Abstract
Purpose
Manganese superoxide dismutase (MnSOD) protects against oxidative damage and modulates the efficacy of chemotherapeutic drugs. A functional single nucleotide polymorphism (SNP) in codon 16 of SOD2 (rs4880), which encodes MnSOD, results in a substitution of valine by alanine (Val16Ala). We hypothesized that this SNP affects breast cancer survival of patients receiving chemotherapy.
Experimental Design
Two patient populations from the United States (n=248) and Norway (n=340) were genotyped for Val16Ala. Kaplan-Meier survival and Cox Proportional-Hazards regression analyses were used to examine the relationship between Val16Ala and disease-specific survival.
Results
Val16Ala was significantly associated with breast cancer outcome in both patient populations. Carriers of the Ala allele had inferior survival rates in the multivariate analysis [Hazard ratio (HR) = 2.44; 95% confidence interval (CI), 1.11–5.37 in United States cohort and 1.91; 95% CI, 1.06–3.45 in Norway cohort for Ala/Ala versus Val/Val]. In an analysis of the combined cohorts, this association was significant for patients receiving adjuvant therapy (HR = 2.47; 95% CI, 1.46–4.19), but not for patients without it (HR = 1.47; 95% CI, 0.57–3.74). After further stratification by type of chemotherapy, the effect of the Ala allele was mostly restricted to cyclophosphamide-containing chemotherapy regimens (HR = 22.0; 95% CI, 5.22–92.9; Ala/Ala versus Val/Val).
Conclusion
The Val16Ala polymorphism affects survival of patients receiving cyclophosphamide-containing chemotherapy. The findings provide the first evidence pointing toward a mechanism for cyclophosphamide-resistance in breast cancer patients.
Introduction
Excess reactive oxygen species (ROS) have been shown to promote tumor development (1–4). Persistent high production of ROS in cancer cells leads to oxidative stress, which increases mutation rates, accelerates tumor progression and activates cancer-related signal transduction pathways. Subsequent adaptation of these cells to oxidative stress is thought to cause resistance to chemotherapy and radiotherapy (5).
MnSOD is a key mitochondrial antioxidant enzyme that protects against ROS and lipid peroxidation. Its main function is the conversion of endogenously produced superoxide into hydrogen peroxide (6). MnSOD also protects against oxidative damage by exogenous factors, such as radiation, and can modify the effects of chemotherapeutics (7,8). In cell culture and animal models, increased MnSOD activity protects against the toxicity of doxorubicin (9,10), which is a widely used anticancer drug for treatment of patients with breast cancer and other malignancies.
MnSOD is encoded by the SOD2 gene. A mitochondrial target sequence (MTS) polymorphism in SOD2 (11), results in a substitution of valine (Val) by alanine (Ala). The substitution disrupts the MTS secondary structure and affects mitochondrial targeting, leading to retention of the Val variant in the mitochondrial membrane, while the Ala variant localizes to the mitochondrial matrix (12,13). This also affects cellular MnSOD activity levels. Cells expressing the Ala variant have been found to have a 30% to 40% higher enzymatic activity than cells expressing the Val variant (13). However, it remains to be shown whether Val16Ala has a dominant effect on MnSOD activity in vivo (14). While results from multiple studies indicate that the Ala variant may confer an increased risk of breast cancer in interaction with the environment (15–20), results from two studies evaluating Val16Ala as a prognostic factor are at variance (21,22).
Any influence of Val16Ala on breast cancer outcome may result from a therapy effect. Val16Ala may solely predict sensitivity to therapy, which is in contrast to a classic prognostic factor (23). To address this issue, we investigated the effect of Val16Ala in cohorts of breast cancer patients receiving either no chemotherapy or different chemotherapy regimens administered as adjuvant or neoadjuvant chemotherapies. With this approach, we found Val16Ala to be predictive of long-term outcome among patients receiving chemotherapy, but not among untreated individuals. Importantly, the effect of Val16Ala on outcome was largely restricted to patients treated with cyclophosphamide-containing regimens. Our findings provide the first data pointing to a mechanism of resistance to cyclophosphamide in breast cancer patients.
Methods
Study population
This study contained two major patient cohorts. Institutional Review Board approval was obtained at the participating institutions in the United States and Norway.
United States (US) cohort
Incident breast cancer cases (n = 248) were recruited between February of 1993 and August of 2003 in the greater Baltimore area, as described previously (24,25). All patients were identified through surgery lists and enrolled into the study prior to surgery. None of them participated in a clinical trial. Information to determine the ER status, disease stage, treatment, and survival was obtained from medical records and pathology reports, the Social Security Death Index, and the National Death Index. Disease staging was performed according to the tumor-node-metastasis (TNM) system of the American Joint Committee on Cancer/the Union Internationale Contre le Cancer (AJCC/UICC). Survival was determined for the period from the date of hospital admission to the date of the last completed search for death entries in the Social Security Death Index (February 3, 2006). Median and mean follow-up times for overall survival in this cohort were 58 and 65 months, respectively.
Norway cohort
Norwegian breast cancer patients (n = 340) were participants of four previously published studies. Ninety-one patients were recruited at the Haukeland University Hospital between 1991–1997 as part of a trial evaluating the effect of adjuvant doxorubicin on locally advanced breast cancer (26). Thirty-six patients were recruited at the same hospital between 1993–2001 as part of a trial to evaluate the response to adjuvant 5-fluorouracil and mitomycin in locally advanced breast cancer (27). One hundred and nine patients were participants in a larger study of 920 breast cancer cases recruited at several Norwegian hospitals between 1995–1998 as part of a trial evaluating the presence of tumor cells in the bone marrow of patients with primarily stage I and II breast cancer (28). One hundred and four patients were recruited into a larger study of 212 breast cancer cases at the Ullevål University Hospital between 1987–1994 as part of an investigation evaluating the relationship between an abnormal tumor p53 status and tumor p21 protein expression in breast cancer patients (29).
