Abstract
Neurogenesis in the adult mammalian nervous system is now well established in the subventricular zone of the anterolateral ventricle and subgranular zone of the hippocampus. In these regions, neurons are thought to arise from neural stem cells, identified by their expression of specific intermediate filament proteins (nestin, vimentin, GFAP) and transcription factors (Sox2). In the present study, we show that in adult rat and mouse, the circumventricular organs (CVOs) are rich in nestin+, GFAP+, vimentin+ cells which express Sox2 and the cell cycle-regulating protein Ki67. In culture, these cells proliferate as neurospheres and express neuronal (doublecortin+, β-tubulin III+) and glial (S100β+, GFAP+, RIP+) phenotypic traits. Further, our in vivo studies using bromodeoxyuridine show that CVO cells proliferate and undergo constitutive neurogenesis and gliogenesis. These findings suggest that CVOs may constitute a heretofore unknown source of stem/progenitor cells, capable of giving rise to new neurons and/or glia in the adult brain.
Keywords: neural stem cells, neurogenesis, gliogenesis, nestin, Sox2, Ki67, vimentin
Introduction
In recent years, it has become increasingly clear that the adult mammalian brain, once thought to be a static structure, in fact retains the capacity to generate new neurons and glia throughout the life of the organism (Alvarez-Buylla and Lim, 2004; Doetsch et al., 1999; Doetsch, 2003b; Doetsch, 2003a). In particular, adult neurogenesis has been found in two discrete regions of the brain: the subventricular zone (SVZ) of the anterolateral ventricular wall and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus (Lie et al., 2004). The neurons produced in these regions are thought to arise from neural stem cells (NSCs) found in highly regulated stem cell niches wherein self-renewal and differentiation of progenitors toward the neural cell fate is determined by local environmental and intrinsic cellular cues (Temple, 2001; Shen et al., 2004; Barkho et al., 2006). In the adult, NSCs are generated for the continuous replacement of specific classes of brain neurons (i.e. periglomerular and granule cells in the olfactory bulb and granule cells of the dentate gyrus) but also in response to neurodegenerative disease, traumatic injury, stroke, etc. (Schouten et al., 2004; Sundholm-Peters et al., 2005; Park et al., 2006).
It has been postulated that NSCs in the SVZ and SGZ derive from a unique cell type, the germinal astrocyte, which is characterized by the presence of specific Class III intermediate filament proteins (i.e. nestin, glial fibrillary acidic protein [GFAP], vimentin) (Doetsch et al., 1999; Doetsch, 2003b; Doetsch, 2003a; Lendahl et al., 1990; Wei et al., 2002; Wiese et al., 2004) and nuclear factors (i.e. Sox1, Sox2, Musashi-1) (Komitova and Eriksson, 2004; Cai et al., 2006; Shin et al., 2007). Still others maintain that additional cells types, such as multiciliated ependymal cells (Johansson et al., 1999) and tanycytes (non-ciliated cells of the ependyma) (Xu et al., 2005) also serve as NSCs in the adult brain and spinal cord (Bruni, 1998; Liu et al., 2002; Mothe and Tator, 2005). Finally, the presence of dividing progenitors has also been reported in a number of discrete regions of the adult brain, such as the cortex (Magavi et al., 2000; Jiang et al., 2001), amygdala (Park et al., 2006), striatum (Benraiss et al., 2001; Pencea et al., 2001; Teramoto et al., 2003; Chmielnicki et al., 2004; Collin et al., 2005; Mohapel et al., 2005), and substantia nigra (Zhao et al., 2003; Van Kampen and Robertson, 2005).
In the present study, we will show that proliferating cells found at specific sites along the third and fourth ventricles express proteins normally associated with germinal astrocytes and their derivatives (transit amplifying cells) of the SVZ and SGZ. We will demonstrate that these cells give rise to neurons and glia both in culture and in vivo. Historically, the midline structures where these cells reside have together been termed circumventricular organs (CVOs) and include the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), median eminence (ME), pineal gland (PG), subcommissural organ (SCO), area postrema (AP) and the choroid plexus. CVOs are unique in structure and function. Their ventricular surfaces are lined with specialized ependymal cells called tanycytes, which act as a partial CSF barrier. In addition, CVOs lack a classic blood-brain-barrier. With the exception of the SCO, all other CVOs contain permeable fenestrated capillaries and are often referred to as the “windows of the brain” (Johnson and Gross, 1993). The CVOs are critically involved in the maintenance of a wide variety of sensory homeostatic and inflammatory pathways in the brain (Moyse et al., 2006). These unique properties indicate that CVOs may represent novel sites for the continued generation of neurons and/or glia in the adult brain.
Results
CVOs express neural stem cell markers
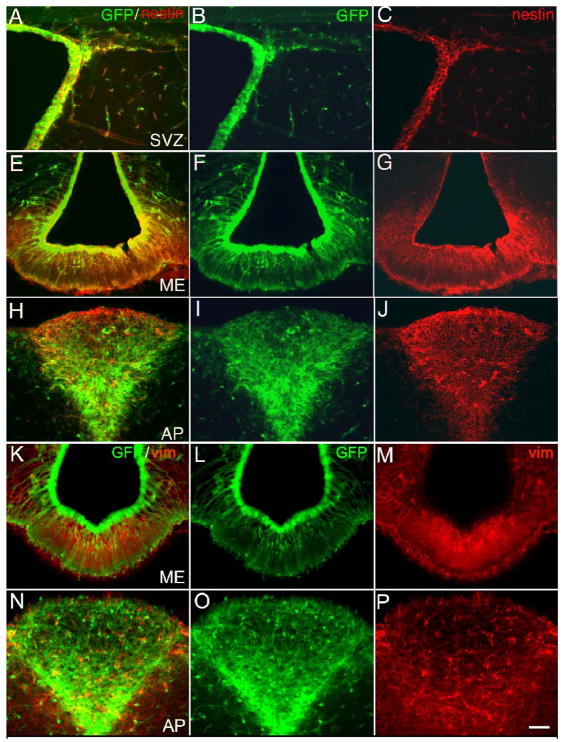
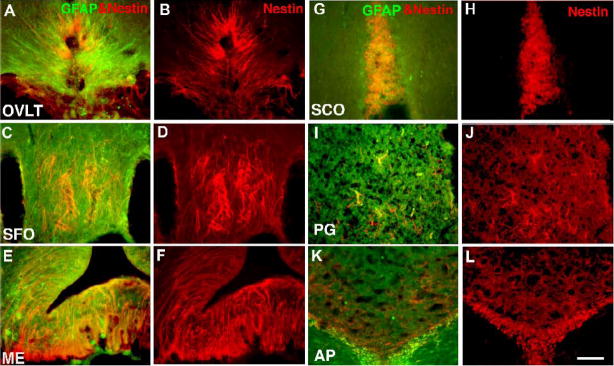
To establish that CVO cells express proteins that identify neural stem cells, we examined CVO regions from both the adult nestin-GFP mouse and adult rat. Using double label immunocytochemistry on nestin-GFP mouse brain sections, we first confirmed in the traditional and most active niche of adult neural stem cells (SVZ shown in panels A-C) that GFP fluorescent cells are nestin-immunoreactive. Likewise, the CVO regions of the nestin-GFP mouse brain (ME in panels E-G and AP in panels H-J of Figure 1) are GFP fluorescent and nestin+. CVO cells from nestin-GFP mice also express the Class III intermediate filament protein vimentin (ME in panels K-M and AP in panels N-P), which has been shown to characterize neural stem cells (Yang et al., 1993; Barry and McDermott, 2005). Robust nestin expression was also demonstrated in the CVOs of the adult rat, as was co-expression of GFAP, a key marker of germinal astrocytes (Figure 2). As evident in the nestin and GFAP merged images of Figure 2(A, C, E, G, I, K), in most cases there was significant overlap in cells stained with both markers.
