Abstract
Although it is well established that hallucinogens act as 5-HT2A and 5-HT2C receptor agonists, little is known about the relative contributions of 5-HT2A and 5-HT2C receptors to the acute behavioral effects of these drugs. The behavioral pattern monitor was used to characterize the effects of the hallucinogen 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) on locomotor and investigatory behavior in mice. Studies were also conducted to assess the contributions of 5-HT2A and 5-HT2C receptors to the behavioral effects of DOI. DOI produced an inverted U-shaped dose response function, with lower doses (0.625–5.0 mg/kg) increasing and higher doses (≥10 mg/kg) decreasing locomotor activity. The increase in locomotor activity induced by 1.0 mg/kg DOI was absent in 5-HT2A receptor KO mice, suggesting the involvement of 5-HT2A receptors. The reduction in locomotor activity produced by 10 mg/kg DOI was potentiated in 5-HT2A KO mice and attenuated by pretreatment with the selective 5-HT2C/2B antagonist SER-082. These data indicate that the decrease in locomotor activity induced by 10 mg/kg DOI is mediated by 5-HT2C receptors, an interpretation that is supported by the finding that the selective 5-HT2C agonist WAY 161,503 produces reductions in locomotor activity that are potentiated in 5HT2A KO mice. These results demonstrate for the first time that 5-HT2A and 5-HT2C receptors both contribute to the effects of DOI on locomotor activity in mice. Furthermore, these data also suggest that 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity.
Keywords: hallucinogen, mice, locomotor activity, knockout, serotonin, DOI
There are seven classes of serotonin (5-HT) receptors (5-HT1 through 5-HT7) that are divided into 14 subfamilies (Nichols and Nichols, 2008). The 5-HT2 class includes three subtypes of G-protein-coupled receptors, classified as 5-HT2A, 5-HT2B and 5-HT2C. Members of the indoleamine and phenylalkylamine classes of serotonergic hallucinogens bind with high-affinity to 5-HT receptor subtypes. Indoleamine hallucinogens such as psilocybin and lysergic acid diethylamide (LSD) are nonselective 5-HT receptor agonists. Phenylalkylamine hallucinogens such as 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) are selective 5-HT2 receptor agonists (Pierce and Peroutka, 1989; Titeler et al., 1988) that are relatively nonselective for 5-HT2A versus 5-HT2C receptors.
Hallucinogen use by humans over the past 30 years has remained relatively stable (Chilcoat and Schtitz, 1996), and the effects of these compounds continue to be of interest from a clinical standpoint (Geyer and Vollenweider, 2008). In humans, serotonergic hallucinogens intensify affective responses and produce profound alterations in visual, auditory, tactile, and olfactory perception. In animals, serotonergic hallucinogens appear to exacerbate neophobia, increase responsiveness to sensory stimulation, and interfere with response habituation across multiple sensory modalities and behavioral responses (for review, see: Geyer and Vollenweider, 2008). The use of animal models of the effects of hallucinogens supports hypothesis testing regarding the neurochemical substrates of hallucinogenesis.
In rats, several lines of evidence indicate that the behavioral effects of phenylalkylamine hallucinogens are mediated primarily by 5-HT2A receptors. For example, an antagonist correlation analysis study has revealed that the potency of 5-HT2 antagonists to block the substitution of R(−)-DOM in rats trained to discriminate LSD is robustly and significantly correlated with their 5-HT2A receptor affinity but not 5-HT2C affinity (Fiorella et al., 1995a). Likewise, the potency of 5-HT2 antagonists to block the head twitch response (HTR) induced by DOI is significantly correlated with their 5-HT2A affinity (Schreiber et al., 1995). Studies employing 5-HT2A- and 5-HT2C-selective antagonists have provided considerable support for the involvement of 5-HT2A receptors in the behavioral effects of phenylalkylamine hallucinogens. The effects of DOI in a number of behavioral paradigms—including drug discrimination, HTR, and prepulse inhibition (PPI) of acoustic startle—are antagonized by the 5-HT2A-selective antagonist M100907 but not by selective 5-HT2C/2B antagonists such as SB 200,646A, SB 206,553, and SER-082 (Smith et al., 1998, 1999; Schreiber et al., 1994, 1995; Sipes and Geyer, 1995; Willins and Meltzer, 1997; Wettstein et al., 1999). Indeed, human studies have confirmed that 5-HT2A receptor activation is responsible for the hallucinogenic effects of psilocybin (Vollenweider et al., 1998).
Previous work in this laboratory has established that the behavioral pattern monitor (BPM) can be used to characterize the acute behavioral effects evoked by hallucinogens in rats. The BPM combines the features of activity and holeboard chambers and is designed to assess both the quantity and several aspects of the quality of unconditioned locomotor and investigatory activity in rats (Geyer et al., 1986). DOM and DOI produce characteristic effects in the BPM consisting primarily of reduced locomotor and investigatory responding and increased avoidance of central areas of the BPM chamber (Adams and Geyer, 1985;Wing et al., 1990; Mittman and Geyer, 1991; Krebs-Thomson et al., 2006). 5-HT2 antagonists have been shown to block the effects of phenylalkylamine hallucinogens in the BPM (Wing et al., 1990; Mittman and Geyer, 1991). The effects of DOI in the BPM are blocked by M100907 but not by SER-082 (Krebs-Thomson et al., 1998), indicating that the ability of DOI to reduce locomotor activity and suppress investigatory responding is attributable to activation of 5-HT2A receptors but not 5-HT2C receptors.
To date, few studies have examined the relative contributions of 5-HT2A and 5-HT2C receptors to the behavioral effects of hallucinogens in mice. It has been reported that the hallucinogen-induced HTR in mice is antagonized by M100907 (Fantegrossi et al., 2005, 2006, 2008) and abolished in 5-HT2A knockout (KO) mice (González-Maeso et al., 2003, 2007). Smith et al. (2003) found that DOI discrimination in mice is completely blocked by pretreatment with M100907. However, SB 206,553 and the 5-HT2C-selective antagonist SB 242,084 significantly attenuated DOI-induced responding, suggesting that 5-HT2C receptors may also play a role in mediating the DOI discriminative stimulus in mice. It is unclear, however, if 5-HT2C receptors play a role only in DOI discrimination, which involves repeated dosing with the drug, or if 5-HT2C receptors also contribute to acute effects of DOI. The objective of the present investigation was to characterize the effects of DOI on exploratory and investigatory responding in mice using a recently developed mouse Behavioral Pattern Monitor (mouse BPM; see: Risbrough et al., 2006). The behavioral effects of DOI were compared with those of the putative 5-HT2C-selective agonist WAY 161,503 (Rosenzweig-Lipson et al., 2006). Finally, a combination of genetic and pharmacological approaches was used to determine whether interactions with 5-HT2A and/or 5-HT2C receptors are responsible for mediating the behavioral effects of DOI on exploratory and investigatory behavior.
