Abstract
Objective
Both obesity and muscle impairment are increasingly prevalent among older persons and negatively affect health and physical functioning. However, the combined effect of coexisting obesity and muscle impairment on physical function decline has been little studied. We examined whether obese persons with low muscle strength experience significantly greater declines in walking speed and mobility than persons with only obesity or low muscle strength.
Design
Community-dwelling adults aged ≥65 years (n = 930) living in the Chianti geographic area (Tuscany, Italy) were followed for 6 years in the population-based InCHIANTI study.
Measurements
On the basis of baseline measurements (1998−2000), obesity was defined as body mass index (BMI) ≥30 kg/m2 and low muscle strength as lowest sex-specific tertile of knee extensor strength. Walking speed and self-reported mobility disability (ability to walk 400 m or climb one flight of stairs) were assessed at baseline and at 3- and 6-year follow-up.
Results
At baseline, obese persons with low muscle strength had significantly lower walking speed compared with all other groups (P≤0.05). In longitudinal analyses, obese participants with low muscle strength had steeper decline in walking speed and high risk of developing new mobility disability over the 6-year follow-up compared with those without obesity or low muscle strength. After the age of 80, the differences between groups were substantially attenuated. The differences seen in walking speed across combination of low muscle strength and obesity groups were partly explained by 6-year changes in muscle strength, BMI and waist circumference.
Conclusions
Obesity combined with low muscle strength increases the risk of decline in walking speed and developing mobility disability, especially among persons <80 years old.
Keywords: aged, disability, longitudinal studies, muscle strength, physical function
Introduction
Over the last decades, we have witnessed a marked increase in the prevalence of overweight and obesity among younger and middle-aged persons living in western countries. Given the current demographic trends, it is expected that the number of obese older persons will increase dramatically in the years to come.1 Obesity is a risk factor for chronic diseases, including type 2 diabetes, hypertension, coronary heart disease and osteoarthritis.2
Obesity in older persons negatively affects physical functioning,3–6 particularly when it is accompanied by low muscle strength. Combination of obesity and muscle impairment has been termed as ‘sarcopenic obesity’. Poor muscle strength relative to excessively high body mass impedes the weight-bearing activities that are vital for maintaining independence in daily life. In spite of this, there are only few studies concerning the combined effect of obesity and muscle impairment on older persons’ physical functioning, disability or mortality, especially in a longitudinal perspective.7–10
On the basis of a 6-year follow-up study of community-dwelling older Italians, we examined the combined effect of low muscle strength and obesity on the rate of decline of walking speed and on the risk of developing new mobility disability. We hypothesized that obese persons with low muscle strength would experience a significantly greater decline in walking speed and would be more likely to develop mobility disability than persons with only obesity or low muscle strength. Finally, we tested the hypothesis that differences in mobility decline would be explained by different rates of change in measures of obesity and muscle strength.
Methods
Study design and participants
InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) is an epidemiological study of factors contributing to loss of mobility in late life carried out in two Italian towns located in the Chianti geographic area. The design of the study and data collection methods have been described earlier in detail.11 The study population consisted of a random sample of 1260 persons aged ≥65 years selected from the population registries of two municipalities. 1155 older adults agreed to participate in the study (participation rate 91.7%) and 930 had information regarding muscle strength, anthropometrics and walking speed at baseline. Of these, 204 did not have either 3- or 6-year follow-up data: 110 (of which 63 were ≥80 years) died, 87 (29 ≥80) refused or were unable to participate in the study, and seven (2 ≥80) moved from the area during the 6-year follow-up. The baseline data were collected in 1998−2000, the 3-year follow-up took place in 2001−2003 and the 6-year follow-up in 2004−2006.
Participants received an extensive description of the study and participated after providing written informed consent. The Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol, which complied with the principles stated in the Declaration of Helsinki.
Measurements of walking speed and mobility disability
Mobility was assessed using identical methods at baseline and at the 3- and 6-year follow-ups using performance-based tests and self-reported information.11 To measure walking speed, participants were asked to walk 4 m at their usual pace, as if they were walking down the street, starting from a standing position. Use of a cane or walker was permitted. Walking speed is a valid and generally used measure of mobility limitation for both healthy and impaired older persons12 with high predictive validity for subsequent disability, hospitalization and mortality.13,14
As a part of the interview, participants were asked whether they had any difficulties in walking 400 m or climbing a flight of stairs, and the responses were coded as (i) no difficulty, (ii) with difficulty, but without help, (iii) with some help from another person and (iv) unable to carry out the activity. Mobility-related disability was defined as need for help or inability to walk 400 m or to climb a flight of stairs.12 Self-reported mobility disability predicts future disability, nursing home admission and mortality.15,16 Participants with mobility disability at baseline were excluded from prospective analyses.
Measurement of obesity
Body Mass Index (BMI, kg/m2) was calculated using objectively measured height and weight. Weight was measured to the nearest 0.1 kg using a high-precision mechanical scale and standing height to the nearest 0.1 cm with a wall measure with participants wearing light indoor clothes and no shoes. Obesity was defined as BMI ≥30 kg/m2.2 Underweight (BMI <18.5 kg/m2) participants were excluded from the analyses (n = 4). Waist circumference was measured to the nearest 0.5 cm by using a non-elastic plastic tape, with the participant standing upright, at the midpoint between the lower rib margin and the iliac crest. The BMI and waist circumference were measured using similar procedures at baseline and at the 3- and 6-year follow-ups.
