Abstract
Objective
Relapsing to overeating is a stubborn problem in obesity treatment. We tested the hypothesis that context cues surrounding palatable food (PF) intake have the power to disrupt caloric regulation even of less PF. Context cues are non-food cues that are in the environment where PF is habitually eaten.
Design
Rats were conditioned to associate intake of Oreo cookies as the PF to cages with distinct context cues that differed from cues in cages were they were only given chow. PF naturally stimulated greater caloric intake. The rats were then tested in the PF cage with only chow available to determine if the PF-paired cues, alone, could elicit overeating of plain chow.
Subjects
Non food-deprived female Sprague-Dawley rats.
Measurements
Intake of plain chow under PF-paired cues vs. chow-paired cues was compared. This was also measured in tests that included a morsel of PF as a priming stimulus. We also controlled for any effect of binge-prone vs. binge–resistant status to predict cued-overeating.
Results
Rats consumed significantly more chow when exposed to context cues previously paired with PF than with chow (p<0.01). This effect occurred using various cues (e.g., different types of bedding or wallpaper). The effect was strengthened by priming with a morsel of PF (p<0.001) and was unaffected by baseline differences in propensity to binge on PF.
Conclusion
Context-cues associated with PF intake can drive overeating even of a less PF and abolish the ability of rats to compensate for the calories of a PF primer. Just as drug-associated context cues can reinstate drug-addiction relapse, PF-paired cues may trigger overeating relapses linked to weight regain and obesity. This model should help identify the reflex-like biology that sabotages attempts to adhere to healthy reduced calorie regimens and call greater attention to the cue-factor in the treatment of binge-eating and obesity.
Keywords: obesity, binge-eating, craving, junk food, external cues, animal model
Efforts to reduce overweight and obesity by adhering to reduced calorie regimens are notoriously sabotaged by relapses to overeating. Most on these regimens will regain their weight within 3 to 5 years [1–3]. Environmental and behavioral factors commonly known to stimulate overeating and weight regain include social situations [4–6], increased TV viewing [7], mere exposure to tempting or “forbidden” foods [6,8–10], negative emotions and stress [4,8,10,11], lack of social support in weight reduction efforts [12], and even the mere cognitive anticipation of caloric deprivation [13]. Another subversive factor to caloric regulation, but one that is less commonly discussed or considered in obesity treatment plans, is the role of context cues to interfere with caloric regulation. While large-scale studies in individuals employing behavioral and surgical methods of weight loss conclude that it is internal and external cues that undermine long-term weight-loss efforts [14–16], context cues, specifically, are rarely mentioned. Context cues are external but relate to fixed objects or stimuli in the surrounding physical environment that distinguish one location from another (e.g., the cues that distinguish our own kitchen or favorite restaurant from someone else’s kitchen or a less favorite restaurant). These types of external cues differ from social cues such as the relationship to, or number of persons present, or from food-related cues such as the sight, smell, or taste of food. For this study we hypothesized that context cues surrounding, in particular, intake of highly palatable food (PF), can acquire the ability to disrupt caloric regulation of a less PF that under sated conditions is always tightly regulated in intake amount. The hypothesis would be confirmed if the cues paired with PF intake elicit overeating of this other food under sated conditions.
PFs which are typically consumed as snacks, “junk food”, and desserts in humans, contribute to obesity by increasing total caloric intake as they are more energy dense than other foods and by their tendency to be passively overconsumed due to their hedonic or “appetizing” nature. Increasing palatability has shown to reduce the satiating properties of PF. There is evidence too that foods high in fat or high in percent sugar/fat content (typical constituents of PFs) are preferred by obese individuals and that even weight-cycling is associated with an increased hedonic preference for these foods ([17] and [18] for review). In previous work with rats we consistently find that access to PF is a necessary trigger to overeat in sated and normal weight rats with a history of caloric restriction [19] and in rats where binge-eating is induced by stress [20,21]. In the stress model, just a morsel of PF is enough to trigger binge eating on plain rat chow [22]. In rat models of diet-induced obesity, the diets used to achieve obesity are highly palatable compared to control diets [23]. We have also used inherent differences in PF intake to develop a model of binge-eating prone and binge-eating resistant rats [24]. Clearly, PF is a powerful inducer of overeating and obesity maintenance in both humans and laboratory animals.