MATERIALS AND METHODS
Subjects
Mice were housed at a vivarium at the University of California San Diego (UCSD), an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Male C57BL/6J mice were obtained from Jackson Labs (Bar Harbor, ME); they were allowed to acclimate for approximately 1 week after arrival. The 5-HT2A wild-type (WT) and knockout (KO) mice were bred in-house; these animals, originally generated at Columbia University (New York, NY) on a 129S6/SvEv background (González-Maeso et al., 2003, 2007), were backcrossed (N10) onto the inbred C57BL/6 line. All breeding was conducted using heterozygous breeding pairs to remove the possibility of genetic drift between WT and KO mice and to ensure that all mice received equivalent maternal care. The 5-HT2A WT and KO mice were weaned at 21–24 days of age, during which a small portion of the tail (1.5 cm) was removed for subsequent genotyping. All mice were housed n=4/cage, separated by sex, in a climate-controlled room with a reversed light cycle (lights on at 2000 hours, off at 0800 hours). Food and water were provided ad libitum, except during behavioral testing. All testing occurred between 1000 and 1800 hours; animal testing was conducted in accord with the “Principles of laboratory Animal Care” NIH guidelines and were approved by the UCSD animal care committee.
Drugs
Drugs used were 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI; Sigma Chemical Co., St. Louis, MO); (+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-metylindolo[1,7-bc][2,6]naphthyridine fumarate (SER-082) and 8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5(6H)-one hydrochloride (WAY 161,503; Tocris Bioscience, Ellisville, MO). DOI and SER-082 were dissolved in isotonic saline. WAY 161,503 was dissolved in saline containing 2.5% Tween 80. DOI and WAY 161,503 were administered intraperitoneally at a volume of 5 ml/kg body weight. SER-082 was administered subcutaneously at a volume of 5 ml/kg body weight.
Apparatus
Investigatory behavior and locomotor activity were measured in 10 mouse behavioral pattern monitors (BPM; San Diego Instruments, San Diego, CA). The design of the mouse BPM system is based on the rat BPM (for a detailed description, see: Geyer et al., 1986). The mouse BPM chamber is a clear Plexiglas box containing a 30 × 60 cm holeboard floor. Each chamber is enclosed in a ventilated outer box to protect it from light and ambient noise from outside the chambers. The chamber contains eleven 1.4-cm holes (three in the floor and eight in the walls), each provided with an infrared photobeam to detect investigatory nosepokes (holepokes). The location of the mouse is obtained from a grid of 12 × 24 photobeams 1 cm above the floor. Rearing is detected by an array of 16 photobeams placed 2.5 cm above the floor and aligned with the long axis of the chamber. The status of the photobeams is sampled every 55 msec. A change in the status of photobeams triggers the storage of the information in a binary data file together with the duration of the photobeam status. Subsequently, the raw data files are transformed into (x,y,t,event) ASCII data files comprised of the (x,y) location of the animal in the mouse BPM chamber with a resolution of 1.25 cm, the duration of each event (t), and whether a holepoke or rearing occurred (event).
Mice were tested in the dark and during the dark phase of their light cycle. The animals were brought into the testing room under black cloth one hour before testing. During testing, a white noise generator produced background noise at 65 dB. Pretreatment and test injections were made under red lights in the testing room. Data were collected for 60 min. The chambers were cleaned thoroughly between testing sessions.
Analysis
Horizontal locomotor activity was quantified as distance traveled. The number of holepokes and rearings were calculated as measures of investigatory behavior. Data were examined in 10-min and 30-min time resolutions. Data were analyzed using three-way ANOVA with pretreatment and treatment as between-factors and time as a repeated measure. Specific post hoc comparisons between selected groups were done using Dunnett’s many-to-one test or Tukey’s studentized range method. Significance was demonstrated by surpassing an alpha level of 0.05. In Experiment 2, genotype was the between-subject variable and drug and time were within-subject variables. Post-hoc simple ANOVAs with the appropriate alpha correction were conducted; significance demonstrated by surpassing an alpha level of 0.025.
Experimental Design
Animals were placed in the mouse BPM chambers 15 min after treatment with DOI, or 10 min after treatment with WAY 161,503. In experiment 1, mice (n=10–11, 63 total) were treated with vehicle, 0.625, 1.25, 2.5, 5.0, or 10 mg/kg DOI. In experiment 2, the 5-HT2A cohort consisted of 14 WT and 13 KO male mice, and 13 WT and 5 KO female mice. The mice were tested in a three-way crossover design with 1 week between tests. Each animal received vehicle as well as 1.0 and 10 mg/kg DOI in a semi-randomized, counterbalanced order, to complete a within-subject design. In experiment 3, mice (n=10, 60 total) were treated with SER-082 (vehicle or 1.0 mg/kg) 15 min before administration of DOI (vehicle, 1.0, or 10 mg/kg). In experiment 4, mice (n=8–10, 38 total) were treated with vehicle or 3, 10, or 30 mg/kg WAY 161,503. In experiment 5, mice (n=8–10, 38 total) were treated with SER-082 (vehicle or 1.0 mg/kg) 20 min before administration of WAY 161,503 (vehicle, 1, or 10 mg/kg). In experiment 6, a new 5-HT2A cohort consisted of 8 WT and 10 KO male mice, and 9 WT and 11 KO female mice. WT and KO mice were injected with either vehicle or WAY 161,503 (10 mg/kg) in a between subjects design.