Measurement of muscle strength
Maximal voluntary isometric strength of knee extensors was measured using a hand-held dynamometer (Nicholas Muscle Tester; Sammon Preston Inc., Chicago, IL, USA) according to a standard assessment protocol that has been proven to be highly reliable (test–retest reliability 0.85, inter-rater reliability 0.74).17 The test was repeated thrice and the best result of either side was used in the analyses. Strength was measured in kilograms (kg) and the lowest sex-specific tertile was used as a marker of low muscle strength. The cutoff points for sex-specific strength tertiles were 17.1 and 21.5 kg (range 3.9−41.7 kg) in men, and 11.3 and 14.3 kg (range 3.5−31.2 kg) in women.
We used baseline BMI and knee extensor strength measurements to categorize persons into four groups, namely (i) neither low muscle strength nor obesity, (ii) low muscle strength, but no obesity, (iii) obesity, but no low muscle strength, (iv) low muscle strength and obesity.
Covariates
Sociodemographic information obtained during the structured interview included age, sex and education (years). Smoking was assessed by self-report, and on the basis of the answers, participants were categorized into never smokers, former smokers and current smokers (smoking within 3 years of interview). The level of physical activity in the 12 months before the interview was assessed through an modified standard interview-administered questionnaire18 and was coded as sedentary (inactivity or light-intensity activity <1 h per week), light physical activity (light-intensity activity 2−4 h per week) and moderate-high physical activity (light-intensity activity at least 5 h per week or moderate activity at least 1−2 h per week). Diseases were ascertained by a trained geriatrician according to standard, pre-established criteria and algorithms that combine information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations and blood tests.19 The following five chronic conditions were used in the analyses: hypertension, angina pectoris, chronic heart failure, diabetes, lung disease (including asthma, chronic bronchitis/emphysema) and hip osteoarthritis.
Pro-inflammatory state is associated with both low muscle strength and obesity, and was therefore controlled in this study as confounding factor.20,21 Blood samples were collected in the morning after 12 h fast. Serum and plasma were stored in a deep freezer at −80°C. Serum interleukin (IL)-6 was determined by high-sensitivity enzyme-linked immunosorbent assay (Human Ultrasensitive, Biosource International Inc., Camarillo, CA, USA) and high-sensitivity C-reactive protein by using an enzyme-linked immunosorbent assay and a colorimetric competitive immunoassay (Roche Diagnostics, GmbH, Mannheim, Germany). The lowest detectable concentration was 0.10 pg ml−1 for IL-6 and 0.3 μg ml−1 for C-reactive protein. Inter-assay coefficients of variations were <9% for all tests.
Statistical analysis
Baseline characteristics according to different combination of low muscle strength and obesity are reported as mean values (standard deviation), medians (inter-quartile range, Q1–Q3) and proportions. Generalized linear models were used to calculate age and sex-adjusted differences across these four categories in baseline characteristics, as well as in change in BMI, waist circumference and muscle strength.
Age-associated longitudinal trajectories of walking speed were examined across combination of low muscle strength and obesity groups using linear mixed-effect regression models.22 Our models decomposed the longitudinal information into between-participant effects (comparing walking speeds between persons who are at different ages) and within-participant effects (comparing walking speeds within the same person as they get older). This method allowed us to examine important differences in the effect of obesity combined with low muscle strength on walking speed decline among young-old person versus oldest-old person.
Logistic regression analysis was used to calculate the incident risk of developing mobility disability. To examine the longitudinal prevalence of mobility disability across the combination of low strength and obesity groups, we used logistic generalized linear mixed models.23 As in the models for walking speed, we decomposed the age effects into between- and within-participant contributions. A short statistical appendix is included detailing our models for interested readers.
All variables in Table 1 that were consistently associated with combination of low muscle strength and obesity groups were included in the multivariate models. As IL-6 and C-reactive protein distributions were highly skewed, log-transformed values and ranked scores were used in the analyses. The SAS 9.1 Statistical Package was used for all analyses (SAS Institute Inc., Cary, NC, USA).
Table 1.
