Abstract
Objective
Interferon alpha (IFN-α) has been implicated in the pathogenesis of juvenile dermatomyositis (JDM). We examined serum IFN-α activity in a cohort of children with JDM to determine relationships between IFN-α and indicators of disease activity and severity.
Methods
39 children with definite/probable JDM were included in the study. Samples were studied from 18 newly diagnosed untreated children, and 11 of these children had a second sample taken at 24 months while they were receiving treatment. 7 of these children also had a third sample available at 36 months, and 21 additional children were studied 36 months after their initial diagnosis. Serum IFN-α was measured using a functional reporter cell assay.
Results
JDM patients had higher serum IFN-α activity than both pediatric and adult healthy controls. In untreated patients, serum IFN-α activity was positively correlated with serum muscle enzymes (p<0.05 for CPK, AST, and aldolase) and inversely correlated with duration of untreated disease (p=0.017). The TNF-α-308A allele was associated with higher serum IFN-α only in untreated patients (p=0.038). At 36 months, serum IFN-α was inversely correlated with muscle enzymes in those patients still requiring therapy, and inversely correlated with skin DAS in those who had completed therapy (p=0.002).
Conclusions
Serum IFN-α activity was associated with higher serum levels of muscle derived enzymes and shorter duration of untreated disease in newly diagnosed patients, and inversely correlated with measures of chronic disease activity at 36 months post-diagnosis. These data suggest that IFN-α could play a role in disease initiation in JDM.
Introduction
Juvenile dermatomyositis (JDM) is a severe multisystem autoimmune disease of childhood which characteristically involves muscle, skin, and vasculature, and frequently results in significant morbidity and pathologic calcification in 15–40% of cases (1). While the pathogenesis of JDM is unknown, genetic factors have been associated with disease susceptibility (2, 3) as well as severity and specific disease manifestations (4). History of an infection, including upper respiratory and/or gastrointestinal complaints, frequently precedes detection of the first definite symptom of JDM, both in Canada (5), and in the US where antibiotics were given to over 64% of cases (6). It is likely that genetic risk factors combine with environmental factors, such as UV light exposure (7) and infectious triggers (6), to result in disease. Current treatment regimens for JDM involve immunosuppressive therapies which confer a significant risk of side effects (1), and improved understanding of the immunopathogenesis of this disease will hopefully drive the development of new therapeutic strategies.
Interferon alpha (IFN-α) is a pleiotropic type I interferon which exerts a number of pro-inflammatory effects upon the immune system, and is classically involved in viral defense (8). Dysregulation of the IFN-α system in autoimmune disease has been observed in a number of autoimmune diseases, including systemic lupus erythematosus (SLE) (9, 10), Sjogren’s syndrome (11), and adult dermatomyositis (12). In SLE and adult dermatomyositis, increased IFN-α signaling has been associated with more severe disease and increased disease activity (12, 13). Additionally, recent work has shown that high serum IFN-α is a heritable risk factor for SLE (14), and a number of SLE genetic risk factors have been shown to influence the high serum IFN-α trait in SLE patients (15, 16). IFN-α dysregulation is implicated in JDM pathogenesis, as microarray studies have shown frequent upregulation of IFN-α-induced mRNA transcripts in muscle biopsies (17) and peripheral blood mononuclear cells (PBMC) from JDM patients (18, 19). Additionally, PBMC from JDM patients show evidence of clonal proliferation, suggesting a response to an antigenic stimulant (20).
In this study, we measure serum IFN-α activity in a cohort of JDM patients at various stages in their disease to determine correlations between clinical parameters and serum IFN-α in the JDM population. Given the associations previously demonstrated between serum IFN-α and autoimmune disease genetic risk factors, we also analyzed serum IFN-α data in the context of the established genetic risk factors for JDM, including the HLA locus (2, 3) and TNF-α-308 promoter polymorphism (4).