RESULTS
Experiment 1: Effect of DOI on locomotor and investigatory behavior
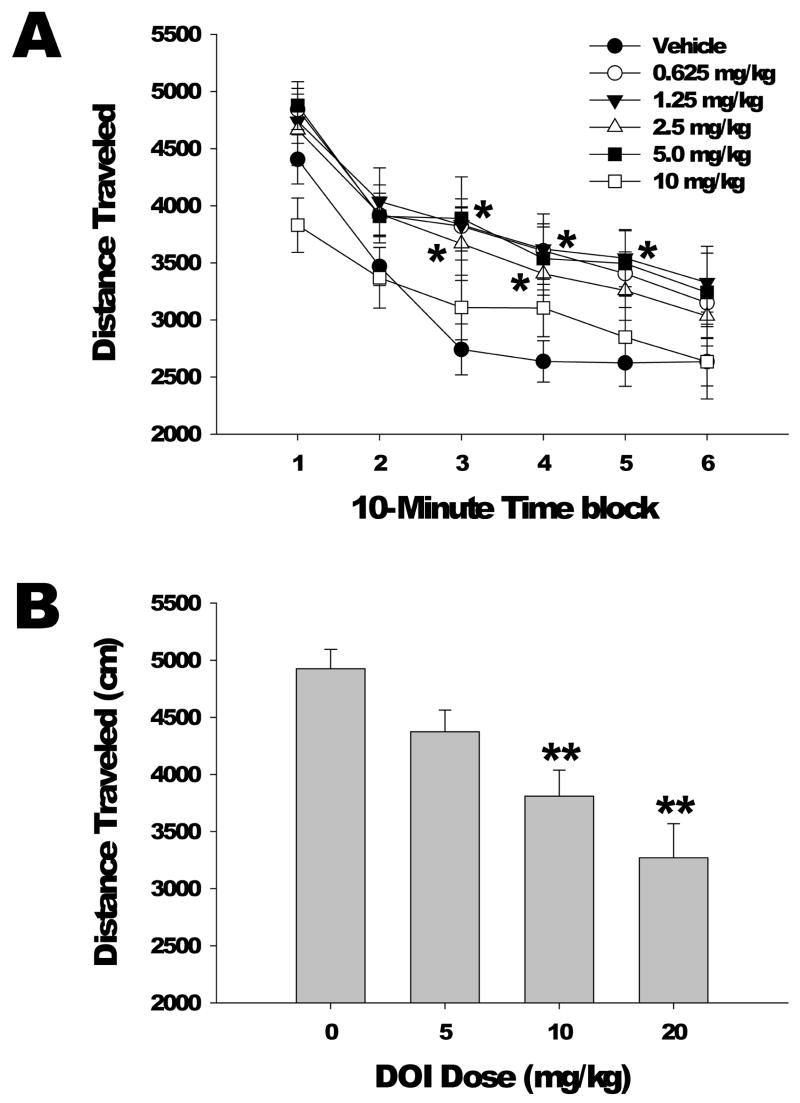
The effect of varying doses of DOI on distance traveled, a measure of locomotor activity, is shown in Figure 1a for successive 10-min intervals of the 1-h test session. DOI administration produced an inverted U-shaped dose-response function on distance traveled (F(5,57)=2.84, p<0.03). The 0.625, 1.25 mg/kg, and 5.0 mg/kg doses of DOI produced a delayed increase in distance traveled compared with vehicle, with post hoc analysis indicating these dosage groups had significantly higher locomotor activity during the last 40 min of the test session (p<0.05, Dunnett’s test). Conversely, compared with vehicle, there was a nonsignificant trend for the highest dose of DOI tested (10 mg/kg) to reduce distance traveled during the initial 10 min of testing (mean ± S.E.M.: vehicle = 4405.9 ± 215.2 cm, 10 mg/kg = 3829.3 ± 238.3 cm, F(1,19)=3.24, p<0.09).
Figure 1.
Effects of DOI on locomotor activity. (a) Dose response of DOI effects on distance traveled (in cm). Mice used were male C57BL/6J. Data are mean±SEM. *p<0.05, Dunnett’s test vs vehicle control. (b) The effects of high doses of DOI on locomotor activity were evaluated in a second dose response experiment. Data shown are the distance traveled (cm) during the first 10 min of testing. Data are mean±SEM. **p<0.01, Dunnett’s test vs vehicle control.
As shown in Table 1, mice treated with DOI made significantly fewer holepokes (F(5,57)=6.69, p=0.0001) and rearings (F(5,57)=10.12, p<0.0001). Specific comparisons revealed that the 10 mg/kg dose of DOI decreased the amount of holepoking behavior (p<0.01, Dunnett’s test), and the 2.5, 5.0 and 10 mg/kg doses of DOI decreased the number of rearings (p<0.01, Dunnett’s test).
Table 1.
Effect of DOI and WAY 161,503 on rearings and holepokes during the first 30 min of testing.
| Rearings | ||||
|---|---|---|---|---|
| 5-HT2A |
SER-082 |
|||
| WT | KO | Veh | 1.0 | |
| DOI: 0 | 197.1±13.3 | 155.2±15.2 | 212.8±13.9 | 184.6±19.2 |
| 1 | 150.0±10.4 | 161.2±11.6 | 193.0±15.9 | 185.8±16.0 |
| 10 | 75.8±7.8 | 104.4±8.7 | 63.3±10.8 | 96.2±12.4 |
| 5-HT2A |
SER-082 |
|||
| WT | KO | Veh | 1.0 | |
| WAY: 0 | 154.8±28.2 | 108.6±21.2 | 103.9±9.1 | 113.1±13.1 |
| 10 | 37.6±11.8 | 28.8±8.6 | 13.5±3.3 | 40.9±5.1 |
| Holepokes | ||||
| 5-HT2A |
SER-082 |
|||
| WT | KO | Veh | 1.0 | |
| DOI: 0 | 99.5±8.4 | 120.0±15.8 | 99.4±9.8 | 94.3±8.0 |
| 1 | 134.0±12.2 | 101.1±10.1 | 102.6±21.0 | 97.6±9.4 |
| 10 | 61.3±8.4 | 62.8±6.7 | 45.4±7.4 | 59.7±8.8 |
| 5-HT2A |
SER-082 |
|||
| WT | KO | Veh | 1.0 | |
| WAY: 0 | 153.1±28.4 | 173.8±17.7 | 119.4±14.2 | 123.8±14.3 |
| 10 | 70.3±22.6 | 24.1±7.0 | 24.3±5.5 | 86.8±13.8 |
To further characterize the decrease in locomotor activity induced by higher doses of DOI, we conduced a second DOI dose-response experiment. Administration of high doses of DOI (5.0, 10 and 20 mg/kg) produced decreases in distance traveled (F(3,43)=3.80, p<0.02) that were most pronounced during the initial blocks of testing (Drug × Time: F(15,215)=4.88, P<0.0001). Post hoc analysis indicated that the 10 mg/kg dose of DOI significantly reduced distance traveled during the initial 20 min of the test session and the 20 mg/kg dose of DOI significantly reduced distance traveled during the first 30 min of the test session (p<0.05, 0.01, Dunnett’s test; Figure 1b).