Figure 1.
Expression of intermediate filaments in CVOs of the adult transgenic nestin-GFP mouse. Using immunocytochemistry for tissue sections, cells in the SVZ (A-C), ME (E-G, K-M) or AP (H-J, N-P) were both GFP fluorescent (B, F, I, L, O) and nestin+ (C, G, J) or vimentin+ (M, P) in merged images (A, E, H, K, N). Calibration bar = 100μm.
Figure 2.
Immunocytochemical localization of intermediate filament proteins in the adult rat CVOs. Shown are nestin staining or merged images of nestin and GFAP staining in the OVLT (A, B), SFO (C, D), ME (E, F), SCO (G, H), PG (I, J) and AP (K, L). Calibration bar = 200μm.
CVO cells proliferate and differentiate in culture
Having demonstrated expression of NSC markers in the CVOs, we next investigated the proliferative potential of CVO cells in culture using the nestin-GFP mouse and adult rat. We first demonstrated proliferation as floating neurospheres of GFP+ cells from adult nestin-GFP mouse in the control neurogenic region SVZ (Figure 3A). Likewise, as shown in Figure 3, GFP+ cells proliferated as floating neurospheres from CVOs ME (B), OVLT (C), and AP (D). Spheres formed over 6–14 days in vitro and were passaged at least three times. CVO spheres exhibited similar growth rates (Table 1; AP and OLVT: 13.5 ± 0.5; SFO: 13.25 ± 0.48 days in vitro to reach 100 μm mean sphere diameter) to control region SVZ (12.75 ± 0.48 days in vitro to reach 100 μm mean sphere diameter), except for the ME region, which exhibited a more rapid growth rate (7 ± 0.41 days in vitro to reach 100 μm mean sphere diameter). CVO sphere yield per 5,000 cells plated was comparable to control region SVZ (Table 1). As evident in Figure 3, the spheres exhibited a heterogeneous nestin-GFP labeling pattern that is characteristic of nestin immunoreactivity of neurospheres from adult mammalian brain (Reynolds and Weiss, 1992). Importantly, spheres did not form from cortical tissue or from neighboring non-CVO ventricular regions of the rat or nestin-GFP mouse (data not shown).
Figure 3.
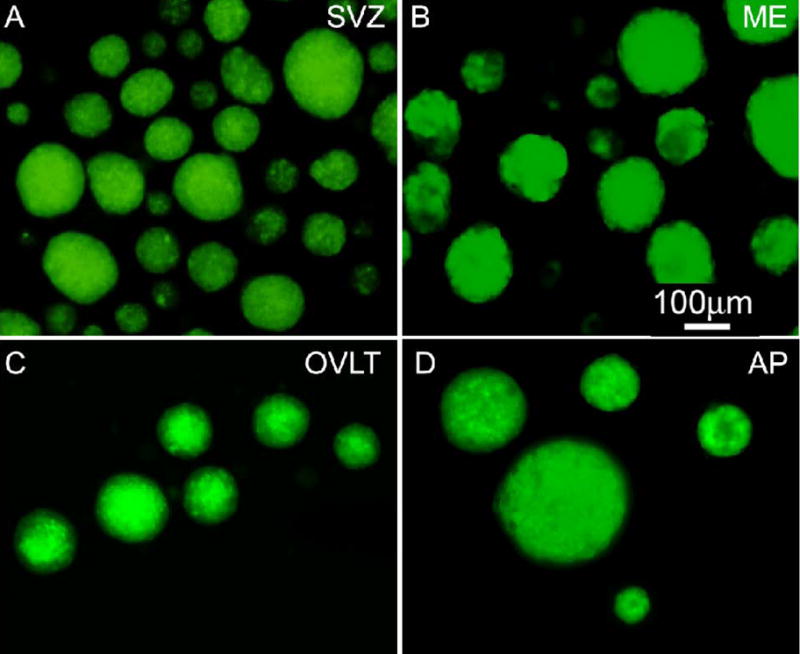
Neurosphere formation from adult transgenic nestin-GFP mouse cells. GFP+ neurospheres from control region SVZ (A) and CVO regions ME (B), OVLT (C), and AP (D) are shown 6–14 days following microdissection and plating of single cells in suspension culture.
Table 1.
Nestin-GFP neurosphere yield from CVOs and SVZ and days in vitro (DIV) to reach 100 μm mean sphere diameter.
| Region | GFP+ |
|
|---|---|---|
| Total Sphere Count* per 5,000 cells plated |
DIV to 100μm mean sphere diameter |
|
| AP | 129.87 ± 3.03 | 13.5 ± 0.5 |
| ME | 93.89 ± 8.30 | 7 ± 0.41 |
| OVLT | 133.31 ± 0.94 | 13.5 ± 0.5 |
| SFO | 83.11 ± 3.99 | 13.25 ± 0.48 |
| SVZ | 125.38 ± 14.24 | 12.75 ± 0.48 |
Spheres of all sizes were quantified. Data is expressed as mean ± S.E.M.
To confirm CVO cell proliferation in vitro we also undertook clonal culture experiments and immunocytochemical staining with the proliferation marker Ki67 (Figure 4). Cells from the adult rat CVO region ME were observed in the act of cytokinesis (A) and forming growing colonies (B, C). Like SVZ cells (D), ME cells expressed nestin and Ki67 in culture (E).