Methods
Patients and Clinical Data
Serum samples from 39 unique patients with JDM were studied. 18 patients had samples available at initial diagnosis prior to any treatment, and 11 of these subjects had a follow up sample available at 24 months while they were still receiving treatment. 28 samples were studied from patients who were 36 months from their initial diagnosis, 14 still required treatment and 14 had resolved and were off all treatment. Those patients who were off medications at 36 months stopped their medications at varying times (mean time off therapy = 9.8 months, SD of 7.4 months). Seven of the patients in the 36 month group were also part of the initial untreated group, and thus had samples available from three time points (initial untreated, 24 months, and 36 months). Twenty-nine patients were female and 11 were male. Six patients were of Hispanic ancestry, 1 was of Asian ancestry, and 32 were of European ancestry. Laboratory data were available from the time of serum sampling, including serum creatine kinase (CPK), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), aldolase, and absolute number of circulating natural killer (NK) cells. Muscle enzymes were measured in the clinical laboratory at Children’s Memorial Hospital, and NK cells were sorted and counted in peripheral blood using flow cytometry on a Flow Cytometer FACSCalibur (BD Bioscience, Mountain View, CA) with fluorescent antibodies to CD16/CD56 and CD3 purchased from BD Biosciences. Clinical data were also available, including prospectively obtained disease activity scores (DAS) for muscle, skin, as well as overall DAS (21), and the duration of symptoms (onset of rash and/or weakness) prior to the start of therapy was also recorded for each patient. Subjects were genotyped at the TNF-α-308 promoter polymorphism (rs1800629), and HLA typing for HLA-DQA*0501 and DQA*0301 alleles was performed at Children’s Memorial Hospital using standard techniques. The study was approved by the institutional review boards at both institutions (University of Chicago IRB #15701B, Children’s Memorial Hospital IRB#10778), and age appropriate informed consent was obtained from all subjects.
Measurement of serum IFN-α activity
We use a sensitive and reproducible bioassay to detect serum IFN-α activity as initially described in (22), which was further validated and tested in a large human population in (14). ELISA methods for detection of IFN-α in human serum have been complicated by both low sensitivity and low specificity (23). In this bioassay, reporter cells (WISH cells, ATCC #CCL125) are used to measure the ability of sera to cause IFN-induced gene transcription. The reporter cells are cultured with patient sera for 6 hours and then lysed, and three canonical IFN-α-induced transcripts (IFIT-1, MX-1, and PKR) are measured using rtPCR. Relative expression data from the three transcripts is then normalized using the mean and SD of healthy donor sera (n=141) run in the same assay, and data are presented as an IFN-α activity score. The IFN-induced transcriptional activity in the reporter cells can be blocked with anti-IFN-α monoclonal antibodies, and no significant functional inhibitors have been detected to date (14). For further details regarding the assay, please see references (14, 22).
Statistical Analysis
Serum IFN-α activity data is not normally distributed, so non-parametric Mann-Whitney t-test is used for comparison of serum IFN-α activity between subject groups. R and p values in correlation analyses are generated using a Spearman’s rank order correlation. X–Y plots demonstrating the raw data for each correlation are shown as semi-log plots with the logarithmic IFN-α data on the X-axis and the clinical variable on the linear Y-axis, with a best-fit semi-log correlation line shown. P values shown are uncorrected for multiple comparisons.
Results
Serum IFN-α activity is elevated in many patients with JDM as compared to controls
JDM patients had higher IFN-α activity in our functional assay than both pediatric (n=19) and adult healthy controls (n=129), as shown in Figure 1A. We sought to confirm that this activity observed in the reporter cell assay was in fact due to IFN-α in the sera by performing blocking experiments in a subset of samples (Figure 1B). In this experiment, the IFN-induced gene expression activity measured in the reporter cells was largely but not completely blocked by anti-IFN-α monoclonal antibody (Figure 1B), confirming that IFN-α was the major type I IFN driving the IFN-induced gene expression observed in the reporter cell assay. We also assayed for potential inhibitors of IFN-α in our samples. When recombinant IFN-α was added to a subset of samples which initially did not show any IFN-α activity in the reporter assay, the expected amount of IFN-α-induced gene expression was observed in the reporter cells. This experiment rules out significant common inhibitors to the assay in these samples, such as anti-IFN-α antibodies which have been reported in autoimmune disease patients (24) (Figure 1B). There were no significant differences in serum IFN-α activity by ancestral background or gender of the subjects, and there was no correlation between serum IFN-α and age of disease onset or age at the time the serum sample was obtained.
Figure 1.