Experiment 2: Effect of 5-HT2A receptor gene deletion on the behavioral response to DOI
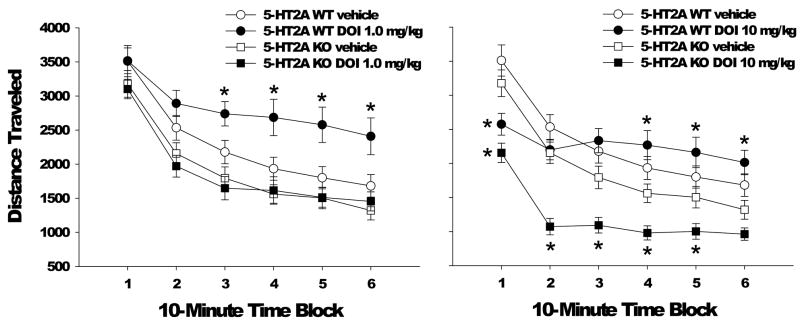
Although there was a main effect of sex on distance traveled (F(1,41)=4.18, p<0.05), there was no interaction between sex and either gene or drug, or between sex, gene, and drug, so data were collapsed across sex. The effect of DOI treatment on distance traveled in 5-HT2A WT and KO mice is illustrated in Figure 2. Treatment with 1 mg/kg DOI had no effect on locomotor activity in 5-HT2A KO mice (Gene × Drug × Time: F(5,215)=2.45, p<0.04). Conversely, 1 mg/kg DOI increased distance traveled in WT mice (F(1,43)=6.65, p<0.02), an effect that occurred primarily during the last 40 min of the 1-h session (Drug × Time: F(5,215)=5.18, p<0.0001). As observed previously in C57BL/6J mice, administration of 10 mg/kg DOI to WT mice reduced distance traveled during the initial blocks of testing (Drug × Time: F(5,215)=20.22, p<0.0001). Interestingly, the duration of DOI-induced hypoactivity was significantly prolonged in 5-HT2A KO mice (Gene × Drug: F(1,43)=17.42, p=0.0001). Post-hoc ANOVAs confirmed that 10 mg/kg DOI significantly reduced distance traveled during the first 50 min of the 1-h session. At baseline, 5-HT2A KO mice display a hypoactive phenotype (F(1,43)=15.66, p=0.0003) relative to their WT littermates. However, post-hoc ANOVAs failed to reveal any 10-min time block during which the 5-HT2A KO mice significantly reduced distance traveled relative to WT mice.
Figure 2.
Effect of 5-HT2A gene deletion on the locomotor response to DOI. Effect of vehicle or 1 mg/kg DOI (left panel) or vehicle or 10 mg/kg DOI (right panel) on distance traveled (in cm) in male and female 5-HT2A WT and KO mice. Data are mean±SEM. *p<0.025 compared to vehicle control
There was an overall effect of 1.0 mg/kg DOI treatment on rearings (F(1,43)=5.46, p<0.03), and an interaction between gene and 1.0 mg/kg DOI (F(1,43)=9.15, p<0.005; Table 1). There was no effect of treatment with 1.0 mg/kg DOI on holepokes. There was an overall effect of 10 mg/kg DOI treatment on rearings (F(1,43)=73.55, p<0.0001), and an interaction between gene and 10 mg/kg DOI (F(1,43)=12.37, p<0.001). Although there was an overall effect of treatment with 10 mg/kg DOI on holepokes (F(1,43)=29.97, p<0.0001), there was no interaction between gene and drug for this behavioral measure.
Experiment 3: Effect of SER-082 on the behavioral response to DOI
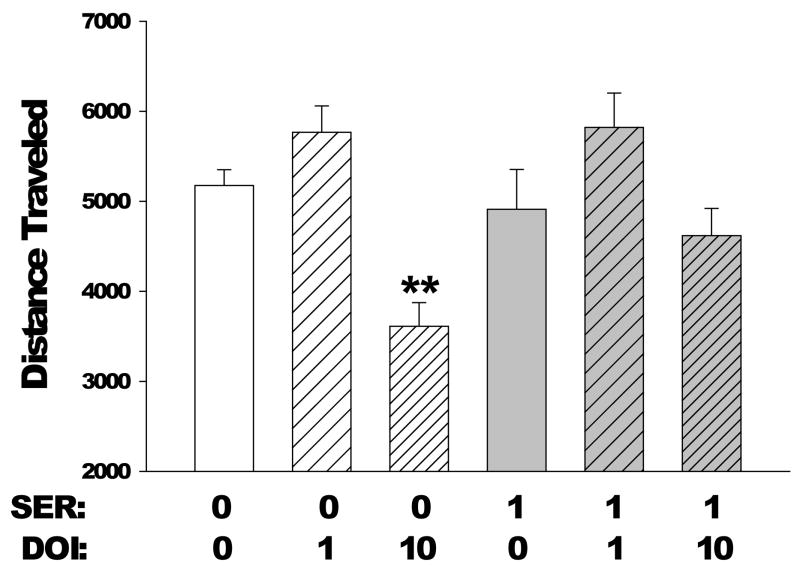
As expected, treatment with 1.0 mg/kg DOI produced an increase in distance traveled (F(1,36)=18.04, p=0.0001), but there was no interaction between SER-082 pretreatment and 1.0 mg/kg DOI treatment. Treatment with a higher dose of DOI (10 mg/kg) reduced distance traveled during the initial blocks of testing, resulting in an interaction between drug and time (F(5,180)=8.44, p<0.0001). Pretreatment with the 5-HT2C/2B antagonist SER-082 attenuated the reduction of locomotor activity induced by 10 mg/kg DOI, resulting in an interaction between SER-082 pretreatment and 10 mg/kg DOI treatment during the first 10 min of testing (F(1,36)=4.63, p<0.04). Post-hoc analysis indicated that there was a trend toward blockade of the DOI-induced decrease in locomotor activity (Figure 3; p<0.1, Tukey’s test). There was an interaction of SER-082 pretreatment with time (F(5,270)=6.16, p<0.0001), but post hoc analysis failed to confirm the effect of SER-082 for any specific time block.
Figure 3.
Effect of pretreatment with SER-082 on the locomotor response (measured as distance traveled) to DOI during the first 10 min of testing. Mice used were male C57BL/6J. Doses are in mg/kg. Data are mean±SEM. **p<0.01, Tukey’s test vs vehicle-vehicle control.
There was no effect of treatment with 1.0 mg/kg DOI on rearings or holepokes (Table 1). By contrast, there was an overall effect of 10 mg/kg DOI treatment on rearings (F(1,36)=67.68, p<0.0001), and an interaction between SER-082 and 10 mg/kg DOI (F(1,36)=4.46, p<0.05). Specific comparisons revealed that 10 mg/kg DOI significantly decreased rearings (p<0.01, Tukey’s test), an effect that was non-significantly attenuated by pretreatment with SER-082. Although treatment with 10 mg/kg DOI had an overall effect on holepoke frequency (F(1,36)=26.79, p<0.0001), there was no specific interaction between SER-082 and 10 mg/kg DOI.