Baseline characteristics of the study population according to combination of low muscle strength and obesity groups
| Overall (n = 930) | Neither low strength nor obesity (n = 458) | Low strength (n = 239) | Obesity (n = 162) | Low strength and obesity (n = 71) | Pa | |
|---|---|---|---|---|---|---|
| Measures of mobility | ||||||
| Usual walking speed, ms−1; mean (s.d.) | 1.04 (0.29) | 1.14 (0.24)* | 0.93 (0.29)* | 1.08 (0.22)* | 0.86 (0.27) | <0.0001 |
| Mobility disabilityb, n (%) | 50 (5.4) | 9 (2.0)* | 24 (10.0) | 7 (4.3)* | 10 (14.1) | 0.021 |
| Muscle strength and obesity | ||||||
| Body mass index, kg/m2; mean (s.d.) | 27.5 (4.0) | 26.2 (2.5)* | 25.0 (2.6)* | 32.6 (2.2) | 33.0 (3.2) | <0.0001 |
| Waist circumference (male), cm; mean (s.d.) | 93.9 (6.8) | 93.9 (6.8)* | 90.5 (7.8)* | 106 (7.1) | 105 (6.7) | <0.0001 |
| Waist circumference (female), cm; mean (s.d.) | 90.7 (10.7) | 87.2 (8.2)* | 84.9 (9.3)* | 100.9 (7.7) | 101.1 (8.7) | <0.0001 |
| Knee extensor strength (male), kg; mean (s.d.) | 19.6 (6.1) | 22.4 (4.9)* | 12.9 (2.9) | 23.5 (4.1)* | 14.1 (2.3) | <0.0001 |
| Knee extensor strength (female), kg; mean (s.d.) | 13.2 (4.2) | 15.0 (3.5)* | 8.9 (1.7) | 15.7 (3.5)* | 9.3 (1.6) | <0.0001 |
| Potential confounders | ||||||
| Age, years; mean (s.d.) | 74.1 (6.8) | 72.5 (6.0)* | 77.7 (7.4) | 71.9 (5.2)* | 77.3 (6.6) | <0.0001 |
| Sex (female), n (%) | 513 (55.2) | 241 (52.6) | 126 (52.7) | 100 (61.7) | 46 (64.8) | 0.037 |
| Education, years; mean (s.d.) | 5.5 (3.3) | 5.8 (3.2)* | 5.2 (3.3) | 5.6 (3.3)* | 4.4 (3.4) | 0.31 |
| Physical activity, n (%) | <0.0001 | |||||
| Sedentary | 154 (16.6) | 49 (10.8)* | 54 (22.7)* | 26 (16.1)* | 25 (35.2) | |
| Moderately active | 410 (44.3) | 189 (41.5) | 107 (45.0) | 80 (49.4) | 34 (47.9) | |
| Highly active | 362 (39.1) | 217 (47.7) | 77 (32.4) | 56 (34.6) | 12 (16.9) | |
| Smoking, n (%) | 0.21 | |||||
| Never | 544 (58.5) | 251 (54.8) | 145 (60.7) | 104 (64.2) | 44 (62.0) | |
| Former | 256 (27.5) | 137 (29.9) | 55 (23.0) | 43 (26.5) | 21 (29.6) | |
| Current | 130 (14.0) | 70 (15.3) | 39 (16.3) | 15 (9.3) | 6 (8.5) | |
| Diseases | ||||||
| Hypertension, n (%) | 334 (35.9) | 141 (30.8)* | 97 (40.6) | 62 (38.3) | 34 (47.9) | 0.050 |
| Angina pectoris, n (%) | 43 (4.6) | 12 (2.6)* | 14 (5.9) | 10 (6.2) | 7 (9.9) | 0.020 |
| Chronic heart failure, n (%) | 42 (4.5) | 9 (2.0)* | 18 (7.5) | 8 (4.9) | 7 (9.9) | 0.013 |
| Diabetes, n (%) | 119 (12.8) | 45 (9.8)* | 31 (13.0) | 27 (16.7) | 16 (22.5) | 0.005 |
| Lung diseasec, n (%) | 84 (9.0) | 37 (7.9) | 28 (12.4) | 11 (6.7) | 8 (11.6) | 0.14 |
| Hip osteoarthritis, n (%) | 49 (5.3) | 16 (3.5)* | 16 (6.7) | 8 (4.9) | 9 (12.7) | 0.024 |
| Inflammation markers | ||||||
| IL-6, pg ml−1; median (IQR) | 1.40 (0.84−2.08) | 1.27 (0.78−1.90)* | 1.50 (0.88−2.80) | 1.45 (0.90−2.09) | 1.69 (0.95−2.45) | 0.019 |
| CRP, μg ml−1; median (IQR) | 2.60 (1.52−3.25) | 2.15 (1.13−4.54)* | 2.40 (1.24−5.48) | 3.91 (2.18−6.39) | 2.99 (1.93−8.48) | <0.0001 |
Abbreviations: CRP, c-reactive protein; IL, interleukin; IQR, interquartile range Q1–Q3; s.d., standard deviation.
Age and sex-adjusted differences in means, proportions and medians across low muscle strength and obesity categories were calculated using generalized linear models. Non-normally distributed continuous variables were ranked before significance examination.
Unable to walk 400 m or climb a flight of stairs without the help of another person.
Lung disease includes asthma, chronic bronchitis/emphysema.
Indicates significant (P < 0.05) difference between ‘Low muscle strength and obesity’ and other groups in pair wise comparisons.
Results
The mean age of the InCHIANTI participants considered in this study was 74.1 years (standard deviation 6.8 years) at baseline, and 55.2% were women. The characteristics of the study population according to low muscle strength and obesity groups are shown in Table 1.
Change over time in walking speed
Figure 1 shows the age-related trajectories of walking speed over 6-year follow-up in different low strength and obesity combinations. At baseline, obese persons with low muscle strength had significantly and consistently lower walking speed compared with all other groups (P≤0.05), except among the persons aged ≥85 years, in whom the difference against low strength group was not statistically significant. Walking speed declined during the 6-year follow-up in all low strength and obesity combinations and in each 5-year age group. Among participants aged 65−80 years, the walking speed decline was most prominent in obese persons with low muscle strength. For example, an average 65-year-old participant with obesity and low muscle strength experienced 0.03 m s−1 decline in walking speed per year, from 1.03 m s−1 at baseline to 0.85 m s−1 at the 6-year follow-up, representing 17% decline compared with 2% decline in the ‘neither low strength nor obesity’ group, 4% decline in the ‘low strength’ group and with 8% decline in the ‘obesity’ group.