Genotyping
For the US cohort, Val16Ala (rs4880) was genotyped at the NCI Genotyping Core Facility, using the Taqman assay conditions described in the SNP500 Cancer database (30). We genotyped genomic DNA from fresh-frozen breast tissue (170 non-tumor tissues and 28 tumors) and buffy coat (n = 50). The genotype assay contained negative and positive controls, with 10% blinded duplicates. We successfully genotyped 98% of the cases (n = 244) and had 100% concordance among blinded duplicates. The Norway cohort was genotyped using the 7900HTFast Real-Time PCR System (Applied Biosystems, Foster City, CA) at the Norwegian Radium Hospital, Norway, using the standard assay conditions for the Applied Biosystems assay ID: C_8709053_10. We genotyped genomic DNA from 56 fresh-frozen breast tumor tissue and 284 buffy coat samples. We successfully genotyped 97% of the cases (n = 329) and had 100% concordance among blinded duplicates.
MnSOD Activity Assay
Human lymphoblast cells were obtained from Coriell (Camden, NJ) and maintained in suspension with RPMI-1640 with 2 mM L-glutamine and 15% FBS. Five of the cell lines were homozygous for the Val allele and the other 5 were homozygous for Ala allele. The MnSOD activity of these cells was measured as described (31). This assay is based upon the reduction of nitroblue tetrazolium (NBT) to blue formazan by the superoxide radical. In the assay, MnSOD activity is discerned from CuZnSOD activity by the addition of 5 mM sodium cyanide.
p53 mutational analysis
TP53 mutations were identified in the US and Norway cohorts, as previously described (24,26,27).
Statistical analysis
Intercooled Stata 9.0 (Stata Corp, College Station, TX) statistical software was used for data analysis. All statistical tests were two-sided and an association was considered statistically significant with P < 0.05. Chi-square and student t-tests were performed to assess differences in the frequency of characteristics between patient subgroups. We performed survival analyses to determine breast cancer-specific survival and censored all causes of death that were not related to breast cancer in our analyses. The Kaplan-Meier method and the log-rank test were used for univariate survival analysis. The Cox Proportional-Hazards regression was used for multivariate survival analysis to calculate adjusted hazard ratios for breast cancer-specific survival. A statistical test for interaction was performed in Stata to determine if the effect of Val16Ala on breast cancer survival is modified by other factors. We tested for interactions in the Cox models containing main effects and an interaction term, assuming a dominant effect of the Ala allele (Ala/Ala and Ala/Val versus Val/Val).
Results
Study population characteristics and genotyping
We studied the relationship between the Val16Ala polymorphism and disease outcome in 248 US and 340 Norwegian breast cancer patients. Four US and 11 Norway patients were excluded because of missing genotype information. Patient characteristics are described in Table 1. The genotype frequency distribution for Val16Ala showed a statistically insignificant variation between US patients and Norway patients, and the survival rates were similar between the two cohorts. Significant differences existed for tumor size, node involvement, tumor grade, and the tumor ER and p53 mutation status. The differences in ER status and tumor grade between US and Norway patients were explained by race/ethnic differences in these cohorts. After adjusting for race/ethnicity, no significant association between cohort and either tumor grade (OR = 1.03; 95% CI, 0.71–1.47) or ER status (OR = 1.09; 95% CI, 0.68–1.75) remained. Fifty-seven percent of women from the US cohort were African-Americans. To further examine possible differences by race/ethnicity, we compared the distribution of the clinical characteristics amongst Norwegians, European-Americans, and African-Americans (Supplementary Table 1). The Val16Ala genotype distribution in the US patient population did not significantly differ between African-Americans and European-Americans (χ2; P = 0.588). African-American patients were significantly more likely to have ER-negative and high grade tumors than patients of European descent. Other differences between the Norway and the US cohort were not explained by race/ethnic differences within the US cohort, e.g., tumor size, node involvement, and p53 mutation status, and similar numbers of the Norwegian, European-American and African-American patients received adjuvant chemotherapy. We also examined the effect of race/ethnicity on survival and compared survival of African-American patients with those of European descent (Supplementary Table 2). Consistent with previous studies (32–34), African-American patients tended to have poorer 5-year and 10-year survival rates than patients of European descent although the differences were not statistically significant at the P < 0.05 level.
Table 1.
Patient Characteristics
| US Cohort (n=244) | Norway Cohort (n=329) | P-value† | |
|---|---|---|---|
| Val16Ala Genotype | |||
| Val/Val | 76 (31%) | 75 (23%) | 0.060 |
| Val/Ala | 121 (50%) | 174 (53%) | |
| Ala/Ala | 47 (19%) | 80 (24%) | |
| Characteristics* | |||
| Race | |||
| African Descent | 139 (57%) | - | <0.001 |
| European Descent | 105 (43%) | 329 (100%) | |
| Age at diagnosis | |||
| (Mean ± S.D.) | 55.0 ± 13.9 | 62.5 ± 13.8 | <0.001 |
| Survival | |||
| Alive | 161 (66%) | 223 (68%) | 0.651 |
| Deceased | 83 (34%) | 106 (32%) | |
| Tumor Size | |||
| T1 | 53 (27%) | 90 (28%) | <0.001 |
| T2 | 93 (47%) | 95 (30%) | |
| T3 | 41 (21%) | 78 (24%) | |
| T4 | 10 (5%) | 56 (18%) | |
| Node Involvement | |||
| No | 141 (62%) | 136 (45%) | <0.001 |
| Yes | 85 (38%) | 167(55%) | |
| Grade | |||
| 1 | 33 (16%) | 41 (13%) | <0.001 |
| 2 | 74 (35%) | 177 (56%) | |
| 3 | 104 (49%) | 100 (31%) | |
| Estrogen Receptor Status | |||
| Negative | 100 (41%) | 96 (31%) | 0.011 |
| Positive | 143 (59%) | 216 (69%) | |
| TP53 Mutation Status | |||
| Negative | 198 (81%) | 226 (70%) | 0.002 |
| Positive | 46 (19%) | 98 (30%) | |
| Adjuvant Chemotherapy | |||
| No | 97 (43%) | 98 (27%) | <0.001 |
| Yes | 130 (57%) | 239 (73%) | |
Cases with missing information are not included
Chi-square test and Student t-test (age at diagnosis) were used to compare differences in patient characteristics
Val16Ala polymorphism and breast cancer-specific survival
The Val16Ala polymorphism was significantly associated with breast cancer-specific survival in both cohorts and in the combined analysis (Figure 1). Univariate and multivariate Cox regression survival analysis (Table 2) indicated that the Ala allele is a predictor of poor outcome. The estimated hazard ratios for the Ala/Ala genotype, when compared with the Val/Val genotype, were remarkably similar between the US and Norwegian cohorts. We also modeled the survival analysis to compare dominant (Val/Val versus Val/Ala and Ala/Ala), recessive (Val/Val and Val/Ala versus Ala/Ala) and additive effects of Val16Ala. Although the Ala/Ala genotype was associated with decreased survival of patients regardless of which model was used, an additive effect by the Ala allele appeared to be the best model for the data.