Figure 4.
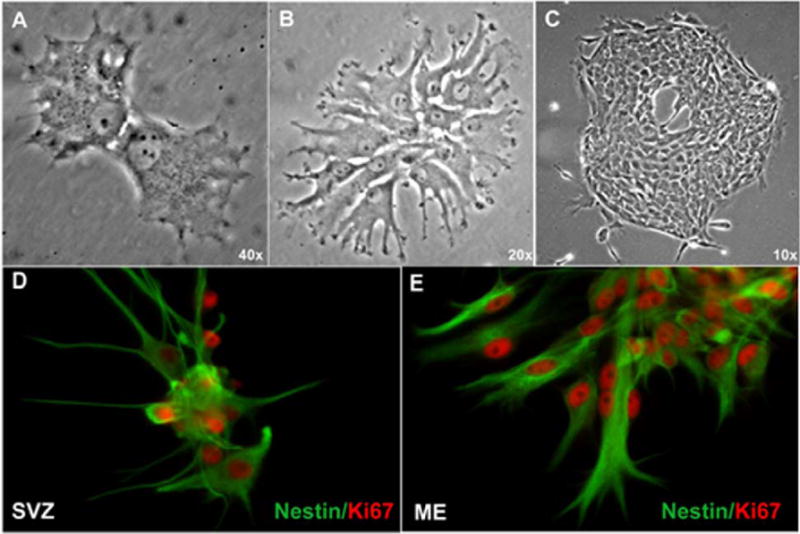
Clonal proliferation of adult rat CVO cells in vitro. Cells from the CVO region ME were plated at low density and single attached cells were marked and observed for colony formation. Here ME cells are shown in the act of cytokinesis (A) and developing expanding colonies (B, C). Immunocytochemical analysis demonstrated that like control SVZ cells (D), ME cells expressed nestin and the nuclear proliferation marker Ki67 (E).
We next sought to determine whether CVO cells had the capacity to differentiate into cells expressing neuronal traits in culture. Cells from the CVO regions ME and OVLT and control SVZ region were dissected from adult nestin-GFP mice and grown as neurospheres. After two passages, cells were plated as adherent cells and differentiated for 14 days in vitro with differentiation medium. To ensure that the cells began the differentiation process from the undifferentiated state, a subset of cells were fixed two days after plating and processed for immunocytochemistry. As shown in Figure 5, undifferentiated cells from the SVZ (A, D), ME (B, E), and OVLT (C, F) expressed nestin and GFP (A-C), but virtually no cells expressed the neuroblast marker doublecortin (DCX, D-F). In contrast, differentiated cells from the SVZ (G, J), ME (H, L) and OVLT (I, M) expressed both DCX and the early neuronal marker β-tubulin III (J-M) but no longer expressed nestin-GFP. Differentiated cells were process-bearing and appeared to lie on a bed of DCX and β-tubulin III-negative cells.
Figure 5.
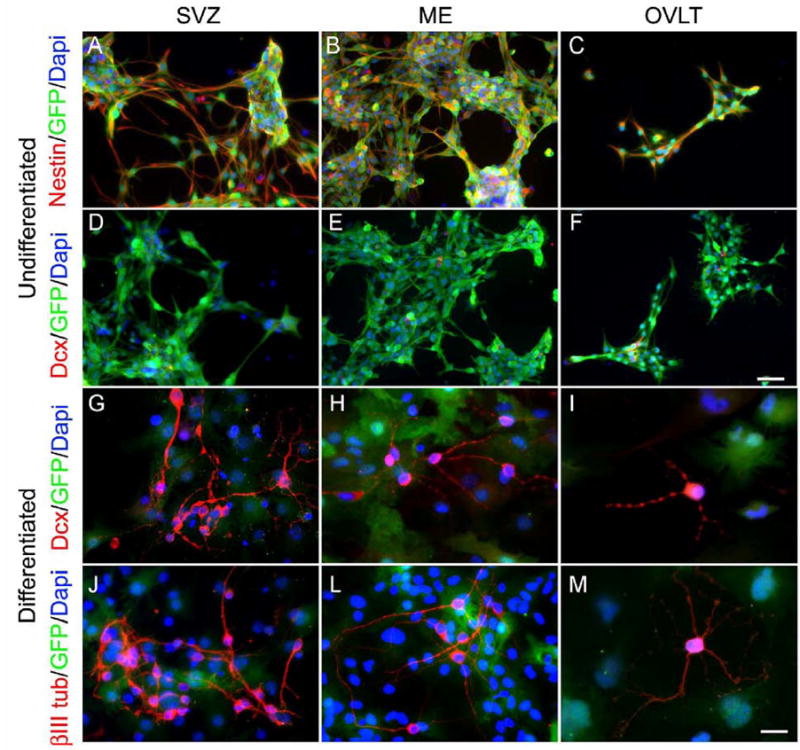
Neuronal differentiation of nestin-GFP mouse CVO cells in vitro. Undifferentiated cells from the SVZ (A, D), ME (B, E), and OVLT (C, F) expressed nestin and GFP (A-C), but few expressed the neuroblast marker doublecortin (DCX, D-F). Following differentiation for 14 days, process-bearing cells expressed neuronal markers DCX (G-I) and β-tubulin III (βIIItub, J-M). Calibration bar = 50 μm for Panels A-F and 25 μm for Panels G-M.
Having demonstrated neuronal phenotypic differentiation of CVO cells, we next tested whether CVO cells from the nestin-GFP mouse could also differentiate towards a glial fate in culture. As described above, GFP+ neurospheres were passaged at least twice, dissociated, and plated as undifferentiated adherent cells. Following treatment with differentiation medium for 10–14 days, cells were assessed for expression of astrocyte markers GFAP and S100β using immunocytochemistry. As seen in Figure 6, cells from the SVZ (A), ME (B), AP (C), and OVLT (D) co-expressed GFAP and S100β but no longer expressed nestin-GFP, consistent with astroglial differentiation. Differentiated cells were also analyzed for expression of the oligodendrocyte marker RIP. As shown in Figure 6, cells from CVO regions ME (E) and OVLT (F) were RIP+ and demonstrated characteristic oligodendrocyte morphology.
Figure 6.