Figure 1.
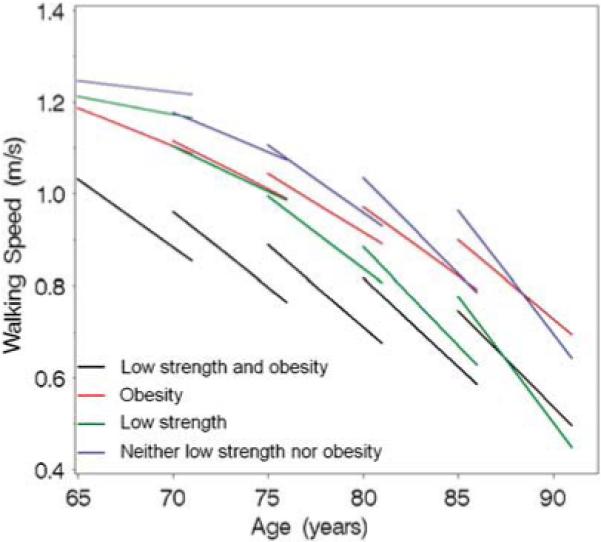
Longitudinal change in walking speed between ages 65−85 years according to the combination of low muscle strength and obesity. Each line plot represents the change in walking speed over 6 years in different baseline age categories. For example, walking speed for an average 65-year-old participant with obesity and low muscle strength was 1.03 m s−1 at baseline and 0.85 m s−1 at 6-year follow-up, and the decline was 0.03 m s−1 year.
After the age of 85 years, walking speed decline was no longer different among obese persons with low muscle strength compared with other groups. The walking speed of an average 85-year-old participant with obesity and low muscle strength declined 0.05 m s−1 each year over 6-year follow-up from 0.96 to 0.64 m s−1, representing 33% decline compared with 33% decline in the ‘neither low strength nor obesity’ group, 42% decline in the ‘low strength’ group and with 23% decline in the ‘obesity’ group.
The existence of different patterns in walking speed decline in different age groups is supported by the significant interaction term, age × time × ‘neither low strength nor obesity’ (P = 0.03), and borderline significant interaction term, age × time × ‘low strength’ (P = 0.07), compared with obese persons with low strength (Model 1, Table 2). After adjustment for lifestyle factors, diseases and inflammatory markers (Model 2), the ‘neither low strength nor obesity’ group remained significantly different from the ‘low strength and obesity’ group, and the difference between the ‘low strength’ and ‘low strength and obesity’ groups was moderately significant.
Table 2.
Linear mixed model estimates of walking speed over time according to baseline age, and combination of low muscle strength and obesity groups
|
Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Estimate | s.e. | P | Estimate | s.e. | P | |
| Intercept | 1.90 | 0.33 | <0.0001 | 1.71 | 0.31 | <0.0001 |
| Age | −0.014 | 0.004 | 0.002 | −0.01 | 0.004 | 0.02 |
| Time | 0.009 | 0.06 | 0.89 | 0.009 | 0.06 | 0.89 |
| Age × time | −0.0006 | 0.001 | 0.43 | −0.0006 | 0.001 | 0.45 |
| Neither low strength nor obesity | 0.25 | 0.36 | 0.49 | −0.04 | 0.32 | 0.90 |
| Low strength | 0.70 | 0.37 | 0.06 | 0.44 | 0.33 | 0.18 |
| Obesity | 0.27 | 0.41 | 0.51 | 0.03 | 0.37 | 0.93 |
| Low strength and obesity | 0 | 0 | ||||
| Age × neither low strength nor obesity | −0.0004 | 0.005 | 0.93 | 0.002 | 0.004 | 0.63 |
| Age × low strength | −0.008 | 0.005 | 0.10 | −0.006 | 0.004 | 0.19 |
| Age × obesity | −0.002 | 0.006 | 0.76 | 0.0007 | 0.005 | 0.88 |
| Age × low strength and obesity | 0 | 0 | ||||
| Time × neither low strength nor obesity | 0.15 | 0.07 | 0.02 | 0.15 | 0.07 | 0.03 |
| Time × low strength | 0.13 | 0.07 | 0.07 | 0.11 | 0.07 | 0.12 |
| Time × obesity | 0.03 | 0.07 | 0.70 | 0.04 | 0.07 | 0.60 |
| Time × low strength and obesity | 0 | 0 | ||||
| Age × time × neither low strength nor obesity | −0.002 | 0.001 | 0.03 | −0.002 | 0.001 | 0.03 |
| Age × time × low strength | −0.002 | 0.001 | 0.07 | −0.001 | 0.001 | 0.13 |
| Age × time × obesity | −0.0002 | 0.001 | 0.82 | −0.0004 | 0.001 | 0.70 |
| Age × time × low strength and obesity | 0 | 0 | ||||
Abbreviations: Age, age at the baseline; CRP, c-reactive protein; IL, interleukin; Time, duration of the follow-up (range 0−6 years). Model 1: unadjusted. Model 2: adjusted for sex, body height, education, physical activity, diseases (hypertension, angina pectoris, chronic heart failure, diabetes and hip osteoarthritis), IL-6 and CRP.