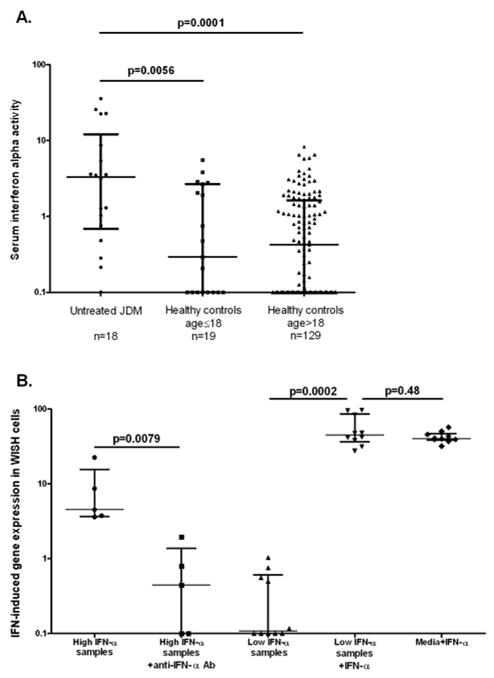
Serum IFN-α activity in JDM patients as compared to controls, and blocking and rule out inhibitor experiments. A. shows serum IFN-α activity in untreated JDM patients (n=18) vs. pediatric healthy controls (n=19) and adult healthy controls (n=129). Each dot represents the IFN-α activity value for one subject (median values: JDM = 3.32, Controls≤18 = 0.29, Controls>18 = 0.43). B. shows blocking experiments in the first two columns, in which samples which had high IFN-α were pre-incubated with anti-IFN-α monoclonal antibody (1ug/mL) and then run in the WISH assay (median values: High IFN-α samples = 4.53, High IFN-α samples + anti-IFN-α = 0.45). Rule out inhibitor experiments are shown in columns 3, 4, and 5 in panel B. Samples which did not show significant IFN-α activity were pre-incubated with recombinant human IFN-α (50U/mL), and then run in the WISH assay. Addition of recombinant IFN-α to the sample resulted in the same IFN-α-induced gene activity in the reporter cells as cells cultured with media and the same amount of IFN-α (median values: Low IFN-α samples = 0.11, Low IFN-α samples + IFN-α = 44.83, Media + IFN-α = 40.25). Lines represent the median and interquartile range, p-values by Mann-Whitney t-test.
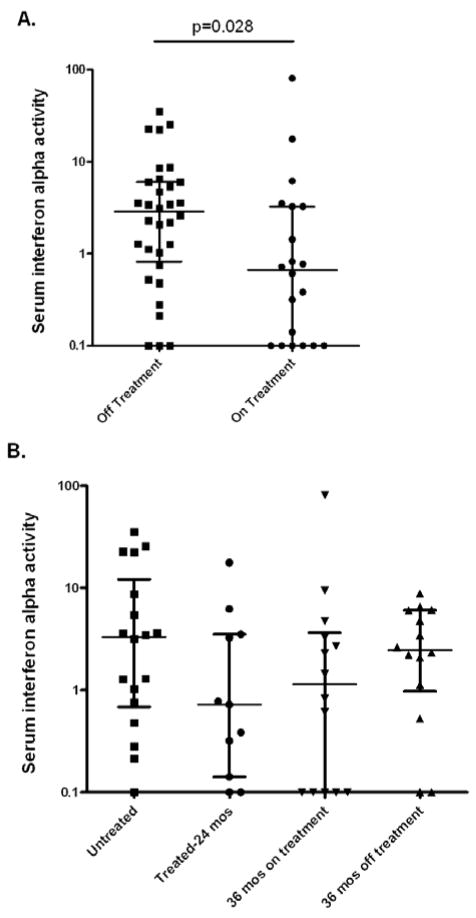
Serum IFN-α activity is higher in subjects who are not receiving therapy
Samples were available from subjects when they were newly diagnosed and untreated, on treatment at 24 months post-diagnosis, or at 36 months post-diagnosis having either completed a course of therapy or still requiring therapy for chronic disease manifestations. As shown in Figure 2A, subjects who were actively receiving treatment had lower serum IFN-α than those who were untreated (p=0.028). When subjects were analyzed by both treatment status and disease duration, newly diagnosed untreated subjects had the highest median serum IFN-α activity, and there was a non-significant trend toward lower serum IFN-α in subjects at 24 months who were on therapy (Figure 2B). At 36 months, there was a non-significant trend toward higher serum IFN-α in those who were off treatment as compared to those who were still receiving treatment (Figure 2B). Interestingly, there were strong but non-significant trends toward higher serum IFN-α in subjects with a family history of SLE and those subjects with a history of antecedent infection preceding the first symptom of JDM (rash or weakness) by 3 months or less (p=0.09 and p=0.12 respectively, data not shown).
Figure 2.
Serum IFN-α activity in JDM patients with different disease duration and treatment status. A. compares serum IFN-α activity in samples from patients who are receiving therapy (either 24 or 36 mos after diagnosis) vs. those who are not receiving therapy (either initial untreated sample or those at 36 months who were off therapy). B. shows serum IFN-α in each separate disease/treatment category. Lines represent the median and interquartile range, all comparisons are non-significant, with p-values >0.25 by Mann-Whitney t-test.