Figure 1.
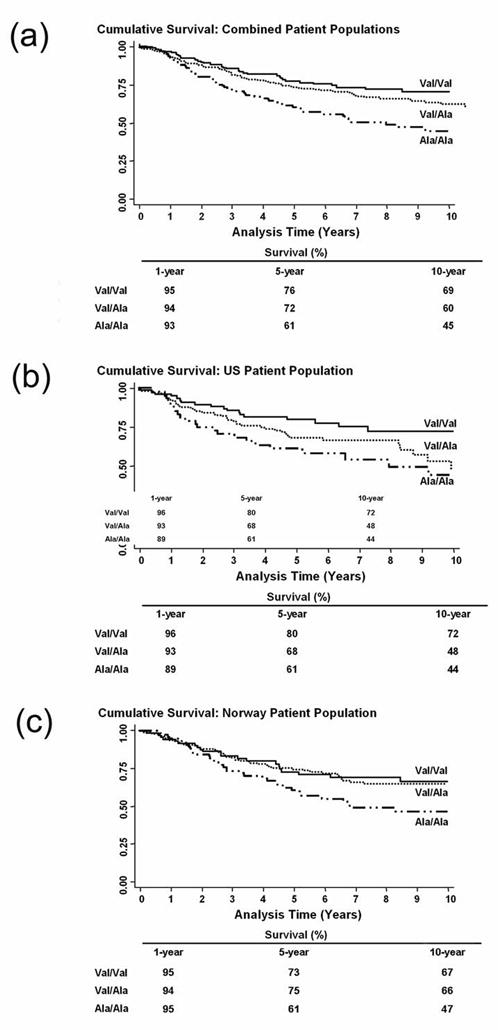
Association between Val16Ala polymorphism and breast cancer survival. Shown are Kaplan-Meier survival curves for 10-year survival. (a) Combined analysis of the US and Norwegian Cohorts (n = 573). Log-rank test: P = 0.003. (b) US Cohort (n = 244). Log-rank test: P = 0.038. (c) Norwegian Cohort (n = 329). Log-rank test: P = 0.039.
Table 2.
Association of Val16Ala with 10-year breast cancer survival
| Univariate | Multivariate* | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Combined analysis | (n=573) | (n=465) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 1.27 (0.87–1.84) | 0.215 | 1.26 (0.83–1.91) | 0.274 |
| Ala/Ala | 1.96 (1.30–2.94) | 0.001 | 2.19 (1.39–3.34) | 0.001 |
| Val/Ala & Ala/Ala | 1.47 (1.03–2.09) | 0.034 | 1.52 (1.03–2.25) | 0.033 |
| Ptrend | 0.001 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 1.67 (1.22–2.27) | 0.001 | 1.91 (1.36–2.67) | <0.001 |
| US Cohort | (n=244) | (n=183) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 1.62 (0.93–2.81) | 0.088 | 1.58 (0.84–2.96) | 0.155 |
| Ala/Ala | 2.24 (1.20–4.18) | 0.011 | 2.44 (1.11–5.37) | 0.027 |
| Val/Ala & Ala/Ala | 1.78 (1.05–3.00) | 0.031 | 1.75 (0.96–3.18) | 0.067 |
| Ptrend | 0.011 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 1.63 (1.00–2.66) | 0.050 | 1.71 (0.96–3.06) | 0.066 |
| Norway Cohort | (n=329) | (n=282) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 1.04 (0.62–1.73) | 0.880 | 0.88 (0.49–1.56) | 0.662 |
| Ala/Ala | 1.73 (1.01–2.97) | 0.047 | 1.91 (1.06–3.45) | 0.031 |
| Val/Ala & Ala/Ala | 1.24 (0.77–2.01) | 0.369 | 1.22 (0.73–2.05) | 0.448 |
| Ptrend | 0.033 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 1.68 (1.12–2.52) | 0.012 | 1.98 (1.30–3.01) | 0.002 |
Cox Proportional-Hazards regression with adjustments for age at diagnosis, cohort, race, tumor size, nodal involvement, tumor grade, estrogen receptor status and p53 mutation.
Tumor ER and p53 status influences the association between Val16Ala and breast cancer-specific survival
Because MnSOD function can be modulated by estrogen and p53 (35,36), we assessed their impact on the relationship between the Val16Ala polymorphism and breast cancer survival (Table 3). This analysis was performed on the combined dataset, due to sample size constraints. In the univariate and multivariate analyses, the Ala/Ala genotype was significantly associated with poor survival in ER-positive patients when compared with the Val/Val genotype. Similar but statistically insignificant trends existed for ER-negative patients. Stratification by tumor p53 mutational status showed that the effect of the Val16Ala polymorphism on breast cancer survival is strongest among patients without p53 mutations. Because the prevalence of p53 mutations was significantly lower in ER-positive than ER-negative tumors (P <0.001, χ2 test), we conducted further analyses, and observed that the increased risk of poor survival in ER-positive patients was only significant when tumors were p53 mutation-negative (HR = 3.90; 95% CI, 1.79–8.51; Ala/Ala versus Val/Val; n = 119). Patients positive for ER and p53 mutations did not experience an increased risk of poor survival (HR = 0.94; 95% CI, 0.28–3.13; n=25). These findings suggest that Val16Ala could be functionally most important among those patients with ER-positive and p53 wild-type tumors. Because hormone replacement therapy may affect the relationship between Val16Ala and survival in ER-positive patients, we further stratified ER-positive patients into those who had received tamoxifen and those who did not. All ER-positive Norwegian patients received tamoxifen and about half of the US patients (Supplementary Table 3). Because of the small number of ER-positive patients who did not receive hormone replacement therapy (n = 64), any finding has to be treated with caution but it appeared from our analysis that Val16Ala may specifically influence survival in ER-positive patients receiving hormone replacement therapy (Supplementary Table 3).