To examine the effect of changes in BMI, waist circumference as well as in muscle strength over follow-up time on studied associations, we carried out additional analyses using linear mixed-effect regression models. After adjusting either for BMI change or waist circumference change, the difference in walking speed decline between ‘low strength and obesity’ and ‘neither low strength nor obesity’ groups disappeared. Neither the BMI change nor the waist circumference change had an independent effect on walking speed decline. Furthermore, after adjusting for muscle strength change, the difference in walking speed decline attenuated between ‘low strength and obesity’ and ‘neither low strength nor obesity’ groups, and change in muscle strength had borderline significance (P = 0.07) independently associated with walking speed decline. Of all the tested variables, change in muscle strength produced best values for model fit, indicating that muscle strength change explains a large portion of the differences in walking speed decline seen between low muscle strength/obesity groups.
More detailed description of the changes in BMI, waist circumference and muscle strength is provided in Table 3. Compared with the other groups, muscle strength decline was greatest in the ‘low strength and obesity’ group. In addition, muscle strength decline was accelerated among oldest-old in the ‘neither low strength nor obesity’ and ‘low strength’ groups. Correspondingly, these are the same groups, in which we saw an accelerated decline of walking speed after age of 80 years (Figure 1).
Table 3.
An average changea of BMI, waist circumference and grip strength over 6-year follow-up according to age, and combination of low muscle strength and obesity groups
| Neither low strength nor obesity | Low strength | Obesity | Low strength and obesity | Main effect of BMI/waist/gripb | Main effect of agec | Interaction effectd | |
|---|---|---|---|---|---|---|---|
| Dependent variable | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P | P | P |
| BMI, kg/m2 | 0.04 | 0.0002 | 0.02 | ||||
| 65−79 years | −0.18 (−0.46; 0.10) | −0.15 (−0.69; 0.39) | −0.23 (−0.81; 0.35) | −0.30 (−1.28; 0.68) | |||
| ≥80 years | −1.15 (−2.21; −0.09) | −0.15 (−1.25; 0.95) | −3.51 (−5.24; −1.78) | −2.56 (−4.82; −0.30) | |||
| Waist circumference, cm | 0.14 | 0.003 | 0.37 | ||||
| 65−79 years | 1.56 (0.78; 2.34) | 2.28 (0.79; 3.78) | 4.67 (3.17; 6.17) | 5.29 (2.42; 8.16) | |||
| ≥80 years | −3.48 (−6.79; −0.16) | 0.37 (−3.34; 4.08) | 1.89 (−5.53; 9.30) | −4.90 (−13.98; 4.18) | |||
| Grip strength, kg | 0.05 | <0.0001 | 0.60 | ||||
| 65−79 years | 0.25 (−0.62; 1.12) | −0.81 (−2.38; 0.76) | 1.19 (−0.29; 2.68) | −2.78 (−5.39; −0.17) | |||
| ≥80 years | −6.72 (−9.41; −4.04) | −5.49 (−8.60; −2.38) | −2.32 (−7.19; 2.55) | −8.87 (−13.81; −3.92) | |||
Abbreviations: BMI, body mass index; CI, confidence interval.
Adjusted for age, sex and baseline value of the dependent variable.
Effect of BMI / waist / grip on dependent variable.
Effect of age on dependent variable.
Interaction effects of BMI × age, waist × age and grip × age on dependent variable.
Risk of mobility disability
The overall incidence of new mobility disability was 6.9% (n = 52) and 13.0% (n = 91), respectively, at 3- and 6-year follow-up. Age- and sex-adjusted probability rates of developing new mobility disability at 3- and 6-year follow-up are shown in Figure 2 for different combinations of low muscle strength and obesity. After 3 years of follow-up, ‘low strength and obesity’ group was no significantly different from the other groups, but after 6-year follow-up, obese persons with low strength had significantly higher rates of new mobility disability compared with persons in the ‘neither low strength nor obesity’ group (P = 0.0004) and with the ‘obese’ group (P = 0.06). In further analysis, we found no significant interaction between low muscle strength and obesity, suggesting that their effect on the risk of developing mobility disability is additive rather than multiplicative.
Figure 2.
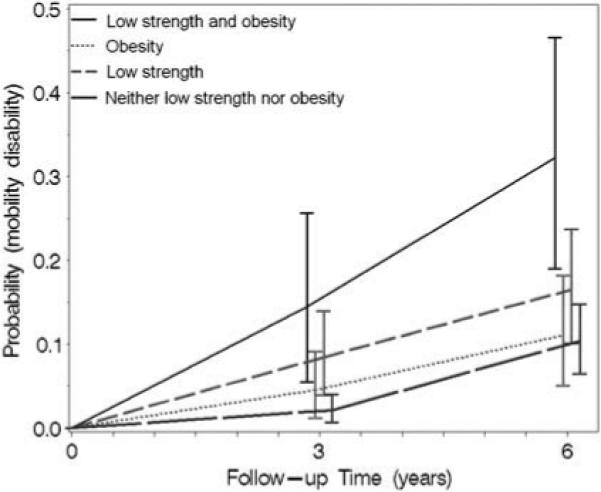
Probability rate of a new mobility disability (95% confidence intervals) according to the combination of low muscle strength and obesity among persons aged 65−85 years. Probabilities are adjusted to represent a 74-year-old female.