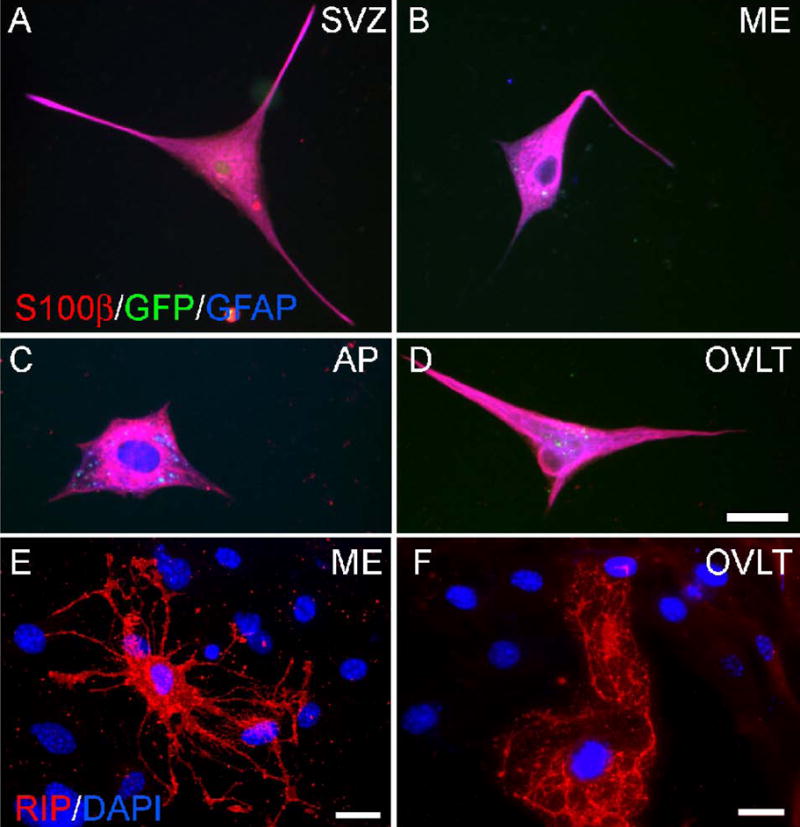
Glial differentiation of nestin-GFP mouse cells in vitro. Undifferentiated nestin-GFP cells plated as adherent culture and differentiated for 9–14 days were analyzed by immunocytochemistry for expression of astrocyte markers S100β and GFAP and oligodendrocyte marker RIP. Shown are cells from the SVZ (A), ME (B), AP (C), and OVLT (D) co-expressing S100β and GFAP. Note that cells have lost expression of GFP, which would appear white if triple-labeled. ME (E) and OVLT (F) cells with characteristic oligodendrocyte morphology are shown expressing RIP. Calibration bar = 25 μm.
CVO cells proliferate and differentiate in vivo
Results in culture indicated that CVO cells possessed the potential to proliferate and differentiate into cells phenotypic of neurons and glia. We therefore examined the adult nestin-GFP mouse and adult rat for evidence of constitutive proliferation and differentiation in vivo. We first assessed the expression of Sox2, a transcription factor mediating multipotency in adult neural stem cells (Komitova and Eriksson, 2004; Ferri et al., 2004; Miyagi et al., 2004), and the cell proliferation marker Ki67. As seen in Figure 7A-C, the adult rat SVZ contained numerous Sox2+ cells, many of which were also Ki67+ (6.53±1.18% Ki67+/Sox2+). Similarly, CVO regions, particularly the OVLT (D-F) and AP (G-I), contained many Sox2+ cells, some of which were also Ki67+ (OVLT: 2.93±1.56% Ki67+/Sox2+; AP: 17.0±9.83% Ki67+/Sox2+). In all cases, labeled cells were found both in the ependymal layer and scattered throughout the parenchyma of the organ but not in neighboring structures. In addition to the CVOs, Sox2+ parenchymal cells were also observed in the caudate nucleus (CN), cerebral cortex, and bed nucleus of the stria terminalis. However, these were not labeled with Ki67 (J-L).
Figure 7.
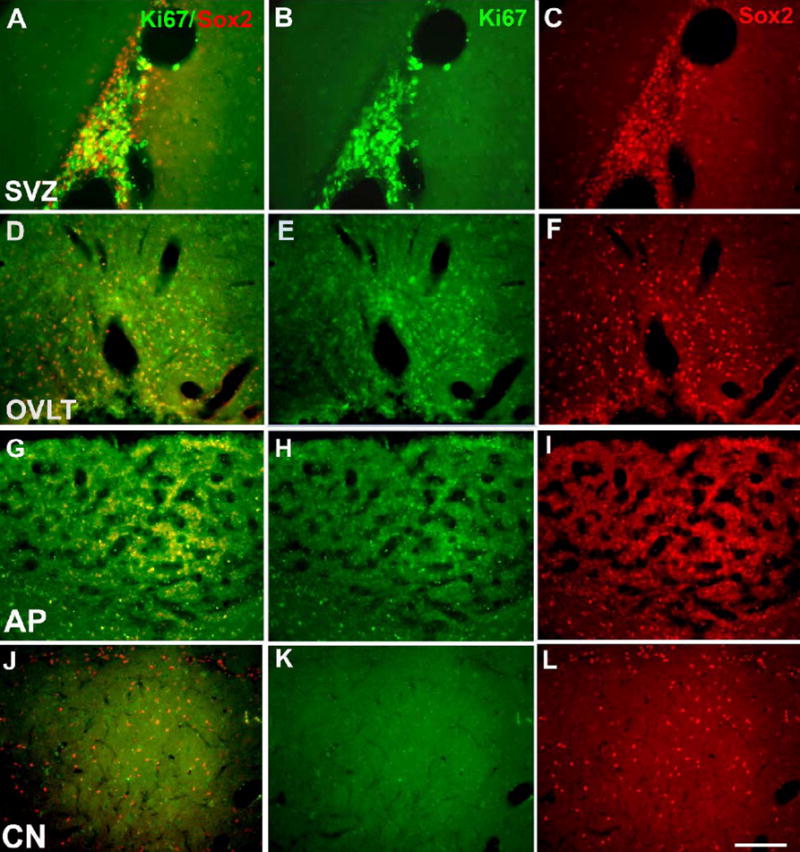
Immunocytochemical localization of transcription and proliferation factors in adult rat CVOs. Shown are merged images (A, D, G, J) for Ki67 (B, E, H, K) and Sox2 (C, F, I, L) staining in the SVZ (A-C), OVLT (D-F), AP (G-I) and in presumptive parenchymal progenitors of the caudate nucleus (J-L). Calibration bar = 200μm.