Table 3.
Effect of ER and p53 mutation on the association of Val16Ala with 10-year breast cancer survival
| Univariate | Multivariate* | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| ER negative | (n=196) | (n=166) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 0.96 (0.54–1.74) | 0.907 | 1.11 (0.56–2.19) | 0.760 |
| Ala/Ala | 1.76 (0.93–3.32) | 0.080 | 1.64 (0.83–3.26) | 0.157 |
| Val/Ala & Ala/Ala | 1.17 (0.68–2.04) | 0.563 | 1.24 (0.67–2.29) | 0.487 |
| Ptrend | 0.060 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 1.84 (1.13–2.87) | 0.014 | 1.76 (1.03–2.99) | 0.035 |
| ER positive | (n=359) | (n=299) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 1.54 (0.93–2.552) | 0.095 | 1.45 (0.83–2.54) | 0.192 |
| Ala/Ala | 2.14 (1.24–3.70) | 0.007 | 2.54 (1.35–4.78) | 0.004 |
| Val/Ala & Ala/Ala | 1.73 (1.07–2.79) | 0.024 | 1.76 (1.04–2.99) | 0.035 |
| Ptrend | 0.005 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 1.60 (1.07–2.42) | 0.024 | 2.06 (1.32–3.21) | 0.001 |
| P53 Mutation negative | (n=424) | (n=340) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 1.38 (0.87–2.20) | 0.171 | 1.64 (0.98–2.73) | 0.060 |
| Ala/Ala | 1.98 (1.20–3.26) | 0.007 | 2.58 (1.49–4.45) | 0.001 |
| Val/Ala & Ala/Ala | 1.58 (1.02–2.44) | 0.039 | 1.90 (1.18–3.53) | 0.008 |
| Ptrend | 0.007 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 1.60 (1.09–2.35) | 0.017 | 1.89 (1.25–2.85) | 0.002 |
| P53 Mutation positive | (n=144) | (n=125) | ||
| Val/Val | 1 (ref.) | 1 (ref.) | ||
| Val/Ala | 0.82 (0.42–1.58) | 0.547 | 0.76 (0.36–1.56) | 0.458 |
| Ala/Ala | 1.97 (0.95–4.08) | 0.069 | 1.38 (0.58–3.26) | 0.468 |
| Val/Ala & Ala/Ala | 1.03 (0.55–1.93) | 0.915 | 0.89 (0.45–1.76) | 0.734 |
| Ptrend | 0.038 | |||
| Val/Val & Val/Ala | 1 (ref.) | 1 (ref.) | ||
| Ala/Ala | 2.40 (1.40–4.12) | 0.001 | 1.81 (0.99–3.33) | 0.054 |
Cox Proportional-Hazards regression with adjustments for age at diagnosis, study site, race, tumor size, tumor grade, nodal involvement, estrogen receptor status and p53 mutation
Val16Ala predicts survival in patients receiving chemotherapy
To determine whether the effect of Val16Ala on survival is dependent on the type of chemotherapy, we combined the cohorts and stratified breast cancer patients by treatment status and performed univariate and multivariate survival analyses within the strata (Table 4). Val16Ala was only significantly associated with disease outcome among patients receiving chemotherapy. In a sub-analysis of these patients, we examined the effect of Val16Ala on patient survival depending on the type of chemotherapy received: doxorubicin-based, 5-fluorouracil (5-FU)-based or cyclophosphamide-based chemotherapy. In all therapy groups, the Ala/Ala genotype significantly increased the risk of poor outcome. Additional modeling of the survival analysis, e.g., using dominant, additive or recessive models, pointed to a recessive effect of the genotype in patients with doxorubicin-based chemotherapy and a dominant effect in those receiving 5-FU-based or cyclophosphamide-based therapy. However, the effect of the genotype was greatest among patients receiving cyclophosphamide-based therapies.
Table 4.
Modification of the association of Val16Ala with 10-year breast cancer survival by receipt of chemotherapy*
| Univariate | Multivariate$ | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | n | HR (95% CI) | P-value | n | |
| No Chemotherapy | (n=221) | (n=161) | ||||
| Val/Val | 1 (ref.) | 58 | 1 (ref.) | 42 | ||
| Val/Ala | 0.96 (0.49–1.87) | 0.898 | 111 | 1.28 (0.56–2.93) | 0.563 | 81 |
| Ala/Ala | 1.31 (0.62–2.75) | 0.480 | 52 | 1.47 (0.57–3.74) | 0.424 | 38 |
| Val/Ala & Ala/Ala | 1.06 (0.57–1.99) | 0.846 | 163 | 1.24 (0.58–2.64) | 0.569 | 119 |
| Ptrend | 0.488 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 169 | 1 (ref.) | 123 | ||
| Ala/Ala | 1.33 (0.73–2.43) | 0.345 | 52 | 1.60 (0.81–3.16) | 0.175 | 38 |
| Chemotherapy | (n=322) | (n=293) | ||||
| Val/Val | 1 (ref.) | 85 | 1 (ref.) | 77 | ||