Experiment 4: Effect of WAY 161,503 on locomotor and investigatory behavior
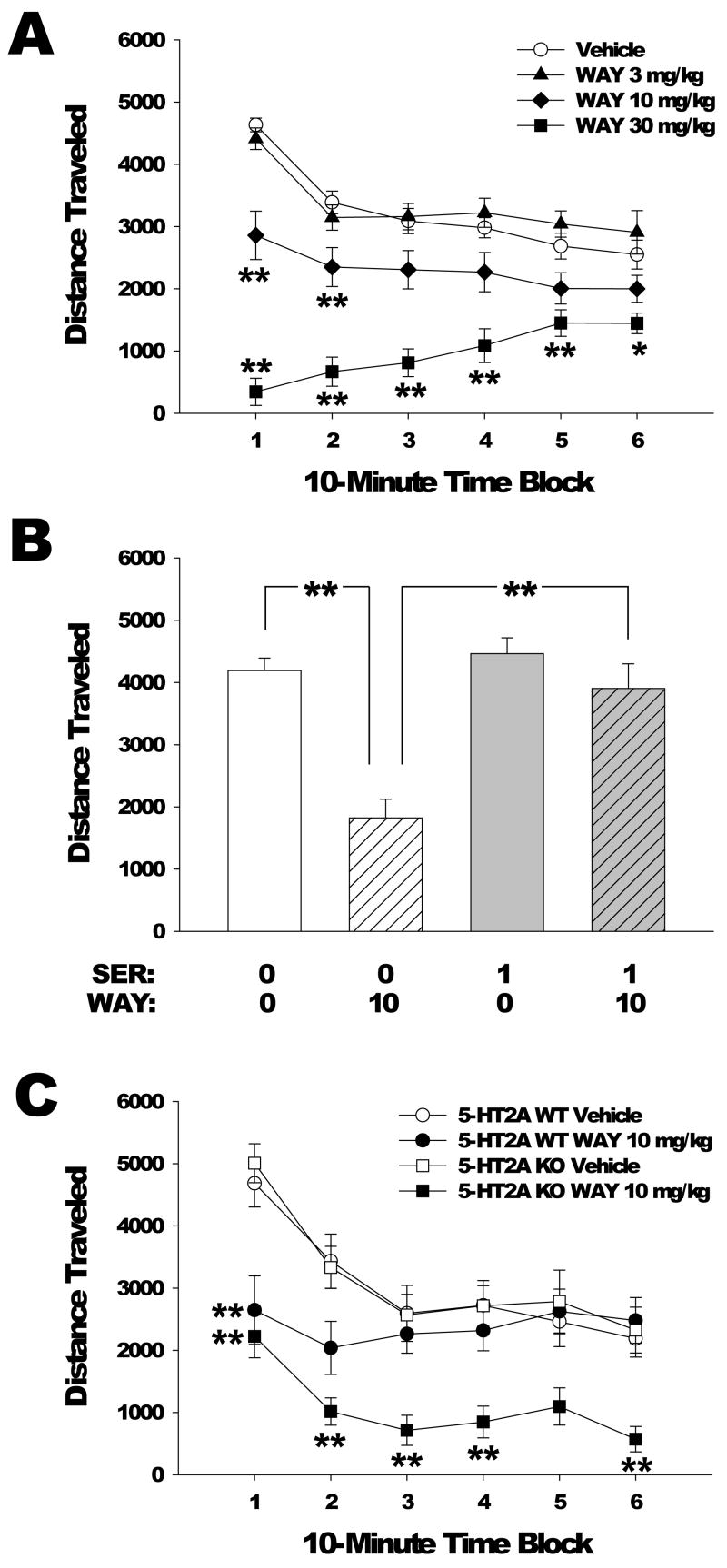
Treatment with the 5-HT2C-selective agonist WAY 161,503 had a significant effect on distance traveled (F(3,42)=26.22, p<0.0001), and there was a significant interaction of WAY 161,503 treatment with time (F(15,170)=10.92, p<0.0001). The high dose, 30 mg/kg, was the most effective, with post hoc analysis indicating this group had significantly lower distance traveled compared with vehicle in all time blocks, whereas the decrease induced by the 10 mg/kg dose reached significance only during the first 20 min of the session (Figure 4a; p<0.01, Dunnett’s test).
Figure 4.
Effects of WAY-161,503 on locomotor activity. (a) Dose response of WAY 161,503 effects on distance traveled (in cm). Mice used were male C57BL/6J. Data are mean±SEM. **p<0.01, Dunnett’s test vs vehicle control. (b) Effect of pretreatment with SER-082 on the locomotor response (measured as distance traveled) to WAY 161,503 during the first 10 min of testing. Mice used were male C57BL/6J. Doses are in mg/kg. Data are mean±SEM. **p<0.01, Tukey’s test. (c) Effect of 5-HT2A gene deletion on the locomotor response to vehicle or 10 mg/kg WAY 161,503 in male and female 5-HT2A WT and KO mice. Data are mean±SEM. **p<0.01, Tukey’s test vs 5-HT2A WT vehicle.
WAY 161,503 reduced the number of holepokes (F(3,34)=28.02, p<0.0001) and rearings (F(3,34)=42.58, p<0.0001). Inspection of the data revealed that all three doses of WAY 161,503 significantly decreased the amount of holepoking behavior (p<0.05, 0.01, Dunnett’s test; data not shown), and the 10 and 30 mg/kg doses of WAY 161,503 significantly decreased the number of rearings (p<0.01, Dunnett’s test).
Experiment 5: Effect of SER-082 on the behavioral response to WAY 161,503
As in the previous study, 10 mg/kg WAY 161,503 reduced distance traveled. This effect was most pronounced during the first 10 min of testing, yielding an interaction of treatment with time (F(5,140)=18.41, p<0.0001). There was a three-way interaction between SER-082 pretreatment, WAY 161,503 treatment, and time (F(5,140)=7.24, p<0.0001). As shown in Fig. 4b, SER-082 pretreatment significantly antagonized the decrease in distance traveled induced by WAY 161,503 during the first 10-min time block (p<0.01, Tukey’s test). In addition, there was an overall effect of SER-082 pretreatment on distance traveled (F(1,28)=5.79, p<0.03), and an interaction of SER-082 pretreatment with time (F(5,140)=7.50, p<0.0001), but post hoc analysis failed to confirm this effect for any specific time block.