In addition to the evidence of the powerful effects of PF to trigger overeating in humans and rodents, our hypothesis concerning the power of PF intake to become conditioned with context cues was inspired by Shepard Siegel’s seminal work on drug addiction. It is now a well-known phenomena that context cues become conditioned with the physiological effects of repeated drug administration such that the cues alone can later instate drug cravings and relapse to drug use [25,26]. This knowledge has translated into a standard and essential consideration of the sabotaging power of context cues in drug addiction treatments and anti-drug relapse strategies [26–28]. Perhaps the same could be true of knowledge regarding context cues to reduce relapses to overeating in the prevention of weight-regain, weight-cycling, and treatment of obesity.
To develop and test the present animal model of cued overeating, we used a PF typical of ‘junk” food in its high fat and sucrose content (Oreo cookies). Rats were fed this food in cages with distinct context stimuli. We then asked if the cues surrounding the PF intake were powerful enough to disrupt the normal amount of regular chow that rats consume. We also tested the effect of PF-“primers” to strengthen cue-conditioned overeating of chow. Finally we determined if a tendency to overeat to conditioned cues was associated with a predisposed propensity to overeat PF under normal conditions. A rodent model of the power of PF-conditioned cues to disrupt caloric regulation offers several advantages. It would suggest that the effect is rooted in reflexive-like biological mechanisms as opposed to mechanisms controlled by higher cognitive processing. This could reshape current cognitive and behavioral strategies to prevent weight-regain, especially those designed to deal with food cravings. The model would also serve to identify neural and endocrine targets unique to cued relapse of overeating. While some mechanism may be shared with those underlying drug relapse, it should point to novel targets specific to food intake. The model could then be used to test the effectiveness of novel pharmacologic, nutritional, and behavioral strategies designed to increase adherence to healthy reduced calorie regimens.
METHODS
Subjects
An initial group of N=50 female Sprague-Dawley, 60 days old (Harlan, IN) were used in Exp. 1. Of these, N=30 were used for the rest of the experiments. They were housed in pairs in standard bedded cages under a 12:12hr dark/light (lights off at 1100). One of the pairs was marked with a black Sharpee dot on its tail to distinguish it from the other cagemate. Identification numbers were marked on a cage tag.
Diets
The maintenance diet was Purina rat chow (Harlan Teklad Global Diets, Indianapolis, IN). This also served as the plain less-palatable food in the studies and contains 3.3 kcal/g; 3.5% kcals from fat, 69.8% from carbohydrate, and 16.7% from protein. The palatable food (PF) was Oreo Double Stuf ® cookies (Nabisco, East Hanover, NJ) containing 4.8 kcal/g; 24% kcals from fat, 72% from carbohydrate, and 3.4% from protein. Ad lib water was available at all times.
Experiment 1. Assignment of Rats as Binge-Eating Prone (BEP) vs. Binge-Eating Resistant (BER)
The purpose of identifying rats as BEP or BER was to later determine if any differences in context-cued overeating could be explained by an inherent penchant to overeat PF (characteristic of BEP status). To assign the rats as BEP or BER, we replicated procedures previously described [24]. Briefly, the rats were given four “cookie + chow feeding tests”. The N=10 rats that consistently ate the most cookie kcals and the 10 rats eating the least cookie kcals in the first 4 hrs of the tests were assigned BEP and BER status, respectively. Chow intake and body weights were also recorded to confirm that in the absence of PF, BEPs and BERs did not differ in chow intake or body weight (i.e., only their difference in amount of PF intake characterizes BEP/BER status). Because the rats were paired in home cages, on days that food intake was measured, a Buddy Barrier was placed into the cage to make it possible to obtain individual food intake readings while still allowing sensory interaction between the rats [29]. Each side contained a separate water bottle. The cookies were always placed on the top on each side of the cage lid and chow was always placed inside the cage on either side of the Buddy Barrier. The rats were acclimated to the Buddy Barrier for 3 days prior to their use in this experiment. Following assignment of BEP/BER status, the rats were left in their home cages without a Buddy Barrier with only chow and water for 2 weeks.