To study more in detail the effects of age, strength and obesity on the risk of developing mobility disability, mixed-effects logistic regression models were constructed (Table 4). Overall, obese persons with low strength were more likely to develop mobility disability compared with ‘neither low strength nor obesity’ (P = 0.04) and ‘low strength’ (P = 0.06) groups. The statistics for the interaction terms, ‘age × neither low strength nor obesity’ (P = 0.05) and ‘age × low strength’ (P = 0.07), indicate that obese persons with low muscle strength are more likely to develop mobility disability compared with the other groups, but this effect tends to be less important at older ages.
Table 4.
Logistic generalized linear mixed models estimating the effect of baseline age, and combination of low muscle strength and obesity on mobility disability
|
Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Estimate | s.e. | P | Estimate | s.e. | P | |
| Intercept | −8.56 | 3.86 | 0.03 | −10.44 | 4.26 | 0.02 |
| Age | 0.06 | 0.05 | 0.19 | 0.07 | 0.05 | 0.19 |
| Time | 0.45 | 0.06 | <0.0001 | 0.53 | 0.06 | <0.0001 |
| Neither low strength nor obesity | −13.89 | 4.67 | 0.003 | −10.53 | 5.06 | 0.04 |
| Low strength | −11.48 | 4.69 | 0.02 | −9.43 | 5.07 | 0.06 |
| Obesity | −9.09 | 5.86 | 0.12 | −5.52 | 6.29 | 0.38 |
| Low strength and obesity | 0 | 0 | ||||
| Age × neither low strength nor obesity | 0.15 | 0.06 | 0.008 | 0.12 | 0.06 | 0.05 |
| Age × low strength | 0.13 | 0.06 | 0.02 | 0.11 | 0.06 | 0.07 |
| Age × obesity | 0.10 | 0.07 | 0.19 | 0.06 | 0.08 | 0.47 |
| Age × low strength and obesity | 0 | 0 | ||||
Abbreviations: Age, age at the baseline; CRP, c-reactive protein; IL, interleukin; Time, duration of the follow-up (range 0−6 years). Model 1: unadjusted. Model 2: adjusted for sex, body height, education, physical activity, diseases (hypertension, angina pectoris, chronic heart failure, diabetes and hip osteoarthritis), IL-6 and CRP.
To examine the effect of abdominal obesity combined with low muscle strength on walking speed decline and risk of developing mobility disability, we carried out parallel analysis that used waist circumference instead of using BMI. Obesity was defined as the highest sex-specific tertile of waist circumference. The results were almost identical compared with using BMI as an indicator of obesity showing that obese persons with low muscle strength had steeped decline in walking speed and high risk of developing mobility disability over the follow-up period compared with those without obesity or low muscle strength. After the age of 80 years, the differences between groups were substantially attenuated.
Discussion
The results of this study provide evidence that older obese persons with low muscle strength have particularly high risk for accelerated decline of walking speed and for the development of new mobility disability. Our data also suggest that this effect is less important in the oldest old. This study also shows that 6-year change in muscle strength, as well as in BMI and waist circumference partly explain the differences seen in walking speed decline across different combinations of low muscle strength and obesity groups. Our results support the notion that muscle strength should be evaluated in obese older patients to recognize persons at the risk of functional decline.
There is only one earlier study regarding the combined effect of obesity and muscle impairment on physical functioning, in which muscle impairment was defined by poor muscle strength. In the cross-sectional Finnish Health 2000 Survey, persons who had high percentage of fat mass and poor muscle strength had higher prevalence of walking limitation compared with those with only high fat or low strength.8
Few other studies have examined the effect of so-called ‘sarcopenic obesity’, defined according to muscle and fat mass on the basis of body composition measurements, on physical functioning. The results, however, were inconsistent. In the New Mexico Elder Health Survey, Baumgartner et al.9 showed that older participants with sarcopenic obesity were more likely to be disabled than participants who were just obese or sarcopenic. In the 8-year follow-up of the same study, sarcopenic obesity at baseline was associated with over twofold higher risk of developing IADL (instrumental activity of daily living) disability compared with non-sarcopenic or non-obese at baseline. However, two cross-sectional studies carried out on the NHANES (National Health and Nutrition Survey) III population24 and on a sample of older women in Verona25 failed to identify a significant association between sarcopenic obesity and poor physical functioning. In fact, they only observed an association between obesity and functional decline, not with low muscle mass. In their studies, Davison et al.24 and Zoico et al.25 both used same categorization to define sarcopenic obesity on the basis of the estimated fat and muscle mass quintiles.
Inconsistencies in findings of studies concerning the prognostic implications of sarcopenic obesity are probably attributable to the choice of using muscle mass as a measure of muscle impairment. In this study, muscle impairment was operationalized as poor muscle strength instead of reduced muscle mass to better capture the effect of muscle impairment on functional limitation and disability.26–28 It has been shown that there is a discrepancy of the age-related loss of muscle mass and strength (greater loss in strength),28,29 and it is partly because of decreased fiber number and size, fat infiltration and modification of the motor units. Muscle quality (strength per unit of cross-sectional area on muscle mass) takes into account changes in both components of the muscle, thus it may be have even better indicator for muscle impairment.29 Unfortunately, we did not have information regarding the total body lean mass.