There was an effect of WAY 161,503 treatment on holepoking (Table 1; F(1,28)=27.88, p<0.0001), and an interaction between SER-082 and WAY 161,503 (F(1,28)=5.43, p<0.03). Specific comparisons revealed that WAY 161,503 significantly decreased holepoking (p<0.01, Tukey’s test), and this effect was antagonized by pretreatment with SER-082 (p<0.01 versus vehicle-WAY 161,503 group, Tukey’s test). There was also a significant effect of SER-082 pretreatment on holepoking (F(1,28)=7.14, p<0.02). For rearings, there was no specific interaction between SER-082 pretreatment and WAY 161,503 treatment. There were significant main effects of SER-082 (F(1,28)=4.6, p<0.05) and WAY 161,503 (F(1,28)=90.72, p<0.0001) on rearings.
Experiment 6: Effect of 5-HT2A receptor gene deletion on the behavioral response to WAY 161,503
WAY 161,503 significantly reduced distance traveled (F(1,34)=19.47, p<0.0001), and this effect was augmented in 5-HT2A KO mice (Gene × Drug: F(1,34)=5.58, p<0.03). Specific comparisons demonstrated that compared with WT mice the effect of WAY 161,503 was significantly potentiated in 5-HT2A KO mice during the third, fourth, and sixth 10-min blocks of the 1-h test session (Figure 4c; p<0.05, 0.01, Tukey’s test). Although there was an overall effect of treatment with 10 mg/kg WAY 161,503 on holepokes (F(1,34)=35.64, p<0.0001) and rearings (F(1,34)=27.99, p<0.0001), there was no interaction between gene and drug for these behavioral measures (Table 1).
DISCUSSION
It is well established that phenylalkylamine hallucinogens such as DOI are potent agonists at 5-HT2 receptors. In the present study, we examined the effect of treatment with DOI on locomotor and investigatory behavior in mice, and assessed the contributions of 5-HT2A and 5-HT2C receptors to these behavioral effects. DOI produced an inverted U-shaped dose response function, with lower doses (~1.0 mg/kg) increasing and higher doses (≥10.0 mg/kg) decreasing locomotor activity. The increased locomotor activity was blocked in 5-HT2A receptor KO mice, suggesting the involvement of 5-HT2A receptors in the locomotor-stimulating effects of DOI. The decreased locomotor activity produced by the 10 mg/kg dose of DOI was mimicked by the selective 5-HT2C agonist WAY 161,503 and blocked by the 5-HT2C antagonist SER-082. Additionally, the decreased locomotor activity produced by 10 mg/kg DOI was potentiated in the 5-HT2A KO mice. These data suggest that the decrease in locomotor activity produced by the 10 mg/kg dose of DOI was mediated by 5-HT2C receptors. Similarly, the reduction in locomotor activity produced by WAY-161,503 was potentiated in 5-HT2A KO mice. The fact that DOI produces dose-dependent effects on locomotion in mice that are mediated by 5-HT2A and 5-HT2C receptors is a novel finding; previous studies in rats have indicated that the locomotor effects of DOI are mediated exclusively by 5-HT2A receptors (Krebs-Thomson et al., 1998). Taken together, these data also suggest that 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice.
The inverted U-shaped dose response function of DOI has not previously been reported in mice. There have been independent reports of DOI increasing (Darmani et al. 1996) and decreasing (González-Maeso et al. 2007) locomotor activity in different strains of mice. DOI at doses of 1 and 2.5 mg/kg increased locomotor activity in young albino ICR mice (Darmani et al. 1996). In contrast, 10 mg/kg DOI decreased locomotor activity in 5-HT2A WT and KO mice on a 129SvJ background (González-Maeso et al. 2007). Hence, there is evidence that lower doses of DOI will increase locomotor activity and that higher doses of DOI will decrease locomotor activity in some strains of mice as we have reported here.
WAY 161,503 is a 5-HT2C agonist that displays approximately six-fold higher affinity for 5-HT2C receptors (Ki = 3.3 nM) than for 5-HT2A receptors (Ki = 18 nM; Rosenzweig-Lipson et al., 2006). The reported affinity values are for human 5-HT2A and 5-HT2C receptors. It is possible that WAY 161,503 may display even greater selectivity for 5-HT2C sites in mice. Two pieces of evidence demonstrate that the decrease in locomotor activity induced by WAY 161,503 is mediated by 5-HT2C receptors and not by 5-HT2A receptors. First, the locomotor effects of WAY 161,503 were blocked by the preferential 5-HT2C/2B antagonist SER-082. Second, WAY 161,503 reduced locomotor activity in 5-HT2A KO mice, indicating that this receptor subtype does not mediate the behavioral effect. In fact, it is possible that the locomotor depressant effect of WAY 161,503 was significantly potentiated in 5-HT2A KO mice because of the loss of countervailing 5-HT2A receptor stimulation by WAY 161,503 in those animals. Taken together, these findings suggest that at the doses tested the locomotor effects of WAY 161,503 are mediated by activation of 5-HT2C receptors.
From the experiment with DOI in 5-HT2A KO mice, it appears that the locomotor-increasing effects of the low dose of DOI are mediated by 5-HT2A receptors. A similar role of 5-HT2A receptors in the behavioral effects of hallucinogens in mice has been reported by other studies. Specifically, hallucinogen-induced HTR in mice is antagonized by M100907 (Fantegrossi et al., 2005, 2006, 2008) and abolished in 5-HT2A knockout (KO) mice (González-Maeso et al., 2003, 2007). Smith et al. (2003) found that DOI discrimination in mice is completely blocked by pretreatment with M100907. However, SB 206,553 and the 5-HT2C-selective antagonist SB 242,084 attenuated DOI-induced responding, suggesting that 5-HT2C receptors may also play a role in mediating the DOI discriminative stimulus in mice. Our data suggest that decreases in locomotor activity produced by 10 mg/kg DOI are mediated by 5-HT2C receptors and not 5-HT2A receptors. First, the selective 5-HT2C agonist WAY 161,503 dose-dependently decreased locomotor activity, an effect that was blocked by the 5-HT2C/2B antagonist SER-082. Second, the DOI-induced hypolocomotion was blocked by SER-082. Third, similar to the reports in 5-HT2A KO mice on a 129SV/J background (González-Maeso et al., 2007), the DOI-induced hypolocomotion was not attenuated in 5-HT2A KO mice. These data are corroborated by other reports in the literature of the locomotor-reducing effects of 5-HT2C agonists. For example, mCPP decreases locomotor activity in mice via 5-HT2C receptors (Gleason et al. 2001), and the 5-HT2C-selective agonists Ro 60-0175 and Ro 60-0332 decrease locomotor activity in rats (Martin et al., 1998).