Experiment 2. Initial Test for Context-Cued Overeating With and Without a PF-Trigger
Conditioning Phase
The rats were now only given cookies in a distinct “Cookie Cage”; never again in their home cage. The Cookie Cages were covered on the outside back and side walls of the cage with black construction paper. On the inside was a Buddy Barrier and a paper towel laid over the usual woodchip bedding on each side of the Buddy Barrier. These cages contained premeasured chow on the top of the cage lid and premeasured cookies on the inside of the cage. The rats would be left in the Cookie Cage for 24 hrs then returned to the home cage. A schedule was made so that each rat would be in the Cookie Cage a total of 7 times (or 7 days) within a span of 22 days. This allowed a minimum of 2–3 days in the home cage between time in the Cookie Cage. Half the rats from each BEP/BER and middle-most group were in the Cookie Cages on any given day that the other half stayed in the home cages. For this first context-cued feeding experiment, we purposely did not counterbalance the context cues across food types. At this stage we simply wished to determine if rats would indeed overeat plain chow, if placed in a location with cues previously and repeatedly associated with cookie intake. If they did not, it would not be necessary to go the extra step of counterbalancing the cue elements across food conditions. Random 4 hr measures of chow and cookie intake were taken on some of the conditioning days to assess if BEPs continued to eat more cookie kcals when in the Cookie Cages compared to BERs (as expected) and to observe whether this amount of consumption escalated or decreased with repeated exposure to the Cookie Cage.
Testing Phase
After the last (7th) exposure to the Cookie Cage, each rat was placed in its home cage for another 2–3 days with only chow. This time, on the 8th placement into the Cookie Cage, only premeasured chow but no cookies was placed in the Cookie Cage. Intake of chow was measured after 4 and 24 hours in this cage to determine if just the context cues previously associated with PF intake would evoke overeating of chow, the less preferred food. In counterbalanced fashion, a similar test was conducted in the same rats when they were in their home cage with only chow, using a Buddy Barrier. This allowed within-subject comparisons of chow intake when placed in the home cage vs. in the Cookie Cage. Care was taken not to conduct the home cage test on the day after time in the Cookie cage as any overeating might affect the next days’ intake in the home cage. When all the rats were tested in this fashion, a similar test in the home cage and Cookie Cage was repeated, this time with a “PF-trigger”. This PF-trigger consisted of a 2 gm (app. 10 kcal) piece of Oreo cookie placed inside both cages. Care was taken to include equal amounts of cookie wafer and cream per piece. Using the PF-trigger allowed us to determine if priming the rats to the PF would reinforce, or strengthen the likelihood of overeating plain chow in the Cookie Cage. Following this experiment, the rats were maintained in their home cages for another 2 weeks with only chow and water.
Experiment 3. Tests for Context-Cued Overeating, With and Without a PF-Trigger, Controlling for Context Cues
Given the favorable results of Exp.2, we proceeded to test the strength of context-cued overeating by controlling for the cues themselves. This was achieved by counterbalancing the cues across food conditions. We also tested how likely and quickly the rats were to re-learn PF associated cues by using completely different cues and giving them only 4 hr (vs. 24 hr access) to the cues at any one time.
Conditioning Phase
The rats were maintained in their usual home cages but for this experiment, they would also spend time in two types of cages on different days: a Chow Cage and a Cookie Cage. Both cages were housed on separate racks in the same colony and were identical to their home cages except they never contained Buddy Barriers and they had distinct types of bedding. The beddings used were Carefresh Pet Bedding ® (PETCO Animal Supplies, Inc., San Diego, CA) and Kaytee Aspen Bedding® (Kaytee Products, Inc., Chilton, WI). For half the rats in each BEP/BER group, the Chow Cage contained Carefresh bedding and the Cookie Cage contained the Aspen bedding. For the other half, the Chow Cage contained Aspen bedding and the Cookie Cage contained Carefresh bedding. Again, PF intake could only occur in the Cookie Cage. The rats were placed into these cages for only 4 hrs (1100–1500) in any given day. After the 4 hrs, they were returned to their home cage with ad lib chow and water. In the Chow Cage, chow was placed on the cage top with a water bottle. In the Cookie Cage, cookies were placed on the cage top with water bottle and chow was placed inside. A schedule was made assigning the rats to be placed daily into one of the 2 cages. The order of the cage placements was randomized for each rat (e.g., some rats were assigned an “A, B, A, A, B…” order while others a “B, A, B, A, A…” or “A, B, B, A, B…” order, etc.). This was done to avoid expectancy effects and to strengthen the saliency of the bedding cue to its respective food condition. The only rules to the schedule were that no rat went into any one cage more than two days in a row and that an equal number of BEP/BER status rats represented any one order. At the end, each rat was exposed to each of the two cages a total of 7 times on consecutive days except on weekends when they stayed in their home cages.