| Val/Ala | 1.42 (0.90–2.25) | 0.130 | 166 | 1.24 (0.76–2.02) | 0.385 | 153 |
| Ala/Ala | 2.41 (1.47–3.94) | <0.001 | 71 | 2.47 (1.46–4.19) | 0.001 | 63 |
| Val/Ala & Ala/Ala | 1.68 (1.09–2.58) | 0.018 | 137 | 1.58 (1.01–2.49) | 0.047 | 216 |
| Ptrend | 0.001 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 251 | 1 (ref.) | 230 | ||
| Ala/Ala | 1.87 (1.29–2.71) | 0.001 | 71 | 1.95 (1.32–2.90) | 0.001 | 63 |
| Doxorubicin containing therapy | (n=160) | (n=149) | ||||
| Val/Val | 1 (ref.) | 44 | 1 (ref.) | 41 | ||
| Val/Ala | 1.12 (0.60–2.10) | 0.709 | 84 | 0.90 (0.47–1.75) | 0.775 | 79 |
| Ala/Ala | 2.76 (1.41–5.40) | 0.003 | 32 | 2.90 (1.33–6.32) | 0.007 | 29 |
| Val/Ala & Ala/Ala | 1.50 (0.84–2.67) | 0.169 | 116 | 1.21 (0.67–2.22) | 0.523 | 108 |
| Ptrend | 0.003 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 128 | 1 (ref.) | 120 | ||
| Ala/Ala | 2.57 (1.53–4.35) | <0.001 | 32 | 2.64 (1.48–4.69) | 0.001 | 29 |
| 5-Fluorouracil containing therapy | (n=121) | (n=112) | ||||
| Val/Val | 1 (ref.) | 25 | 1 (ref.) | 24 | ||
| Val/Ala | 2.95 (1.03–8.50) | 0.045 | 64 | 2.87 (0.94–8.74) | 0.064 | 59 |
| Ala/Ala | 4.63 (1.56–13.7) | 0.006 | 32 | 5.99 (1.71–21.0) | 0.005 | 29 |
| Val/Ala & Ala/Ala | 3.49 (1.25–9.74) | 0.017 | 96 | 4.12 (1.40–12.1) | 0.010 | 88 |
| Ptrend | 0.004 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 89 | 1 (ref.) | 83 | ||
| Ala/Ala | 1.90 (1.05–3.42) | 0.033 | 32 | 2.07 (1.10–3.88) | 0.023 | 29 |
| Cyclophosphamide containing therapy | (n=142) | (n=123) | ||||
| Val/Val | 1 (ref.) | 35 | 1 (ref.) | 31 | ||
| Val/Ala | 2.72 (1.03–7.14) | 0.042 | 74 | 1.92 (0.69–5.29) | 0.208 | 64 |
| Ala/Ala | 5.82 (2.17–15.51) | <0.001 | 33 | 22.0 (5.22–92.9) | <0.001 | 28 |
| Val/Ala & Ala/Ala | 3.56 (1.41–8.98) | 0.007 | 107 | 3.22 (124–8.38) | 0.016 | 92 |
| Ptrend | <0.001 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 109 | 1 (ref.) | 95 | ||
| Ala/Ala | 2.86 (1.62–5.06) | <0.001 | 33 | 4.60 (2.29–9.21) | <0.001 | 28 |
Analysis included 543 patients. Information on receipt of chemotherapy was not available for 12 Norwegian patients and 18 US patients.
Cox Proportional-Hazards regression with adjustments for age at diagnosis, study site, race, tumor size, tumor grade, nodal involvement, p53 mutation and estrogen receptor status.
Because many patients had received combination therapies, an additional analysis was performed to examine the possibility of confounding effects by multiple chemotherapeutics on the relationship between Val16Ala and breast cancer survival (Table 5). For that, 4 patient groups were identified that received either (a) doxorubicin monotherapy, (b) cyclophosphamide/doxorubicin or cyclophosphamide/doxorubicin/5-FU combinations, (c) mitomycin/5-FU, or (d) the combination of cyclophosphamide/methotrexate/5-FU (CMF). The combinations of cyclophosphamide and doxorubicin (b) and of cyclophosphamide, doxorubicin, and 5-FU (c) are also commonly referred to as AC and FAC regimens, respectively, in clinical practice. Multivariate survival analyses revealed that patients receiving doxorubicin monotherapy were at a significantly increased risk of poor survival in the recessive model. Addition of cyclophosphamide to doxorubicin containing chemotherapy regimens (AC and FAC) resulted in a significantly increased risk of poor survival if patients were carriers of the Ala/Ala genotype (unadjusted HR = 6.33; 95% CI, 1.58–25.7; adjusted HR = 139.5; 95% CI, 2.77–7017; Ala/Ala versus Val/Val). In patients who received mitomycin/5-FU chemotherapy there was no significant association with survival in any model. Patients receiving cyclophosphamide/methotrexate/5-FU had an increased risk of poor survival if they were carriers of the Ala/Ala genotype, similar to AC/FAC patients (unadjusted HR = 6.02; 95% CI, 1.35–26.8; adjusted HR = 24.27; 95% CI, 2.84–207; Ala/Ala versus Val/Val). Together, these results suggest that Val16Ala affects most significantly the disease outcome of patients who received a cyclophosphamide-based regimen.
Table 5.