We have shown previously that the effects of DOI in rats in the BPM are blocked by M100907 but not by SER-082 (Krebs-Thompson et al., 1998), indicating that the effects are mediated exclusively by 5-HT2A receptors. In contrast to those previous findings in rats, it is now apparent that both 5-HT2A and 5-HT2C receptors are involved in mediating the locomotor effects of DOI in mice. These results suggest that 5-HT2C receptors make a much more significant contribution to the behavioral effects of DOI in mice than they do in rats. Similar findings have been reported by drug discrimination studies. Specifically, whereas DOI-induced stimulus control in rats is mediated exclusively by 5-HT2A receptors (Schreiber et al., 1994; Smith et al., 1999), in mice there is evidence of involvement of both 5-HT2A and 5-HT2C receptors (Smith et al., 2003).
Our data suggest that 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. As discussed above, it appears that 5-HT2A receptor activation increases locomotor activity; whereas 5-HT2C receptors decrease locomotor activity. One of the more interesting findings from these studies is that the locomotor-decreasing effect of the high dose of DOI is potentiated in 5-HT2A KO mice, suggesting that the 5-HT2A receptor serves to mask the effect of DOI at reducing locomotor activity via the 5-HT2C receptor. A similar pattern of results was reported in 5-HT2A KO mice on a 129SV/J background (González-Maeso et al., 2007). The lower dose of DOI tested in that study (2 mg/kg), which failed to decrease locomotor activity in 5-HT2A WT mice, did decrease locomotor activity in 5-HT2A KO mice, similar to what was reported with the higher dose of DOI (10 mg/kg; González-Maeso et al., 2007).
The behavioral profile of DOI which we observed in the current studies differs from that observed in rats. In rats, DOI consistently decreases locomotor activity in the BPM at doses as low as 0.3 mg/kg (Hameleers et al., 2007; Krebs-Thomson & Geyer 1998; Mittman and Geyer, 1991; Wing et al., 1990). Similar to our data, however, other hallucinogens have been shown to increase locomotor activity in mice. For example, LSD produces a delayed increase in activity in 129/Sv mice, an effect that is partially mediated by 5-HT5A receptors (Grailhe et al., 1999). Similarly, in our hands DOM increases locomotor activity in C57BL/6 mice (unpublished observations). Experiments are underway to better characterize the behavioral effects of other hallucinogens in mice and examine the contribution of 5-HT2 receptors.
There is evidence that the 5-HT2C receptor may act to mask behavioral effects induced by 5-HT2A receptor activation in rats. For example, Ro 60-0175, a 5-HT2 agonist displaying approximately 30-fold selectivity for 5-HT2C receptors versus 5-HT2A receptors (Martin et al., 1998), does not induce the HTR unless administered in combination with a 5-HT2C-selective antagonist (Vickers et al., 2001). Given that the HTR is 5-HT2A receptor-mediated behavior (Schreiber et al., 1995; González-Maeso et al., 2003, 2007), this finding indicates that the ability of Ro 60-0175 to induce behavioral effects via the 5-HT2A receptor is suppressed by its interaction with the 5-HT2C receptor. DOI reduces PPI through a 5-HT2A-dependent mechanism (Sipes and Geyer, 1995), and it has been reported that the effect of DOI on PPI is attenuated when it is administered in combination with the 5-HT2C-selective agonist WAY-163909 (Marquis et al., 2007). There have been other reports in the literature indicating that 5-HT2A and 5-HT2C receptors can produce opposing behavioral effects. For example, selective 5-HT2A antagonists decrease impulsive responding in the five-choice serial reaction time test in rats, whereas selective 5-HT2C antagonists have the opposite effect (Winstanley et al., 2004). The present results indicate that in mice 5-HT2A and 5-HT2C receptors exert functionally antagonistic influences on locomotor activity, with activation of the former receptor producing an increase in distance traveled and activation of the latter receptor producing a decrease.
The 5-HT2A and 5-HT2C sites are Gαq-coupled receptors that activate the phosphoinositide hydrolysis signaling cascade, leading to neuronal depolarization and increases in excitability (reviewed by Aghajanian and Sanders-Bush, 2002). Activation of 5-HT2A and 5-HT2C receptors excites GABAergic interneurons in the dorsal raphe nucleus, leading to inhibition of serotonergic cell firing (Boothman et al. 2003; Boothman et al. 2006; Sharp et al. 2007). Given that 5-HT2A and 5-HT2C receptors play similar physiologic roles, it is somewhat paradoxical that these two receptors produce opposing effects on locomotor activity in mice. It should be noted, however, there are differences in the distribution of 5-HT2A and 5-HT2C receptors. The 5-HT2A receptor is expressed in cortex, olfactory tubercle, midbrain, and cerebellum, with particularly high concentrations in the proximal apical dendrites of layer V pyramidal cells in prefrontal cortex (Willins et al., 1997; Jakab and Goldman-Rakic, 1998; Cornea-Hébert et al., 1999; Xu and Pandey, 2000; Miner et al., 2003). The 5-HT2C receptor has a wider distribution in the CNS and is heavily expressed in the striatum, thalamus, and hippocampus (Clemett et al., 2000). 5-HT2A and 5-HT2C receptors have been shown to have opposite effects on activity within specific brain regions and neurochemical pathways. Bath application of 5-HT to rat piriform cortex slices induces IPSPs in layer II pyramidal cells by activating 5-HT2A receptors on GABAergic interneurons (Sheldon and Aghajanian, 1990, 1991). However, 5-HT also excites the pyramidal cells directly, by activating 5-HT2C receptors (Sheldon and Aghajanian, 1991; Marek and Aghajanian, 1994). It has also been reported that activity in the mesocortical dopaminergic pathway is regulated differentially by 5-HT2A and 5-HT2C receptors; specifically, dopamine release in frontal cortex is tonically inhibited by 5-HT2C receptors (Millan et al., 1998) and phasically facilitated by 5-HT2A receptors (Gobert and Millan, 1999). Likewise, 5-HT2A receptors increase stimulated release of dopamine from the mesoaccumbens and nigrostriatal projections (Schmidt et al., 1992; Porras et al., 2002), whereas 5-HT2C receptors inhibit dopamine release from those pathways under stimulated and basal conditions (Di Matteo et al., 1998; Di Giovanni et al., 1999; Porras et al., 2002). Thus, there is extensive evidence that 5-HT2 receptor subtypes have differing electrophysiological and neurochemical effects. The opposite effects of 5-HT2A and 5-HT2C receptor activation on locomotor activity may be linked to the fact that these receptors exert an opposing regulatory influence on dopamine release. Experiments are in progress to determine whether 5-HT2A receptors in the mesoaccumbens and mesocortical pathways are involved in the DOI-induced increase in locomotor activity, and whether the effect can be blocked by dopamine antagonists.