Owing to the lack of total body composition measures, we used, in this study, BMI and waist circumference as a measure of obesity instead of using information regarding body fatness, which has been used in other studies.9,24,25 However, it must be stated that, although widely used, the validity of BMI and waist circumference as a measure of body fatness may be reduced because of age-related changes in body composition. On the other hand, our goal was to use a combination of measures that are easily available and enable screening of weak obese persons in the clinical setting. BMI, waist circumference and hand-held dynamometer are very cost-effective and easy to use with older persons. Future studies are needed to examine the effect of low muscle strength and high body fatness on functional decline, measured with more sophisticated methods, such as isokinetic dynamometer or leg press, and DXA (dualenergy X-ray absorptiometry) or computed tomography, respectively.
Combination of low muscle strength and obesity lays at the cross-road between two major trends affecting to public health: the increasing number of older persons and increase in prevalence of obesity. The progressive decline of muscle mass and strength is perhaps the most ineluctable anatomical change occurring with aging. Factors that contribute to age-related muscle mass/strength decline include progressive reduction of physical activity, hormonal changes and a chronic pro-inflammatory state, malnutrition, loss of neuromuscular function and chronic diseases.30–32 The high rate of obesity in the older population is a relatively recent trend. Fat accumulation and redistribution among older persons are because of progressive decline in total energy expenditure stemming from hormonal changes, decreased physical activity and basal metabolic rate.33
Given the age-related changes in body composition, obesity and low muscle mass/strength may coexist in same person simply by chance. However, there are several factors that speak for the common etiology or at least for the causal connection between obesity and muscle impairment, such as physical activity, low-grade inflammation, insulin resistance and malnutrition.20,21,34 The factors that can lead to development of the condition of low muscle strength and obesity are discussed in more detailed in the recent review.35 There is a paucity of studies in which obesity, potential mediating factors and low muscle strength have been measured repeatedly in the same individuals. Comprehensive longitudinal studies are needed to shed light on the critical factors that lead to development of this important geriatric syndrome.
As older obese persons with low muscle strength have increased risk of losing their walking ability and to experience accelerated decline of lower extremity performance, individuals with excess body weight should be carefully screened for low muscle strength. On the basis of our findings, we suggest that interventions aimed at reducing excess body weight and/or improving physical performance may reduce the risk of mobility disability. However, the type of intervention most adequate for these individuals should be selected with caution and on the basis of future studies conducted in obese individuals. Evidence from observational cohort studies suggests that weight loss in old age may adversely affect health and functional status.36,37 However, in a few studies, weight loss through a combination of diet and exercise led to improved physical functioning among obese older people.38–40
Some limitations of this study need to be discussed. First, this study is based on a sample of older Italians living in two cities in the Tuscany area. It is likely that InCHIANTI participants differ from other population-based samples and this may affect the generalizability of our results. An additional problem, typical of longitudinal studies, is that some participants were lost to follow-up because of selective mortality or other reasons. Participants who did not participate in the 3- or 6-year follow-up were more likely to have mobility disability at baseline (P<0.0001). In addition, they had lower walking speed, knee extensor strength and they were older than those who participated in the 6-year follow-up (P<0.0001). Furthermore, the majority of those who did not participate in the 3- or 6-year follow-up were in the lowest tertile of muscle strength, with many affected by obesity and low muscle strength. The selective exclusion of those participants from the study population may have caused an underestimation of the effect of the combination of low muscle strength and obesity on walking speed decline or mobility disability.
Finally, our finding, suggesting an attenuation of mobility disability risk among obese persons with low muscle strength aged ≥85 years old, is based on a small number of participants aged ≥85 years in the InCHIANTI study population. Thus, this finding, although interesting and challenging, should be interpreted with caution. One explanation for this finding can relate to the so-called ‘obesity-paradox’—obesity may not be as harmful among the oldest-old as in younger persons, and may even provide some protection against disability and mortality.41,42 Correspondingly, as the results of this study indicate, the decline in strength is smallest among obese oldest-old persons. Further cross-sectional and longitudinal studies that specifically focus on the oldest-old are needed to clarify how body composition affects functional status in extremely long-lived individuals.
In conclusion, older community-dwelling obese persons with decreased lower extremity muscle strength experience a steeper decline in walking speed and have significantly higher risk of developing mobility disability compared with those without obesity and low muscle strength, especially among persons aged <80 years old. Further prospective studies are needed to examine the biological mechanism leading to accelerated functional decline among obese persons with low muscle strength, and to test the effect of interventions designed to reduce fat and increase muscle mass in older persons.
Acknowledgements
The InCHIANTI study baseline (1998−2000) was supported as a ‘targeted project’ (ICS110.1/RF97.71) by the Italian Ministry of Health and, in part, by the US National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001−2003) was funded by the US National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-up 2 (2004−2006) was financed by the US National Institute on Aging (Contract: N01-AG-5-0002); supported, in part, by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland, USA. This work was also supported by grants from the Finnish Academy (no. 125494 SS) and the Robert Wood Johnson Foundation (DA). None of the sponsoring institutions interfered with the collection, analysis, presentation or interpretation of the data reported here.
