [DOI] [PubMed] [Google Scholar]
- 75.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Larrick JW, Wright SC. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990;4:3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- 77.Matthews N, Neale ML, Jackson SK, Stark JM. Tumour cell killing by tumour necrosis factor: inhibition by anaerobic conditions, free-radical scavengers and inhibitors of arachidonate metabolism. Immunology. 1987;62:153–155. [PMC free article] [PubMed] [Google Scholar]
- 78.Yamauchi N, Kuriyama H, Watanabe N, Neda H, Maeda M, Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989;49:1671–1675. [PubMed] [Google Scholar]
- 79.Zimmerman RJ, Chan A, Leadon SA. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res. 1989;49:1644–1648. [PubMed] [Google Scholar]
- 80.Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene. 2002;21:3917–3924. doi: 10.1038/sj.onc.1205489. [DOI] [PubMed] [Google Scholar]
- 81.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Sejas DP, Qiu Y, Williams DA, Pang Q. Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence. J Cell Sci. 2007;120:1572–1583. doi: 10.1242/jcs.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]