Similar to our findings with DOI, the increase in locomotor activity induced by (+)amphetamine also follows an inverted U-shaped dose-response function (Paulus and Geyer, 1991). The fact that higher doses of (+)-amphetamine fail to increase locomotor activity is likely attributable to the induction of stereotypies such as sniffing, licking, and biting (Randrup and Munkvad, 1967) which tend to compete with ambulation. This conclusion is supported by the fact that high doses of (+)-amphetamine produce increases in spatial d, a measure of the complexity of locomotor paths, indicating an increase in local perseverative movements (Paulus and Geyer, 1991). Although there are structural similarities between DOI and amphetamine, it does not appear that the decrease in locomotor activity induced by high doses of DOI (≥10.0 mg/kg) is a consequence of the induction of stereotypies because DOI does not reliably produce increases in spatial d (unpublished observations). DOI is known to induce abnormal behavioral responses in mice such as HTR and ear scratch response (ESR) (Darmani et al., 1990a,b; González-Maeso et al., 2007). It is possible that after administration of high doses of DOI these behaviors occur so frequently that they significantly reduce the amount of time spent locomoting. The previous finding that DOI-induced HTR and ESR are completely abolished in 5-HT2A KO mice (González-Maeso et al., 2003, 2007) argues against this interpretation since we found that 10 mg/kg DOI reduces locomotor activity in 5-HT2A KO mice (see Figure 2).
In summary, the effects of DOI on locomotor activity in mice are dose-dependent and are mediated by 5-HT2A and 5-HT2C receptors. Taken together with previous studies, these results indicate that 5-HT2C receptors make an important contribution to the behavioral effects of phenylalkylamine hallucinogens in mice. These findings demonstrate that rats and mice differ in their locomotor response to DOI, both in terms of the nature of the behavioral profile and the contribution of 5-HT2C receptors to the effect. It also appears that 5-HT2A and 5-HT2C receptors have opposing influences on locomotor activity in mice.
Acknowledgments
We would like to thank Mahalah Buell and Vincent Quinn for technical assistance, and Virginia Lehmann-Masten for assistance with data analysis. These studies were supported by R01DA02925.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that over the past three years Mark Geyer has received compensation from Abbott, Acadia, Addex, Amgen, AstraZeneca, Bristol-Myers Squibb, Jazz, Omeros, Organon, Serono, and Wyeth-Ayerst and holds an equity interest in San Diego Instruments (San Diego, CA). Adam Halberstadt, Jay Gingrich, Iris van der Heijden, Michael Ruderman, Victoria Risbrough, and Susan Powell have no conflict of interest, financial or otherwise, to declare.
References
- Adams L, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT2C receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol. 2006;149:861–869. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Schtitz CG. Age-specific patterns of hallucinogen use in the US population: an analysis using generalized additive models. Drug Alcohol Dep. 1996;43:143–153. doi: 10.1016/s0376-8716(96)01297-5. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990a;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Pharmacological characterization of ear-scratch response in mice as a behavioral model for selective 5-HT2-receptor agonists and evidence for 5-HT1B- and 5-HT2-receptor interactions. Pharmacol Biochem Behav. 1990b;37:95–99. doi: 10.1016/0091-3057(90)90047-l. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Shaddy J, Gerdes CF. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol Behav. 1996;60:1495–1500. doi: 10.1016/s0031-9384(96)00323-x. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdére P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. Selective blockade of serotonin2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience. 1999;91:587–597. doi: 10.1016/s0306-4522(98)00655-1. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Selective blockade of serotonin2C/2B receptors enhances dopamine release in the rat nucleus accumbens. Neuropharmacology. 1998;37:265–272. doi: 10.1016/s0028-3908(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Reissig CJ, Rabin RA, Winter JC. A 5-HT2C receptor-mediated interaction between 2,5-dimethoxy-4-methylamphetamine and citalopram in the rat. Pharmacol Biochem Behav. 2004;79:25–30. doi: 10.1016/j.pbb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–365. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology. 1995a;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley S, Lorrain DS, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology. 1995b;121:364–72. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Lucaites VL, Shannon HE, Nelson DL, Leander JD. m-CPP hypolocomotion is selectively antagonized by compounds with high affinity for 5-HT2C receptors but not 5-HT2A or 5-HT2B receptors. Behav Pharmacol. 2001;12:613–620. doi: 10.1097/00008877-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Hameleers R, Blokland A, Steinbusch HW, Visser-Vandewalle V, Temel Y. Hypomobility after DOI administration can be reversed by subthalamic nucleus deep brain stimulation. Behav Brain Res. 2007;185:65–67. doi: 10.1016/j.bbr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2 receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, Barrett JE, Stack G, Meltzer HY, Harrison BL, Rosenzweig-Lipson S. WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: a novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther. 2007;320:486–496. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bös M, Jenck F, Moreau J-L, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HHG, Broekkamp CLE, Ruigt GSF, Köhler C, Van Delft AML. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1988;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial acaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology. 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989;97:118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia. 1967;11:300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, Stack G, Welmaker G, Barrett JE, Dunlop J. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res. 2006;1073–1074:240–251. doi: 10.1016/j.brainres.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Fadayel GM, Sullivan CK, Taylor VL. 5-HT2 receptors exert a state-dependent regulation of dopaminergic function: studies with MDL 100,907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. Eur J Pharmacol. 1992;223:65–74. doi: 10.1016/0014-2999(92)90819-p. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effect of DOI by MDL 100,907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sheldon PW, Aghajanian GK. Serotonin (5-HT) induces IPSPs in pyramidal layer cells of rat piriform cortex: evidence for the involvement of a 5-HT2-activated interneuron. Brain Res. 1990;506:62–69. doi: 10.1016/0006-8993(90)91199-q. [DOI] [PubMed] [Google Scholar]
- Sheldon PW, Aghajanian GK. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse. 1991;9:208–218. doi: 10.1002/syn.890090307. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. DOI disruption of prepulse inhibition in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/−)DOI] in C57BL/6J mice. Psychopharmacology. 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology. 1999;144:248–254. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–330. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT2A receptor-mediated head-twitch behaviour in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical antipsychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog Neuro-Psychopharmacol Biol Psychiat. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin2A (5HT2A) receptors in the rat brain. Brain Res Bull. 2000;51:499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]