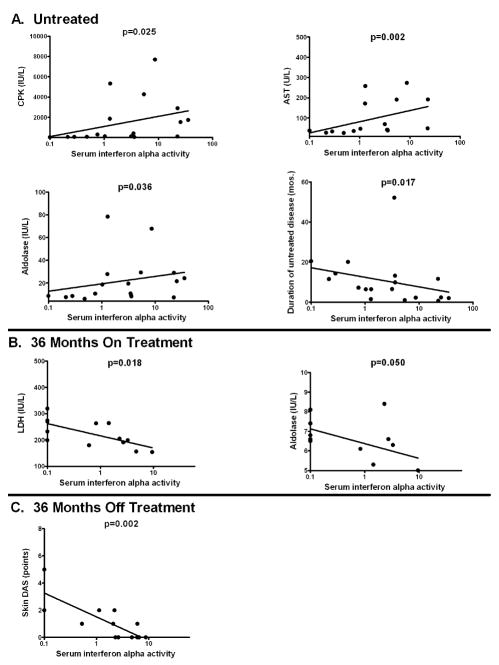
Serum IFN-α activity is correlated with serum muscle enzyme levels in untreated patients
We next analyzed serum IFN-α in relation to a number of quantitative clinical variables using correlation analysis (Table 1). In the untreated patients, serum IFN-α was positively correlated with serum muscle enzymes (p<0.05 for CPK, AST, and aldolase), and was inversely correlated with duration of untreated disease (p=0.017). Patients who were on treatment at 24 months showed no correlations between serum IFN-α and other parameters. Those subjects who required continuing therapy beyond 36 months showed an inverse correlation between serum aldolase and serum LDH and serum IFN-α activity, for those subjects with higher muscle enzymes had lower serum IFN-α. In patients who had completed therapy by 36 months, there was a strong inverse correlation between disease activity score in the skin (skin DAS) and serum IFN-α (p=0.002), and those subjects with persistent skin involvement had lower IFN-α. The inverse correlation between serum IFN-α and total DAS score in these subjects was due to the skin DAS score reflecting persistent skin involvement, as muscle DAS score did not show any correlation with serum IFN-α in this patient group and the total DAS is the sum of the skin and muscle DAS scores. X–Y plots showing the relationships in the raw data for those variables that were significantly correlated with serum IFN-α are shown in Figure 3.
Table 1.
Correlation between serum IFN-α activity and clinical and biologic measures of disease activity and severity
| CPK | AST | LDH | Aldo. | NK Abs | DAS SKIN | DAS WEAK | DAS | DUD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial Untreat. n=18 | Rho | 0.525* | 0.705* | 0.395 | 0.447* | −0.292 | −0.205 | 0.011 | −0.043 | −0.556# |
| P value | 0.025* | 0.002 * | 0.117 | 0.036* | 0.256 | 0.415 | 0.964 | 0.864 | 0.017# | |
| Treated at 24 mos n=11 | Rho | −0.159 | −0.068 | −0.342 | −0.280 | −0.232 | −0.225 | 0.338 | −0.216 | 0.014 |
| P value | 0.662 | 0.863 | 0.334 | 0.434 | 0.492 | 0.506 | 0.310 | 0.523 | 0.968 | |
| On therapy at 36 mos n=14 | Rho | −0.121 | −0.460 | −0.639# | −0.575# | 0.278 | −0.103 | −0.074 | −0.189 | −0.156 |
| P value | 0.692 | 0.114 | 0.018# | 0.050# | 0.380 | 0.725 | 0.801 | 0.516 | 0.592 | |
| Off therapy at 36 mos n=14 | Rho | −0.412 | −0.152 | 0.036 | −0.124 | 0.046 | −0.781# | 0.087 | −0.614# | −0.323 |
| P value | 0.162 | 0.619 | 0.908 | 0.687 | 0.876 | 0.002# | 0.777 | 0.026# | 0.260 |
indicates significant positive correlations, and
indicates significant inverse correlations. Treatment status/disease duration groups are indicated at the left. Rho = Spearman’s rho value, with p-value derived Spearman’s correlation. CPK = creatine phospokinase, AST = aspartate aminotransferase, LDH = lactate dehydrogenase, Aldo = aldolase, NK Abs = absolute natural killer cell count, DAS Skin = disease activity score in the skin, DAS Weak = disease activity score in the muscle, DAS = total disease activity score, DUD = duration of untreated disease
Figure 3.
Correlation plots showing significant relationships between serum IFN-α activity and clinical variables. A. shows correlation plots for untreated patients between serum IFN-α activity and CPK, AST, aldolase, and duration of untreated disease respectively. B. shows correlation plots for patients still receiving treatment at 36 months between serum IFN-α activity, aldolase and LDH. C. shows correlation plot for patients who were off treatment at 36 months between serum IFN-α activity and skin DAS. Plots are in semi-log format, Y-axis is linear and X-axis is log, best fit semi-log line is shown on each plot.