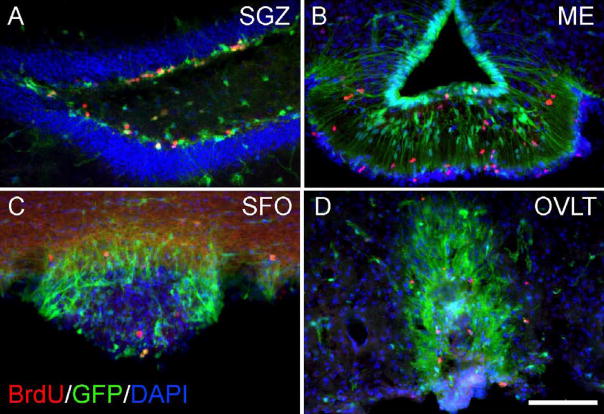
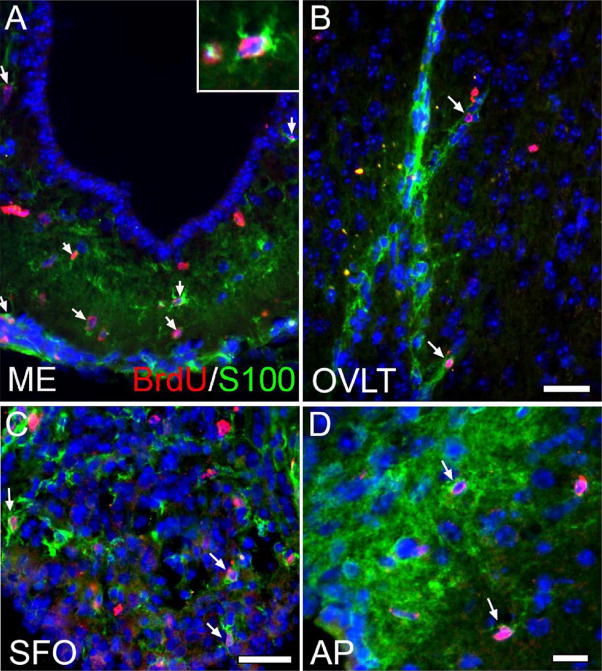
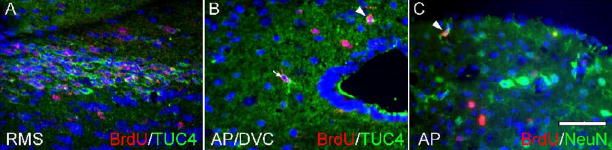
To further establish CVO cell proliferation in vivo, we also performed studies using the thymidine analog bromodeoxyuridine (BrdU) in adult nestin-GFP mice. In these studies, mice were administered BrdU for one week, and tissue was processed for immunocytochemistry. As shown in Figure 8, BrdU+ nuclei were seen scattered throughout the known neurogenic SGZ region of the hippocampus (A) as well as the CVOs: shown here are the ME (B), SFO (C), and OVLT (D). Like cells of the SGZ, some BrdU-labeled cells in the proliferative CVO zones also appeared to express nestin-GFP. Following our observation of BrdU+ cells in the CVOs, we next conducted further studies in an effort to determine whether proliferating (BrdU+) CVO cells went on to differentiate. CVOs were evaluated in nestin-GFP− littermates for expression of neuronal and glial markers following BrdU administration for four weeks. As shown in Figure 9, we found that many BrdU-labeled cells in the CVO regions ME (A), OVLT (B), SFO (C), and AP (D) co-expressed the glial marker S100β, likely indicating constitutive gliogenesis at these sites. S100β+/BrdU+ cells represented a substantial proportion of total BrdU+ cells in the CVOs (OVLT: 45.2 ± 5.23%; SFO: 29.6 ± 4.12%; ME: 30.3 ± 3.52%; AP: 21.7 ± 3.59%; Table 2). We also probed the ME, OVLT, SFO, and AP for expression of TUC-4 (TOAD [Turned On After Division]/Ulip/CRMP-4), a very early neuronal marker (Cameron and McKay, 2001; von Bohlen Und Halbach, 2007) and NeuN, a more mature neuronal marker. As evident in Figure 10, like control cells of the rostral migratory stream (A), BrdU+ cells of the AP (B, arrowhead) and surrounding dorsal vagal complex (B, arrow) also expressed TUC-4. However, the proportion of BrdU+ cells co-expressing Tuc-4 in the AP (15.6 ± 3.16%) was far lower than at the RMS (95.2 ± 0.04%; Table 3). BrdU+ cells of the AP also co-expressed NeuN (panel C of Figure 10). BrdU+/NeuN+ cells represented 22.8 ± 3.73% of the total BrdU+ cell population in the AP (Table 3). Constitutive neurogenesis was not observed at the other CVO regions examined in the intact mouse.
Figure 8.
Constitutive proliferation of CVO cells in the nestin-GFP mouse. BrdU-labeled nuclei are seen in control region SGZ (A) and CVO regions ME (B), SFO (C), and OVLT (D) following administration of BrdU for one week. Calibration bar = 100μm.
Figure 9.
Constitutive gliogenesis in CVOs of the nestin-GFP mouse. To track the differentiative fate of proliferating (BrdU+) CVO cells, intact Nestin-GFP animals were administered BrdU for four weeks. As indicated by the arrows, many BrdU+ cells co-labeled with the astrocyte marker S100β in the ME (A, see inset for enlargement of BrdU+, S100β+ cell), OVLT (B), SFO (C), and AP (D), indicating active gliogenesis at these sites. Scale bars = 25 μm.
Table 2.
Quantification of BrdU-labeled and BrdU/S100β double-labeled cells from the CVO regions following BrdU treatment for four weeks.
| Region | BrdU+ per section* | S100β+/BrdU+ per section* | % S100β +/BrdU+ vs. BrdU+ |
|---|---|---|---|
| OVLT | 11.65 ± 3.37 | 5.20 ± 1.61 | 45.2 ± 5.23 |
| SFO | 15.88 ± 3.82 | 4.60 ± 1.18 | 29.6 ± 4.12 |
| ME | 35.36 ± 4.22 | 10.72 ± 1.69 | 30.3 ± 3.52 |
| AP | 34.03 ± 8.68 | 7.08 ± 1.86 | 21.7 ± 3.59 |
Values indicate average number cells immunopositive per section. Data is expressed as mean ± S.E.M.
Figure 10.