Sub-analysis of the association of Val16Ala with 10-year breast cancer survival by specific chemotherapy regimen
| Therapy* | Univariate | Multivariate$ | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | n | HR (95% CI) | P-value | n | |
| Doxorubicin monotherapy (n=86) | ||||||
| Val/Val | 1 (ref.) | 22 | 1 (ref.) | 22 | ||
| Val/Ala | 0.65 (0.30–1.45) | 0.300 | 43 | 0.70 (0.31–1.61) | 0.402 | 43 |
| Ala/Ala | 1.73 (0.77–3.87) | 0.180 | 21 | 2.03 (0.84–4.87) | 0.115 | 21 |
| Val/Ala & Ala/Ala | 0.95 (0.47–1.92) | 0.893 | 64 | 1.05(0.50–2.20) | 0.892 | 64 |
| Ptrend | 0.189 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 65 | 1 (ref.) | 65 | ||
| Ala/Ala | 2.18 1.12–4.22) | 0.020 | 21 | 2.35 (1.18–4.66) | 0.015 | 21 |
| Cyclophosphamide/Doxorubicin or Cyclophosphamide/Doxorubicin/5-FU (n=63) | ||||||
| Val/Val | 1 (ref.) | 21 | 1 (ref.) | 18 | ||
| Val/Ala | 2.61 (0.71–9.51) | 0.145 | 32 | 2.49 (0.39–15.8) | 0.330 | 28 |
| Ala/Ala | 6.33 (1.58–25.7) | 0.010 | 10 | 139.5 (2.77–7017) | 0.013 | 8 |
| Val/Ala & Ala/Ala | 3.37 (0.98–11.59) | 0.054 | 42 | 2.60 (0.54–12.5) | 0.233 | 36 |
| Ptrend | 0.005 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 53 | 1 (ref.) | 46 | ||
| Ala/Ala | 3.51 (1.32–9.33) | 0.012 | 10 | 16.1 (3.22–80.3) | 0.001 | 8 |
| Mitomycin/5-FU (n=35) | ||||||
| Val/Val | 1 (ref.) | 8 | 1 (ref.) | 8 | ||
| Val/Ala | 2.44 (0.52–11.4) | 0.256 | 19 | 2.80 (0.54–14.4) | 0.218 | 19 |
| Ala/Ala | 1.62 (0.27–9.75) | 0.598 | 8 | 1.91 (0.26–13.9) | 0.521 | 8 |
| Val/Ala & Ala/Ala | 2.20 (0.49–9.85) | 0.305 | 27 | 2.20 (0.47–10.3) | 0.316 | 27 |
| Ptrend | 0.797 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 27 | 1 (ref.) | 27 | ||
| Ala/Ala | 0.79 (0.22–2.85) | 0.721 | 8 | 0.57 (0.14–2.26) | 0.428 | 8 |
| Cyclophosphamide/Methotrexate/5-FU (n=77) | ||||||
| Val/Val | 1 (ref.) | 14 | 1 (ref.) | 14 | ||
| Val/Ala | 3.05 (0.69–13.4) | 0.141 | 41 | 2.15 (0.45–10.3) | 0.338 | 36 |
| Ala/Ala | 6.02 (1.35–26.8) | 0.018 | 22 | 24.27 (2.84–207) | 0.004 | 19 |
| Val/Ala & Ala/Ala | 3.99 (0.95–16.8) | 0.059 | 63 | 4.61 (1.06–20.1) | 0.042 | 55 |
| Ptrend | 0.004 | |||||
| Val/Val & Val/Ala | 1 (ref.) | 55 | 1 (ref.) | 50 | ||
| Ala/Ala | 2.53 (1.21–5.26) | 0.013 | 22 | 4.17 (1.77–9.81) | 0.001 | 19 |
11 patients who received doxorubicin and 3 patients who received cyclophosphamide as part of other therapy regimens than described were not included in the analyses
Cox Proportional-Hazards regression with adjustments for age at diagnosis, study site, race, tumor size, tumor grade, nodal involvement, p53 mutation and estrogen receptor status
Interaction analysis
We applied a test for interaction and examined interactions between Val16Ala and known risk factors in breast cancer survival. We did not find an interaction with age at diagnosis, race/ethnicity, tumor size, node involvement, tumor grade, p53 mutation status, or tumor ER and PR status. However, we observed a significant interaction between this genotype and cyclophosphamide-based therapies (AC, FAC, and CMF) on breast cancer survival (Pinteraction= 0.023), assuming a dominant effect of the Ala allele (Ala/Ala and Ala/Val versus Val/Val). This observation is consistent with our previous data that the relationship between Val16Ala and breast outcome is due to a specific interaction between this genotype and cyclophosphamide-based therapy.
MnSOD activity is higher in lymphoblastoid cell lines with the Ala/Ala genotype
We compared MnSOD activity between five lymphoblastoid cell lines carrying the Val/Val genotype and five lymphoblastoid cell lines carrying the Ala/Ala genotype (Supplementary Figure 1). We observed an approximate increase of 50% in MnSOD activity in cells with the Ala/Ala genotype (34.8 ± 17.7 relative units; mean ± S.D.) when compared to the Val/Val genotype (23.3 ± 8.1).
Discussion
In our study of 248 US and 340 Norwegian women with incident breast cancer, a polymorphism in the SOD2 gene, Val16Ala, was associated with breast cancer survival. This association was restricted to patients receiving chemotherapy and was most significant for those patients who received a cyclophosphamide-containing therapy. Our findings suggest that Val16Ala influences the effect of chemotherapy in breast cancer and modifies disease-specific survival.
While several studies have reported that Val16Ala is associated with the risk to develop breast and prostate cancer (15–20,37–40), only few studies examined the effect of this polymorphism on breast cancer outcome. One study found an association between the Val allele and an increased risk of disease progression and breast cancer-specific death among 95 patients with metastatic breast cancer who received high dose chemotherapy and autologous stem cell support (22). A second study, conducted in 279 breast cancer patients undergoing either adjuvant chemotherapy, radiation, or both, did not find a significant association between Val16Ala and outcome (21). In contrast, we observed a significant association of the Ala/Ala genotype with decreased breast cancer survival in two independent patient populations. Perhaps, differences in therapy and patient population may account for the opposite finding between the first study and ours. However, many patients in the second study were treated with cyclophosphamide, and there is no obvious difference in the patient populations other than the Arkansas patients were recruited through a tumor registry that could explain the difference in findings between the second study and ours. Another possible explanation for the conflicting results may relate to the tumor redox status. It is possible that this status changes with disease progression, which may affect intrinsic ROS production and the relative effect of the Val16Ala genotype on therapy outcome and overall survival. Thus, future studies in larger data sets are required to further examine the relationships between Val16Ala, patient survival, and response to therapy in breast cancer.
We tested the hypothesis that Val16Ala would affect breast cancer survival when ROS levels are increased, which is seen in patients receiving chemotherapy. Our cell culture studies with human lymphoblastoid cells showed that the Ala allele encodes a higher MnSOD activity which is consistent with previous studies that studied the effect of this genotype on MnSOD expression and activity using over-expression of a transgene (12,13). Alkylating agents, such cyclophosphamide, and anthracyclines, such as doxorubicin, are examples of chemotherapeutic drugs that generate ROS in cancer cells during treatment (41). Cyclophosphamide generates abundant ROS when metabolically activated in cells, inducing oxidative stress and lipid peroxidation (42–44). The mechanism by which cyclophosphamide increases ROS is not fully understood, and it may involve glutathione depletion and activation of signaling pathways such as p66Shc. Consistent with cyclophosphamide-induced ROS generation, we detected a significant interaction between Val16Ala and cyclophosphamide in breast cancer survival and found that the Ala/Ala genotype predicts a poor outcome among patients treated with this drug. Carriers of this genotype represent 20% to 25% of the patient population that would be candidates for therapies other than cyclophosphamide. Mechanistically, the Ala allele may increase resistance to chemotherapeutic drugs because it increases MnSOD activity and detoxification of drugs, whose anticancer activity involves generation of reactive oxygen species. Previous in vitro studies have indicated that high expression of MnSOD increases resistance to doxorubicin (9,10) and 5-FU (45–47), suggesting that increased MnSOD activity may have similar effects. Since our data indicate that Val16Ala has its strongest outcome effect with cyclophosphamide therapy, future studies are needed that examine the relationship between MnSOD and cyclophosphamide cytotoxicity in human cancer cells.