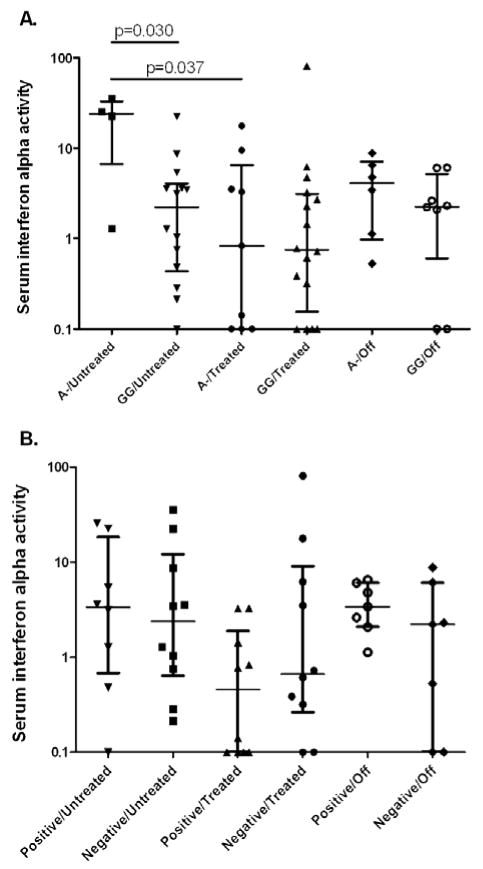
Serum IFN-α activity is higher in untreated subjects with the TNF-α-308 A allele
When subjects were stratified by treatment status and TNF-α-308 genotype, untreated subjects carrying the minor A allele had higher serum IFN-α activity than those with GG genotypes as shown in Figure 4A (p=0.038). There was a similar trend in the subjects who were off treatment at 36 months, which was not statistically significant. These subjects had been off therapy for varying periods of time, and this heterogeneity could explain why we do not see a significant effect of the TNF-α-308 genotype in this group. We also examined genotype at the HLA locus DQA*0501, however there was no difference in serum IFN-α by HLA DQA*0501 genotype, as shown in Figure 4B. Similarly, HLA DQA*0301 genotype did not show any significant relationship to serum IFN-α activity, and HLA DRB*0301 was typed in 20 of the 39 subjects and only 2 of the 20 were risk allele carriers (data not shown). Thus, while the TNF gene is contained within the HLA locus and potential for long-range linkage exists, it appears that the association of the TNF-α-308 A allele with higher IFN-α in untreated patients is largely separable from DQA*0501 and DQA*0301 in this cohort.
Figure 4.
Serum IFN-α activity in JDM patients stratified by disease duration/treatment status and TNF-α and HLA*DQA 0501 genotypes. A. shows serum IFN-α activity in JDM patients stratified by disease duration/treatment status and TNF-α-308 promoter polymorphism genotype. A- = AA or GA genotype, A is the risk allele. B. shows serum IFN-α activity in JDM patients stratified by disease duration/treatment status and HLA DQA*0501 genotype. Positive indicates a subject carrying the risk allele of HLA DQA*0501, and negative indicates a non-risk allele genotype. 24 month on treatment and 36 month on treatment groups are combined into one group for both of these analyses, and the off treatment group consists of subjects who are 36 months from initial diagnosis and able to successfully discontinue therapy. Lines represent the median and interquartile range, p-values by Mann-Whitney t-test.
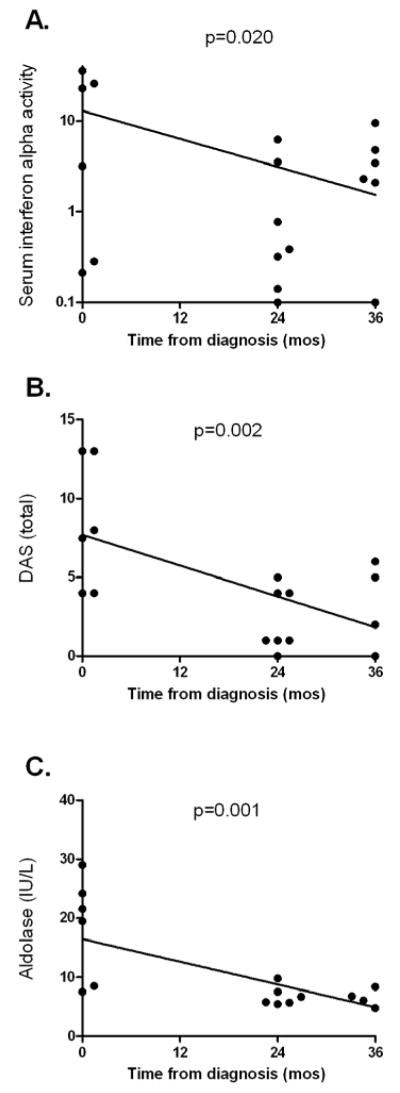
Longitudinal data in subjects with 0, 24, and 36 month samples available
Seven subjects had samples available in the initial untreated stage, 24 months post diagnosis while on treatment, and at 36 months (5 still on therapy, 2 off therapy). Longitudinal data for each individual is shown in Figure 5. Serum IFN-α activity decreased as time from diagnosis increased in these 7 subjects (p=0.020), although many of these seven patients still had detectable IFN-α activity in our assay at 36 months. Total DAS and muscle enzymes showed a strong inverse correlation with time from diagnosis, likely reflecting effective treatment during this period. Although the number of subjects in this analysis was limited, the longitudinal trends in these 7 individuals with data from multiple time points support our cross-sectional analysis indicating that serum IFN-α and muscle enzymes are correlated and elevated early in the disease course in JDM.