Appendix
This appendix contains a more detailed description of the models specified in the statistical analysis section. Let ‘i’ denote the ith participant, ‘1’ denote the baseline evaluation, ‘j’ the jth longitudinal evaluation (j = 1, 2, 3), and SO, the four categories of combination of low muscle strength and obesity (1 = low strength and obesity, 2 = obesity, 3 = low strength, 4 = neither low strength nor obesity) defined for each participant at baseline. We decomposed the overall information on age effects (Ageij) into the baseline ‘cross-sectional’ between-participant age effect (Agei1) and the effect of ‘longitudinal’ changes in age within the same person (Δageij = Ageij–Agei1).43 To analyze the effects of these conditions on Walking Speed (WS), we formulate the following linear mixed model:
| (1) |
| (2) |
| (3) |
Where the β(SO) notation denotes a term specific to each SO category, b0i is a participant-specific random intercept and b1i is a random slope over age. This model allows the primary longitudinal age effects of interest (changes in outcomes within a person as that person ages over time) to depend on a participant's age at first measurement (that is, letting age effects differ for younger-old versus older-old). Furthermore, the implied marginal model at the centered adjustors is:
Within a SO category, the effect of aging 3 years (Δageij = 3) from, say, 60−63 years is:
whereas the effect of aging 3 years from, say, 80 to 83 years is:
and thus, the interaction term β3 models the difference in within-participant aging across different baseline ages. Nonlinear effects could be estimated similarly with additional data, but with three observations per person the linear terms were adequate.
The generalized linear mixed (logistic) models used to explore the effect of combination of low muscle strength and obesity categories on mobility disability were similar; however, we did not estimate random slope terms or the Agei1 × Δageij interaction term as there were only three binary outcomes per person to support the model and the inclusion of these terms led to non-convergence.
Line plots of the age-related trajectories of walking speed were drawn on the basis of the estimates of linear mixed-effect regression models (Figure 1). To show the differences between age groups, and low strength and obesity groups, the line plots were drawn separately for five 5-year age groups between 65 and 85 years. For all regression models, continuous covariates were centered against their mean values and categorical variables against the mode values (Table 1).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging US Population. Obesity (Silver Spring) 2007;15:2855–2865. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO; Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- 3.Stenholm S, Rantanen T, Alanen E, Reunanen A, Sainio P, Koskinen S. Obesity history as a predictor of walking limitation at old age. Obesity. 2007;15:929–938. doi: 10.1038/oby.2007.583. [DOI] [PubMed] [Google Scholar]
- 4.Mendes de Leon CF, Hansberry MR, Bienias JL, Morris MC, Evans DA. Relative weight and mobility: a longitudinal study in a biracial population of older adults. Ann Epidemiol. 2006;16:770–776. doi: 10.1016/j.annepidem.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988−2004. JAMA. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 6.Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes. 2006;30:364–373. doi: 10.1038/sj.ijo.0803130. [DOI] [PubMed] [Google Scholar]
- 7.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 8.Stenholm S, Rantanen T, Heliövaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008;56:462–469. doi: 10.1111/j.1532-5415.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner RN. Body composition in healthy aging. Ann NY Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macci C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Reuben DB, Seeman TE, Keeler E, Hayes RP, Bowman L, Sewall A, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59A:M1050–M1061. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 17.Bandinelli S, Benvenuti E, Del Lungo I, Baccini M, Benvenuti F, Di Iorio A, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging (Milano) 1999;11:287–293. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 18.Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk physical activity questionnaire. Int J Epidemiol. 2002;31:168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik J, Fried L, Simonsick E, Kasper J, Lafferty M. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. NIH Publication No. 95−4009. [Google Scholar]
- 20.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 21.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. 2nd edn Springer-Verlag; New York: 2001. [Google Scholar]
- 23.Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. 2nd edn Springer; New York: 2005. [Google Scholar]
- 24.Davison KK, Ford E, Cogswell M, Dietz W. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 25.Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28:234–241. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 26.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:M324–M333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 27.Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, et al. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann NY Acad Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- 28.Lauretani F, Russo C, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61A:M1059–M1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 30.Doherty T. Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 31.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 32.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58A:M1012–M1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 33.Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr. 2000;54:S92–S103. doi: 10.1038/sj.ejcn.1601030. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity—definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JS, Kritchevsky SB, Tylavsky F, Harris T, Simonsick EM, Rubin SM, et al. Weight change, weight change intention, and the incidence of mobility limitation in well-functioning community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2005;60A:M1007–M1012. doi: 10.1093/gerona/60.8.1007. [DOI] [PubMed] [Google Scholar]
- 37.Al Snih S, Raji MA, Markides KS, Ottenbacher KJ, Goodwin JS. Weight change and lower body disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1730–1737. doi: 10.1111/j.1532-5415.2005.53522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 39.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:866–871. doi: 10.1093/gerona/62.8.866. [DOI] [PubMed] [Google Scholar]
- 41.Inelmen EM, Sergi G, Coin A, Miotto F, Peruzza S, Enzi G. Can obesity be a risk factor in elderly people? Obes Rev. 2003;4:147–155. doi: 10.1046/j.1467-789x.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferrucci L, Alley D. Obesity, disability, and mortality: a puzzling link. Arch Intern Med. 2007;167:750–751. doi: 10.1001/archinte.167.8.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. 2nd edn Oxford University Press; New York, USA: 2002. [Google Scholar]