We observed that Val16Ala could be functionally most significant among patients with ER-positive and p53 wild-type tumors. MnSOD is induced by p53 in response to stress (36), and can suppress radiation-induced neoplastic transformation, induction of hypoxia-inducible factor-1α and vascular endothelial growth factor (7,48,49). From these data, we hypothesize that Val16Ala may become functional in the p53-induced stress response when breast tumors are treated with chemotherapeutic drugs, e.g., cyclophosphamide and doxorubicin, with a subsequent induction of p53 and MnSOD. This pathway is consistent with previous research observing resistance to anthracycline therapy among breast cancer patients with a mutant p53 tumor status (50). Our analyses did not find that race/ethnicity is a confounder of the relationship between Val16Ala and survival. However, because of the higher frequency of ER-negative tumors among African-American breast cancer patients, Val16Ala may affect African-American women differently than it is affecting women of European ancestry.
Our study has strengths and limitations. We conducted the analysis in two independent populations, limiting the likelihood of a false-positive discovery. We were also able to assess the implication of the tumor p53 mutational status on the association between Val16Ala and breast cancer survival in both patient populations. However, the existing sample size did not allow a more in-depth examination of the effect of Val16Ala using stratification of subsets, e.g., analysis of interactions between Val16Ala and specific chemotherapeutics after stratification of patients by ER and p53 status. We also pooled the cohorts to increase power when we performed subgroup analyses, e.g., to examine whether the effect of Val16Ala on survival is dependent on the type of chemotherapy. Even in this combined analysis, some subgroups consisted of small patient numbers, leading to wide confidence intervals and perhaps unstable risk estimates. Furthermore, information on patients’ therapy was obtained from medical records in the US cohort and for some of the Norway patients. This therapy information and the therapy regimens across both study populations were grouped by type of combination therapy, but we are aware that there is substantial heterogeneity among the patients by choice of combination (e.g., combination of hormone replacement therapy and chemotherapy), dosage, and treatment duration within each subgroup.
In conclusion, we found that a functional polymorphism in the SOD2 gene affects the survival of breast cancer patients. Carriers of the common Ala/Ala genotype were at a significantly increased risk of poor survival when treated with specific chemotherapeutic drugs especially cyclophosphamide. Our findings suggest that Val16Ala modifies patients’ response to chemotherapy.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the Research Council of Norway and the Norwegian Cancer Society. Sharon A. Glynn is a recipient of an All-Ireland Cancer Consortium NCI Cancer Prevention Fellowship, sponsored in part by the Health Research Board of Ireland. The authors thank personnel at the University of Maryland and the Baltimore Veterans Administration, and the Surgery and Pathology Departments at the University of Maryland Medical Center, Baltimore Veterans Affairs Medical Center, Union Memorial Hospital, Mercy Medical Center, and Sinai Hospital for their contributions.
References
- 1.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–81. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 2.Forsberg L, de Faire U, Morgenstern R. Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys. 2001;389:84–93. doi: 10.1006/abbi.2001.2295. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 4.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 5.Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3:323–7. doi: 10.1186/bcr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–9. [PubMed] [Google Scholar]
- 7.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog. 1992;6:238–42. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- 8.Oberley LW. Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomed Pharmacother. 2005;59:143–8. doi: 10.1016/j.biopha.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Hur GC, Cho SJ, Kim CH, et al. Manganese superoxide dismutase expression correlates with chemosensitivity in human gastric cancer cell lines. Clin Cancer Res. 2003;9:5768–75. [PubMed] [Google Scholar]
- 10.Suresh A, Guedez L, Moreb J, Zucali J. Overexpression of manganese superoxide dismutase promotes survival in cell lines after doxorubicin treatment. Br J Haematol. 2003;120:457–63. doi: 10.1046/j.1365-2141.2003.04074.x. [DOI] [PubMed] [Google Scholar]
- 11.Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem Biophys Res Commun. 1996;226:561–5. doi: 10.1006/bbrc.1996.1394. [DOI] [PubMed] [Google Scholar]
- 12.Sutton A, Imbert A, Igoudjil A, et al. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics. 2005;15:311–9. doi: 10.1097/01213011-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogen. 2003;13:145–57. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 14.Bastaki M, Huen K, Manzanillo P, et al. Genotype-activity relationship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics. 2006;16:279–86. doi: 10.1097/01.fpc.0000199498.08725.9c. [DOI] [PubMed] [Google Scholar]
- 15.Slanger TE, Chang-Claude J, Wang-Gohrke S. Manganese superoxide dismutase Ala-9Val polymorphism, environmental modifiers, and risk of breast cancer in a German population. Cancer Causes Control. 2006;17:1025–31. doi: 10.1007/s10552-006-0043-5. [DOI] [PubMed] [Google Scholar]
- 16.Tamimi RM, Hankinson SE, Spiegelman D, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism, plasma antioxidants, cigarette smoking, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:989–96. [PubMed] [Google Scholar]
- 17.Ambrosone CB, Freudenheim JL, Thompson PA, et al. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59:602–6. [PubMed] [Google Scholar]
- 18.Mitrunen K, Sillanpaa P, Kataja V, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis. 2001;22:827–9. doi: 10.1093/carcin/22.5.827. [DOI] [PubMed] [Google Scholar]