Neurogenesis in the area postrema of the intact nestin-GFP mouse. Shown are BrdU-labeled cells co-expressing the early neuronal marker TUC-4 within the boundary of the AP (B, arrowhead), in the dorsal vagal complex (DVC, B, arrow), and in the rostral migratory stream, a known region of migrating neuroblasts (RMS, A). BrdU-labeled cells in the AP also express the neuronal marker NeuN (C, arrow). Scale bar = 50 μm.
Table 3.
Quantification of BrdU-labeled and BrdU/Tuc-4 or BrdU/NeuN double-labeled cells from the AP and RMS following BrdU treatment for four weeks.
| Region | Marker | BrdU+ per section* | Marker+/BrdU+ per section* | % Marker+/BrdU+ vs. BrdU+ |
|---|---|---|---|---|
| AP | Tuc-4 | 37.86 ± 7.24 | 5.67 ± 1.26 | 15.6 ± 3.16 |
| NeuN | 31.19 ± 8.07 | 7.08 ± 3.55 | 22.8 ± 3.73 | |
| RMS | Tuc-4 | 454.7 ± 38.2 | 434.2 ± 40.9 | 95.2 ± 0.04 |
Values indicate average number cells immunopositive per section. Data is expressed as mean ± S.E.M.
Discussion
The present findings demonstrate that specific sites along the third and fourth ventricles in the adult brain, regions historically known as the CVOs, contain cells that express intermediate filament proteins (nestin, vimentin, GFAP) and transcription factors (Sox2), markers that typically characterize self-renewing multipotent stem/progenitor cells of the embryonic neuroepithelium (Cai et al., 2006; Shin et al., 2007) and adult SVZ and SGZ (Mignone et al., 2004). Grown in culture these cells proliferate as neurospheres and become cells phenotypic of neurons and glia when treated with differentiation medium. Moreover, our in vivo studies show that a pool of Sox2+ CVO cells contain nuclei that also label with the proliferation factor Ki67, indicating that cell division persists in these regions in the adult brain. Likewise, our BrdU studies confirm that these regions contain constitutively proliferating cells that go on to differentiate into neurons and glia. These findings raise the intriguing possibility that CVOs function not only to mediate sensory/inflammatory homeostatic mechanisms in the adult brain, but also serve a stem cell function with the potential to give rise to differentiated neural and glial cell types.
Although earlier studies have not examined the CVO structures as a collective group, a number of inquiries of individual CVOs have reported findings consistent with a stem cell role. Thus, Arochena and colleagues (Arochena et al., 2004) have shown that, in the adult gray mullet fish, the OVLT and AP possess the radial glial markers GFAP and vimentin. In addition, GFAP+ and nestin+ cells have been observed in the adult mouse SFO, ME, and AP (Wei et al., 2002; Pecchi et al., 2007). In vitro, cells isolated from the AP, choroid plexus and third and fourth ventricles of adult mice have generated neurospheres of multipotent stem cells (Weiss et al., 1996; Bauer et al., 2005; Charrier et al., 2006; Chouaf-Lakhdar et al., 2003; Itokazu et al., 2006).
Consistent with these findings, our studies in culture demonstrated that CVO cells possessed the potential to become neurons and glia. Interestingly, however, our in vivo studies showed that in the intact animal, proliferating cells of the ME, OVLT, and SFO gave rise to astrocytes, while cells of the AP gave rise to both neurons and astrocytes. It is possible that, as in the known neurogenic SVZ and SGZ regions, the CVO microenvironment may possess factors critical for maintaining progenitors and regulating differentiation. Future studies will be necessary to identify factors that instruct CVO cell fate. In addition, although S100β is used as the standard marker for mature non-germinal astrocytes, it should be noted that S100β-expressing astrocytes have been reported to divide in the adult brain (Seri et al., 2004; Ihrie and Alvarez-Buylla, 2008). Therefore, it must be considered that at least a portion of the BrdU+/S100β+ population identified in the CVOs may not be fully differentiated.
In vivo, we observed the spontaneous proliferation (Ki67+) of a small subset of multipotent (Sox2+) cells in all CVO regions examined. Proliferating Sox2+ cells in the CVOs may represent a pool of latent stem cells which are relatively quiescent in the intact brain, but which possess the potential for amplification and differentiation under specific circumstances. In this regard, CVOs, with their abundance of fenestrated capillaries and their proximity to ventricles, may be ideally positioned to react to pathological (i.e. inflammatory) or physiological (i.e. autonomic) perturbations. In support of this notion, Xu et al. (Xu et al., 2005) found that the intraventricular injection of the mitogen bFGF resulted in the proliferation of ME cells and subsequent generation of new neurons of the hypothalamus. Likewise, Moyse and colleagues showed that vagotomy promoted a transient but substantial increase in the production of new (BrdU+/NeuN+) neurons in the dorsal vagal complex (Moyse et al., 2006; Bauer et al., 2005). From a physiological standpoint, our observation of constitutive gliogenesis in the ME is particularly interesting given the reported role of astrocytes in this region in the regulation of reproductive neuroendocrine output. Hypothalamic glia have been shown to regulate gonadotropin-releasing hormone release by both structural and chemical mechanisms (Prevot et al., 2007; Garcia-Segura et al., 2008; Ojeda et al., 2008).
Although we have demonstrated CVO cell proliferation and differentiation toward neuronal and glial phenotypes, differentiative potential of these cells has not yet been fully tested. Recently, Gleiberman and colleagues (Gleiberman et al., 2008) found that nestin-expressing precursors/stem cells of the adult mouse pituitary gland differentiate to become neuroendocrine cells of the anterior pituitary, which the authors argue may adapt the gland for dynamic responses to physiological and pathological stimuli. Given that CVOs also contain neuroendocrine cells, it is an intriguing possibility that nestin+ CVO cells may help maintain this cell population within the CVO. Future studies will be needed to make such determinations. Similarly, Vivard and colleagues have recently characterized a platelet derived growth factor receptor alpha-expressing glial cell population in the adult rat neurohypophysis able to proliferate as neurospheres and give rise to mature astrocytes and oligodendrocytes in vitro (Virard, et al., 2008). Future studies are needed to examine the full expression profile of the primary progenitors within the CVOs. In addition, it will be important to identify conditions that promote CVO cell proliferation and govern their differentiation in the normal and diseased brain.
Experimental methods
Animals and treatments
All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and under approval of Thomas Jefferson University’s Institutional Animal Care and Use Committee.
BrdU administration
Nestin-GFP male and female mice (8–14 weeks old) were administered BrdU (Fisher Scientific, Fair Lawn, NJ; 1 mg/ml) in their drinking water for one (proliferation study) or four weeks (differentiation studies), then immediately perfused and brains processed for tissue sectioning and immunocytochemistry.