Figure 5.
Longitudinal data for serum IFN-α, total DAS, and aldolase in children with JDM who had sera available at all time points. Serum IFN-α, total DAS, and aldolase (A, B, and C respectively) are plotted against time in months from diagnosis in seven patients with samples available at these time points. Regression lines and analyses are performed with semi-log (A, log distributed IFN-α data on Y-axis, linear time on X-axis) or linear (B. and C.) models, with p-values calculated from Spearman’s rho.
Discussion
We show that serum IFN-α activity is significantly elevated in many children with JDM as compared to healthy adult and pediatric donors. These high circulating levels of IFN-α are similar to those observed in other autoimmune diseases such as SLE and Sjogren’s syndrome (14, 25). Previous studies have documented up-regulation IFN-α-induced transcripts in muscle biopsies (17) and PBMC (18, 19) from JDM patients, and the “interferon signature” observed in about 30% of JDM PBMC is similar to that seen in children with SLE (26). Our observation of high serum IFN-α activity in JDM could reflect IFN-α production in target tissues in which there is an active inflammatory response. In other autoimmune diseases characterized by high serum IFN-α, there is similar evidence for IFN-α production in affected tissues, including increased IFN-α-induced gene transcription in salivary glands of Sjogren’s syndrome patients (11), and high levels of IFN-α-induced gene expression in SLE skin biopsies (27).
Serum IFN-α activity was correlated with a number of important biological and clinical measures of disease activity in our JDM population, suggesting that IFN-α is intimately involved in JDM pathogenesis. The correlation between serum IFN-α and muscle inflammation is supported by the very high level of IFN-a gene expression in JDM muscle biopsies (17, 28). Additionally, gene expression studies in JDM PBMC demonstrate up-regulation of the IFN-α-induced transcript MXA, which was correlated with muscle involvement but not skin involvement at both diagnosis and after therapy (18). In untreated patients, serum IFN-α activity was highest in those patients with a short duration of untreated disease, suggesting that IFN-α may be important in the early phase of disease. This is highly interesting given the data implicating microbial infections in the onset of JDM (5, 6). There is no consistent evidence to support ongoing viral or bacterial infection in JDM (29, 30) despite serological data, including case control studies, which implicate viral antigens (31–33). It is possible that a microbial infection could trigger high IFN-α production, which is then not down-regulated as would normally be expected following clearance of the infection by the host. We did see a trend toward higher serum IFN-α in the 43% of subjects who had a history of infection preceding the onset of JDM symptoms by 3 months or less in our cohort (p=0.12, data not shown). Infection with group A beta hemolytic strep can reactivate myositis in a child with quiescent symptoms (34). Additionally, molecular dissection has identified an HLA-DNA binding region of the Streptococcal M5 protein (amino acids 367–375), homologous with smooth muscle heavy chain myosin (amino acids 114–122), which elicited both cytotoxic responses and proliferation in peripheral blood from children with active symptoms of JDM, but not other rheumatic diseases (35).
At 24 months, there was a non-significant trend toward lower serum IFN-α activity, which is likely at least partially due to treatment. Subjects at 36 months who were still on treatment had similar IFN-α activity to those at 24 months on treatment, while there was some evidence for a “rebound” in serum IFN-α activity at 36 months in those who have discontinued treatment, suggesting either continued immune system stimulation or a higher level of constitutive IFN-α expression in those children. Treatment normalized many other parameters as well, and no correlation was seen between IFN-α and any other variable in subjects who were on treatment 24 months post-diagnosis. Interestingly, there was a strong trend toward higher serum IFN-α in untreated subjects with a family history of lupus (p=0.09, data not shown), and we have previously provided evidence supporting both heritability (14) and genetic influence (15, 16) on serum IFN-α activity in lupus patients, supporting the concept of intrinsic or constitutive differences in serum IFN-α between individuals.