- 19.Cai Q, Shu XO, Wen W, et al. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Breast Cancer Res. 2004;6:R647–R655. doi: 10.1186/bcr929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millikan RC, Player J, de Cotret AR, et al. Manganese superoxide dismutase Ala-9Val polymorphism and risk of breast cancer in a population-based case-control study of African Americans and whites. Breast Cancer Res. 2004;6:R264–R274. doi: 10.1186/bcr786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosone CB, Ahn J, Singh KK, et al. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res. 2005;65:1105–11. [PubMed] [Google Scholar]
- 22.Bewick MA, Conlon MS, Lafrenie RM. Polymorphisms in manganese superoxide dismutase, myeloperoxidase and glutathione-S-transferase and survival after treatment for metastatic breast cancer. Breast Cancer Res Treat. 2008;111:93–101. doi: 10.1007/s10549-007-9764-8. [DOI] [PubMed] [Google Scholar]
- 23.Lonning PE, Knappskog S, Staalesen V, Chrisanthar R, Lillehaug JR. Breast cancer prognostication and prediction in the postgenomic era. Ann Oncol. 2007;18:1293–306. doi: 10.1093/annonc/mdm013. [DOI] [PubMed] [Google Scholar]
- 24.Boersma BJ, Howe TM, Goodman JE, et al. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J Natl Cancer Inst. 2006;98:911–9. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 25.Martin DN, Boersma BJ, Howe TM, et al. Association of MTHFR gene polymorphisms with breast cancer survival. BMC Cancer. 2006;6:257. doi: 10.1186/1471-2407-6-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisler S, Lonning PE, Aas T, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res. 2001;61:2505–12. [PubMed] [Google Scholar]
- 27.Geisler S, Borresen-Dale AL, Johnsen H, et al. TP53 gene mutations predict the response to neoadjuvant treatment with 5-fluorouracil and mitomycin in locally advanced breast cancer. Clin Cancer Res. 2003;9:5582–8. [PubMed] [Google Scholar]
- 28.Wiedswang G, Borgen E, Karesen R, et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469–78. doi: 10.1200/JCO.2003.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen AL. Relationship between abnormal p53 protein and failure to express p21 protein in human breast carcinomas. J Pathol. 1997;181:140–5. doi: 10.1002/(SICI)1096-9896(199702)181:2<140::AID-PATH745>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Packer BR, Yeager M, Staats B, et al. SNP500Cancer: a public resource for sequence validation and assay development for genetic variation in candidate genes. Nucleic Acids Res. 2004;32(Database issue):D528–D532. doi: 10.1093/nar/gkh005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 32.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–54. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 33.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 34.Polite BN, Cirrincione C, Fleming GF, et al. Racial differences in clinical outcomes from metastatic breast cancer: a pooled analysis of CALGB 9342 and 9840--Cancer and Leukemia Group B. J Clin Oncol. 2008;26:2659–65. doi: 10.1200/JCO.2007.13.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussain SP, Amstad P, He P, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–6. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 37.Woodson K, Tangrea JA, Lehman TA, et al. Manganese superoxide dismutase (MnSOD) polymorphism, alpha-tocopherol supplementation and prostate cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study (Finland) Cancer Causes Control. 2003;14:513–8. doi: 10.1023/a:1024840823328. [DOI] [PubMed] [Google Scholar]
- 38.Kang D, Lee KM, Park SK, et al. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol Biomarkers Prev. 2007;16:1581–6. doi: 10.1158/1055-9965.EPI-07-0160. [DOI] [PubMed] [Google Scholar]
- 39.Arsova-Sarafinovska Z, Matevska N, Petrovski D, et al. Manganese superoxide dismutase (MnSOD) genetic polymorphism is associated with risk of early-onset prostate cancer. Cell Biochem Funct. 2008;26:771–7. doi: 10.1002/cbf.1504. [DOI] [PubMed] [Google Scholar]
- 40.Mikhak B, Hunter DJ, Spiegelman D, et al. Manganese Superoxide Dismutase (MnSOD) Gene Polymorphism, Interactions with Carotenoid Levels, and Prostate Cancer Risk. Carcinogenesis. 2008;29:2335–40. doi: 10.1093/carcin/bgn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–96. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 42.Look MP, Musch E. Lipid peroxides in the polychemotherapy of cancer patients. Chemotherapy. 1994;40:8–15. doi: 10.1159/000239163. [DOI] [PubMed] [Google Scholar]
- 43.al Bekairi AM, Shah AH, Chaudhry MA, Qureshi S. Uric acid as an inhibitor of biochemical changes induced by cyclophosphamide in mice. Toxicol Lett. 1991;58:69–75. doi: 10.1016/0378-4274(91)90192-9. [DOI] [PubMed] [Google Scholar]
- 44.Sudharsan PT, Mythili Y, Selvakumar E, Varalakshmi P. Cardioprotective effect of pentacyclic triterpene, lupeol and its ester on cyclophosphamide-induced oxidative stress. Hum Exp Toxicol. 2005;24:313–8. doi: 10.1191/0960327105ht530oa. [DOI] [PubMed] [Google Scholar]
- 45.Ueta E, Yoneda K, Kimura T, et al. Mn-SOD antisense upregulates in vivo apoptosis of squamous cell carcinoma cells by anticancer drugs and gamma-rays regulating expression of the BCL-2 family proteins, COX-2 and p21. Int J Cancer. 2001;94:545–50. doi: 10.1002/ijc.1513. [DOI] [PubMed] [Google Scholar]
- 46.Ueta E, Yoneda K, Yamamoto T, Osaki T. Manganese superoxide dismutase negatively regulates the induction of apoptosis by 5-fluorouracil, peplomycin and gamma-rays in squamous cell carcinoma cells. Jpn J Cancer Res. 1999;90:555–64. doi: 10.1111/j.1349-7006.1999.tb00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung YM, Park S, Park JK, Kim Y, Kang Y, Yoo YD. Establishment and characterization of 5-fluorouracil-resistant gastric cancer cells. Cancer Lett. 2000;159:95–101. doi: 10.1016/s0304-3835(00)00535-8. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Kirk JS, Venkataraman S, et al. Manganese superoxide dismutase suppresses hypoxic induction of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Oncogene. 2005;24:8154–66. doi: 10.1038/sj.onc.1208986. [DOI] [PubMed] [Google Scholar]
- 49.Kattan Z, Minig V, Leroy P, Dauca M, Becuwe P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res Treat. 2008;108:203–15. doi: 10.1007/s10549-007-9597-5. [DOI] [PubMed] [Google Scholar]
- 50.Aas T, Borresen AL, Geisler S, et al. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811–4. doi: 10.1038/nm0796-811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