Cell culture
CVO and SVZ regions were visualized under a fluorescent dissection microscope and GFP+ tissue was dissected from 8–14 week-old male and female nestin-GFP mice (12–16 animals per culture). Cells were dissociated in 1 mg/ml papain (Roche, Indianapolis, IN) in HBSS-CMF (Mediatech, Herndon, VA) and plated as suspension culture in NeuroCult NSC Basal Medium with added NeuroCult NSC Proliferation Supplement for mouse (STEMCELL Technologies, Vancouver, BC, Canada) at the following densities: SVZ (150,000–175,000 cells/ml), OVLT (50,000–75,000 cells/ml), ME (75,000–125,000 cells/ml), SFO (25,000–50,000 cells/ml), and AP (25,000–50,000 cells/ml). Cultures were supplemented with 20 ng/ml bFGF (R&D, Minneapolis, MN), 20 ng/ml EGF (R&D), and 0.36 units/ml heparin sodium (Abraxis, Schaumburg, IL). Cultures were passaged by trituration with a P200 pipette until dissociation of neurospheres was achieved. For differentiation experiments, cells were plated on poly-ornithine or Geltrex (Invitrogen, Carlsbad, CA)-coated chamber slides. Medium was then changed to NeuroCult NSC Basal Medium with added NeuroCult NSC Differentiation Supplement for mouse (STEMCELL Technologies) for 10–14 days.
For clonal analysis of adult rat cells, the ME was dissected from 4 adult rats (Fischer 344; 250–350g) using a dissection microscope. Tissue was dissociated in 1 mg/ml papain (Roche, Indianapolis, IN) in HBSS-CMF and plated at low density (10,000–25,000 cells/well) on 6-well tissue culture plates in NeuroCult NSC Basal Medium with added NeuroCult NSC Proliferation Supplement for rat (STEMCELL Technologies). Cultures were supplemented with 20 ng/ml bFGF, 20 ng/ml EGF, and 0.36 units/ml heparin sodium. Single adherent cells were marked and examined daily for colony formation.
For quantification of spheres, GFP+ cells were counted from each region using a hemacytometer to obtain plating density immediately following microdissection and plating of CVO and control (SVZ) regions in culture. When spheres reached an average of 100 μm in diameter, absolute GFP+ sphere yield from each region from each dissection (n=4) was counted using an eyepiece reticle. Sphere yield was then expressed as number of GFP+ spheres formed per 5,000 GFP+ cells plated.
Immmunocytochemistry
Tissue sections
Adult male rats (Fischer 344; 250–350g) or 8–14 week-old male and female nestin-GFP mice were deeply anesthetized with 70 mg/kg Nembutal (rats) or a cocktail of ketamine (100 mg/kg) and xylazine (5 mg/kg, mice) and perfused with cold (4°C) periodate-lysine-paraformaldehyde (4%). Brain sections were cut at 30μm on a freezing microtome (rats) or at 14μm on a cryostat (mouse) and sections were incubated with primary antibodies in a 0.01 M phosphate-buffered saline (PBS) solution (pH 7.4) containing 0.3% Triton X100 and 2% normal donkey serum (NDS). After two days at room temperature (rat sections) or overnight at 4° C (mouse sections), sections were washed five times for ten minutes each with a 0.01 M PBS solution (pH 7.5). Secondary immunofluorescent antibodies were incubated for one (mouse sections) or three hours (rat sections) at room temperature in 0.01 M PBS solution (pH 7.5) containing 2% NDS. The nuclear dye Hoechst 33258 (Invitrogen, 1 mg/ml) was added to secondary antibody solution for nuclear staining. Sections were then washed and mounted on slides and examined on a Nikon Scanalytics Image System or an Olympus IX2-UCB with a Sensicam digital camera system (Cooke) and Slidebook imaging software (Intelligent Imaging Innovations).
For BrdU studies, tissue sections were washed with 0.01 M PBS, then treated with 2M HCl for 30 minutes at room temperature. Sections were then washed with 0.01 M PBS followed by incubation for 30 minutes at room temperature with 0.1 M sodium tetraborate decahydrate. Sections were then washed with 0.01 M PBS and processed for immunocytochemistry as above.
For quantification of rat sections, representative Ki67/Sox2 immunostained coronal sections (30 μm thickness) were sampled from SVZ and CVO regions OVLT and AP from three adult male Fischer 344 rats, and immunopositive nuclei were counted per section, averaged, and expressed as mean ± S.E.M. For quantification of mouse sections, coronal sections of 14 um thickness were cut through the rostrocaudal extent of all CVOs used for quantification (OVLT, ME, SFO, and AP; n=3 nestin-GFP mice per staining). Quantification of immunopositive cells was carried out on every section (OVLT), every other section (ME, SFO), or every third section (AP) for each staining (S100B/BrdU, Tuc-4/BrdU, NeuN/BrdU). Immunopositive cells were counted per section, averaged, and expressed as mean ± S.E.M.
Culture
Cultures were rinsed and then fixed with 4% paraformaldehyde for 30 minutes at room temperature. Cultures were incubated in 0.3% Triton X100 in 0.01 M PBS for fifteen minutes at room temperature followed by incubation for 30 minutes at room temperature with 3% NDS in 0.01 M PBS. Primary antibodies were incubated overnight at 4° C in 0.01 M PBS with 1% NDS. Cultures were then washed and incubated with secondary antibodies for 30 minutes at room temperature in 0.01 M PBS with 1% NDS. The nuclear dye Hoechst 33258 (Invitrogen, 1 mg/ml) was added to secondary antibody solution for nuclear staining. Cultures were then washed, coverslipped, and analyzed using an Olympus IX2-UCB with a Sensicam digital camera system (Cooke) and Slidebook imaging software (Intelligent Imaging Innovations).