At 36 months, we saw a number of inverse correlations between serum IFN-α activity and clinical variables. In subjects who were still on treatment at 36 months, serum IFN-α and muscle enzymes were inversely correlated, which is opposite to the relationship observed in untreated patients. While muscle enzymes were lower in general at 36 months than in untreated patients, this result may indicate that those with chronic low grade muscle inflammation have lower serum IFN-α. In patients who had discontinued treatment at 36 months, there was a strong inverse correlation between serum IFN-α and skin DAS. Those subjects with evidence of disease activity in the skin at 36 months off therapy had lower serum IFN-α activity. These inverse correlations between serum IFN-α at 36 months may represent disease progression or evolution. A previous study has identified significant variation in gene expression profiles in muscle biopsies from JDM patients in subjects with greater 2 months of symptoms compared to those with less than 2 months of symptoms (28), supporting the idea of an evolving immune response over time. IFN-α may be more important in the initiation of disease, while other factors which are inversely correlated with IFN-α may play a more important role in the later phase of disease. TNF-α has been implicated in some of the chronic manifestations of JDM, such as pathologic calcifications and duration of disease activity (4). There is evidence for in vivo cross-regulation of IFN-α and TNF-α in Sjogren’s syndrome (36), SLE (16), and adult dermatomyositis (37), and in vitro experiments support the idea that high levels of TNF-α can inhibit IFN-α production (38). If TNF-α is more important to chronic disease manifestations, then it is possible that cross-regulation of IFN-α and TNF-α may result in the inverse correlations we observe at 36 months.
The finding of higher serum IFN-α activity in untreated carriers of the TNF-α-308 A allele is curious, however, as 50% of the carriers of this allele would be expected to have increased TNF-α production (4). This may relate to differences in cytokine regulation in early vs. late disease, as this association between the TNF-α genotype and higher serum IFN-α is only seen in initial untreated subjects. Indeed, most studies in JDM strongly support an evolution of the immunopathogenesis of the disease over time (28, 39), and it is likely that our findings in this study reflect some of these longitudinal changes. The relationships we demonstrate strongly support a role for IFN-α in JDM disease pathogenesis, and suggest that measurement of serum IFN-α may provide useful diagnostic and prognostic information. Serum IFN-α activity was most strongly associated with early events in the disease, potentially implicating IFN-α in disease initiation.
Acknowledgments
Grant Support and Disclosures: TB Niewold - NIH CTSA K12 Scholar Award RR025000-02, NIAID Clinical Research Loan Repayment AI071651, Arthritis Foundation PostDoctoral Fellowship Award, Arthritis National Research Foundation Scholar Award; SN Kariuki none; G Morgan - nothing to disclose; S Shrestha - nothing to disclose; LM Pachman - NIAMS RO-1 AR48289, The CureJM Foundation, and Macie’s Miracle Foundation.
References
- 1.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371(9631):2201–12. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 2.Reed AM, Pachman L, Ober C. Molecular genetic studies of major histocompatibility complex genes in children with juvenile dermatomyositis: increased risk associated with HLA-DQA1 *0501. Hum Immunol. 1991;32(4):235–40. doi: 10.1016/0198-8859(91)90085-n. [DOI] [PubMed] [Google Scholar]
- 3.Mamyrova G, O’Hanlon TP, Monroe JB, Carrick DM, Malley JD, Adams S, et al. Immunogenetic risk and protective factors for juvenile dermatomyositis in Caucasians. Arthritis Rheum. 2006;54(12):3979–87. doi: 10.1002/art.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43(10):2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Manlhiot C, Liang L, Tran D, Bitnun A, Tyrrell PN, Feldman BM. Assessment of an infectious disease history preceding juvenile dermatomyositis symptom onset. Rheumatology (Oxford) 2008;47(4):526–9. doi: 10.1093/rheumatology/ken038. [DOI] [PubMed] [Google Scholar]
- 6.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53(2):166–72. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 7.Reefman E, Kuiper H, Limburg PC, Kallenberg CG, Bijl M. Type I interferons are involved in the development of UVB-induced inflammatory skin lesions in Systemic Lupus Erythematosus (SLE) patients. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.070359. [DOI] [PubMed] [Google Scholar]
- 8.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8(6):907–22. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 9.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 10.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25(4):401–6. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 11.Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, et al. Activation of the type I interferon system in primary Sjogren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52(4):1185–95. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 12.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56(11):3784–92. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 14.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58(8):2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kariuki SN, Crow MK, Niewold TB. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activityand low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum. 2008;58(9):2818–2823. doi: 10.1002/art.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168(8):4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor KA, Abbott KA, Sabin B, Kuroda M, Pachman LM. MxA gene expression in juvenile dermatomyositis peripheral blood mononuclear cells: association with muscle involvement. Clin Immunol. 2006;120(3):319–25. doi: 10.1016/j.clim.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baechler EC, Bauer JW, Slattery CA, Ortmann WA, Espe KJ, Novitzke J, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med. 2007;13(1–2):59–68. doi: 10.2119/2006-00085.Baechler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abramson LS, Albertini RJ, Pachman LM, Finette BA. Association among somatic HPRT mutant frequency, peripheral blood T-lymphocyte clonality, and serologic parameters of disease activity in children with juvenile onset dermatomyositis. Clin Immunol. 1999;91(1):61–7. doi: 10.1006/clim.1998.4675. [DOI] [PubMed] [Google Scholar]
- 21.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49(1):7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 22.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–16. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 23.Jabs WJ, Hennig C, Zawatzky R, Kirchner H. Failure to detect antiviral activity in serum and plasma of healthy individuals displaying high activity in ELISA for IFN-alpha and IFN-beta. J Interferon Cytokine Res. 1999;19(5):463–9. doi: 10.1089/107999099313901. [DOI] [PubMed] [Google Scholar]
- 24.Suit BE, Axelrod D, Moutsopoulos HM, Decker JL, Hooks JJ. Detection of anti-interferon antibodies in systemic lupus erythematosus. Clin Exp Rheumatol. 1983;1(2):133–5. [PubMed] [Google Scholar]
- 25.Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum. 2008;58(2):541–6. doi: 10.1002/art.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual V, Patel P, McVicker V, Abbott K, Gurhsahaney A, Pachman LM. Peripheral Blood Mononuclear Cell Gene Expression Profiles in Children with Juvenile Dermatomyositis/Polymyositis (JDM/JPM) Share Type-1 Interferon (IFN) Signatures with Systemic Lupus Erythematosus (SLE) but are Distinct. Arthritis Rheum. 2006;54(9 supple):S695–6. [Google Scholar]
- 27.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159(1):237–43. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YW, Shi R, Geraci N, Shrestha S, Gordish-Dressman H, Pachman LM. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunol. 2008;9:43. doi: 10.1186/1471-2172-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pachman LM, Litt DL, Rowley AH, Hayford JR, Caliendo J, Heller S, et al. Lack of detection of enteroviral RNA or bacterial DNA in magnetic resonance imaging-directed muscle biopsies from twenty children with active untreated juvenile dermatomyositis. Arthritis Rheum. 1995;38(10):1513–8. doi: 10.1002/art.1780381019. [DOI] [PubMed] [Google Scholar]
- 30.Bowles NE, Dubowitz V, Sewry CA, Archard LC. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987;1(8540):1004–7. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- 31.Koch M, Brody JA, Nemo GJ, Sever JL. Antibody levels to parainfluenza, rubella, measles, and influenza A virus in children with polymyositis. Arthritis Rheum. 1975;18(4):353–5. doi: 10.1002/art.1780180410. [DOI] [PubMed] [Google Scholar]
- 32.Christensen ML, Pachman LM, Schneiderman R, Patel DC, Friedman JM. Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986;29(11):1365–70. doi: 10.1002/art.1780291109. [DOI] [PubMed] [Google Scholar]
- 33.Pachman LM, Hayford JR, Hochberg MC, Pallansch MA, Chung A, Daugherty CD, et al. New-onset juvenile dermatomyositis: comparisons with a healthy cohort and children with juvenile rheumatoid arthritis. Arthritis Rheum. 1997;40(8):1526–33. doi: 10.1002/art.1780400822. [DOI] [PubMed] [Google Scholar]
- 34.Martini A, Ravelli A, Albani S, Viola S, Scotta MS, Magrini U, et al. Recurrent juvenile dermatomyositis and cutaneous necrotizing arteritis with molecular mimicry between streptococcal type 5 M protein and human skeletal myosin. J Pediatr. 1992;121(5 Pt 1):739–42. doi: 10.1016/s0022-3476(05)81905-5. [DOI] [PubMed] [Google Scholar]
- 35.Massa M, Costouros N, Mazzoli F, De Benedetti F, La Cava A, Le T, et al. Self epitopes shared between human skeletal myosin and Streptococcus pyogenes M5 protein are targets of immune responses in active juvenile dermatomyositis. Arthritis Rheum. 2002;46(11):3015–25. doi: 10.1002/art.10566. [DOI] [PubMed] [Google Scholar]
- 36.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren’s syndrome treated with etanercept. Arthritis Rheum. 2007;56(12):3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dastmalchi M, Grundtman C, Alexanderson H, Mavragani CP, Einarsdottir H, Barbasso Helmers S, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- 38.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102(9):3372–7. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58(2):571–6. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]