Antibodies
Sections were incubated with primary antibodies to Millipore rabbit anti-GFAP (Billerica, MA, AB5804; 1:300) or DAKO rabbit anti-GFAP (Carpinteria, CA, Z0334, 1:1000), Millipore mouse anti-nestin (MAB353; 1:200), DSHB mouse anti-vimentin (Iowa City, Iowa, 40E-C; 1:200), Abcam rabbit anti-Ki67 (Cambridge, MA, AB833; 1:50), Millipore chicken anti-GFP (AB16901, 1:200), Santa Cruz rabbit anti-doublecortin (Santa Cruz, CA, sc-28939, 1:500), R&D mouse anti-β-tubulin III (MAB1195, 1:200), Sigma mouse anti-S100β (St. Louis, MO, S2532, 1:200) or Abcam mouse anti-S100β (Ab4066, 1:250), R&D Systems mouse anti-Sox2 (MAB2018; 1:800), Accurate Chemical rat anti-bromodeoxyuridine (Westbury, NY, OBT0030, 1:100), Millipore rabbit anti-TUC-4 (AB5454, 1:100), Millipore mouse anti-RIP (MAB1580, 1:1000), or Millipore mouse anti-NeuN (MAB377, 1:100). All secondary antibodies were Alexa Fluor antibodies from Invitrogen: donkey anti-rabbit 594, 1:300; donkey anti-rabbit 488, 1:200; donkey anti-mouse 594, 1:300; donkey anti-mouse 488, 1:200; donkey anti-rat 594, 1:300; goat anti-chicken 488, 1:200; goat anti-rabbit 350, 1:200.
Acknowledgments
This work was supported by NIH NS32519, NS43309 and the Hassel Foundation. This project is funded, in part, under a grant with the Pennsylvania Department of Health: SAP4100026302 C.U.R.E. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. Lori Bennett was supported in part by a Measey MD/PhD Student Fellowship from Thomas Jefferson University. The authors thank Xiaotao Wei, Dr. Rupal Mehta, and Steve Borson for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arochena M, Anadon R, Diaz-Regueira SM. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (chelon labrosus), an advanced teleost. J Comp Neurol. 2004;469:413–436. doi: 10.1002/cne.11021. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- Bauer S, Hay M, Amilhon B, Jean A, Moyse E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 2005;130:75–90. doi: 10.1016/j.neuroscience.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni JE. Ependymal development, proliferation, and functions: A review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cai J, Shin S, Wright L, Liu Y, Zhou D, Xue H, Khrebtukova I, Mattson MP, Svendsen CN, Rao MS. Massively parallel signature sequencing profiling of fetal human neural precursor cells. Stem Cells Dev. 2006;15:232–244. doi: 10.1089/scd.2006.15.232. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Charrier C, Coronas V, Fombonne J, Roger M, Jean A, Krantic S, Moyse E. Characterization of neural stem cells in the dorsal vagal complex of adult rat by in vivo proliferation labeling and in vitro neurosphere assay. Neuroscience. 2006;138:5–16. doi: 10.1016/j.neuroscience.2005.10.046. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaf-Lakhdar L, Fevre-Montange M, Brisson C, Strazielle N, Gamrani H, Didier-Bazes M. Proliferative activity and nestin expression in periventricular cells of the adult rat brain. Neuroreport. 2003;14:633–636. doi: 10.1097/00001756-200303240-00022. [DOI] [PubMed] [Google Scholar]
- Collin T, Arvidsson A, Kokaia Z, Lindvall O. Quantitative analysis of the generation of different striatal neuronal subtypes in the adult brain following excitotoxic injury. Exp Neurol. 2005;195:71–80. doi: 10.1016/j.expneurol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003a;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003b;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Lorenz B, DonCarlos LL. The role of glia in the hypothalamus: Implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction. 2008;135:419–429. doi: 10.1530/REP-07-0540. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–91. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- Itokazu Y, Kitada M, Dezawa M, Mizoguchi A, Matsumoto N, Shimizu A, Ide C. Choroid plexus ependymal cells host neural progenitor cells in the rat. Glia. 2006;53:32–42. doi: 10.1002/glia.20255. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–86. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang Z, Wang H, Zhang Y. Nestin expression and proliferation of ependymal cells in adult rat spinal cord after injury. Chin Med J(Engl) 2002;115:339–341. [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Miyagi S, Saito T, Mizutani K, Masuyama N, Gotoh Y, Iwama A, Nakauchi H, Masui S, Niwa H, Nishimoto M, Muramatsu M, Okuda A. The sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Mol Cell Biol. 2004;24:4207–4220. doi: 10.1128/MCB.24.10.4207-4220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Moyse E, Bauer S, Charrier C, Coronas V, Krantic S, Jean A. Neurogenesis and neural stem cells in the dorsal vagal complex of adult rat brain: New vistas about autonomic regulations--a review. Auton Neurosci. 2006:126–127. 50–58. doi: 10.1016/j.autneu.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- Park JH, Cho H, Kim H, Kim K. Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience. 2006;140:673–684. doi: 10.1016/j.neuroscience.2006.02.076. [DOI] [PubMed] [Google Scholar]
- Pecchi E, Dallaporta M, Charrier C, Pio J, Jean A, Moyse E, Troadec JD. Glial fibrillary acidic protein (GFAP)-positive radial-like cells are present in the vicinity of proliferative progenitors in the nucleus tractus solitarius of adult rat. J Comp Neurol. 2007;501:353–368. doi: 10.1002/cne.21259. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Dehouck B, Poulain P, Beauvillain JC, Buee-Scherrer V, Bouret S. Neuronal-glial-endothelial interactions and cell plasticity in the postnatal hypothalamus: Implications for the neuroendocrine control of reproduction. Psychoneuroendocrinology. 2007;32(Suppl 1):S46–51. doi: 10.1016/j.psyneuen.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Schouten JW, Fulp CT, Royo NC, Saatman KE, Watson DJ, Snyder EY, Trojanowski JQ, Prockop DJ, Maas AI, McIntosh TK. A review and rationale for the use of cellular transplantation as a therapeutic strategy for traumatic brain injury. J Neurotrauma. 2004;21:1501–1538. doi: 10.1089/neu.2004.21.1501. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, Xu QX, Davidson BP, Stice SL, Smith AK, Goldman SA, Reubinoff BE, Zhan M, Rao MS, Chesnut JD. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem Cells. 2007;25:1298–1306. doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen JM, Robertson HA. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience. 2005;136:381–386. doi: 10.1016/j.neuroscience.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Virard I, Gubkina O, Alfonsi F, Durbec P. Characterization of heterogeneous glial cell populations involved in dehydration-induced proliferation in the adult rat neurohypophysis. Neuroscience. 2008;151:82–91. doi: 10.1016/j.neuroscience.2007.10.035. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329:409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- Wei LC, Shi M, Chen LW, Cao R, Zhang P, Chan YS. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res Dev Brain Res. 2002;139:9–17. doi: 10.1016/s0165-3806(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Yang HY, Lieska N, Shao D, Kriho V, Pappas GD. Immunotyping of radial glia and their glial derivatives during development of the rat spinal cord. J Neurocytol. 1993;22:558–571. doi: 10.1007/BF01189043. [DOI] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]