Testing Phase 1
The same procedures used in Exp. 2 were used except that the chow-only intake was taken while the rats were in the Chow Cage instead of in their home cages. Due to results with the PF-trigger in Exp. 2, we counterbalanced the following four experimental conditions in a repeated measures design, counterbalancing for order across BEP/BER groups: 1) intake of chow in the Chow Cage without a PF trigger, 2) intake of chow in the Chow Cage with a PF trigger, 3) intake of chow in the Cookie Cage without a PF trigger, and 4) intake of chow in the Cookie Cage with a PF trigger. The tests were conducted over four consecutive days. We hypothesized that the rats would eat the most chow when placed in the Cookie Cage with a PF trigger.
Testing Phase 2
Following the 4-conditions test above, the rats were left in their home cages over two days then re-tested again, this time only under the two PF-triggered conditions in counterbalanced fashion: 1) intake of chow in the Chow Cage with PF trigger, and 2) intake of chow in the Cookie Cage with PF trigger. This was conducted over two subsequent days. This test would determine if context-cued overeating could be retained without intermittent reconditioning sessions. The test was then performed again on the next 2 days (PF-triggered conditions only).
Testing Phase 3
Following the tests in Phase 2 which did not involve re-conditioning the rats with cookies in the Cookie Cage, we allowed the rats 4 more placements in each cage, the Cookie Cage with cookies and chow, and the Chow Cage with only chow. We then re-tested the rats in these cages with only chow available and a PF-trigger. This was done to determine if rats could be shortly reconditioned (in a matter of one week’s time) to overeat to context cues.
Experiment 4. Verification of the Stability of BEP/BER Status
At the end of the context-cued feeding experiments, the rats were weighed and a final “cookies + chow feeding test” was performed in their home cages, using the same procedures used to assign them BEP/BER status (Exp.1). Intake of chow and PF was recorded after 4 hrs from lights out. This was done to verify the integrity of their assigned BEP/BER status after being subjected to the context-cued feeding manipulations of Exp. 2 and 3.
Statistical Analyses
For Exp. 1 and 4, between-groups ANOVAs compared mean chow intake, PF intake, and body weight of BEP vs. BER vs. middle-most groups to verify BEP/BER status. For Exp. 2 and 3, separate repeated measures ANOVAs were conducted using cage and PF-trigger conditions as the within-subjects variables and group assignment (BEP/BER/middle-most) as the between-groups factor. Only BEP/BER data are illustrated. For all experiments, food intake was reported in kcals ± SEM and body weight in grams ± SEM. Alpha was set at p<0.05 for statistical significance. The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved all animal procedures.
RESULTS
Experiment 1. Assignment of Rats as Binge-Eating Prone (BEP) vs. Binge-Eating Resistant (BER)
The average median split value of PF intake in N = 50 rats was 35 kcals/4hrs. Rats assigned BEP status consistently ate a minimum mean of 30% more PF than BERs during the “cookie + chow feeding tests” used to assign BEP/BER status (Figure 1; p<0.01). BEPs were confirmed to not simply be “big” eaters because tests where only chow was provided in the cage confirmed that they ate no more chow than did BERs (data taken a week after the 4 “cookie + chow feeding tests”: BEP: 50.7 ± 2.5 vs. BER: 50.1 ± 2.6 chow kcals/4hrs, ns; not shown). Body weights recorded after the feeding tests confirmed no difference between groups (BER: 220.7 ± 3.2 vs. BEPs: 226.7 ± 3.8 g, ns; not shown). During the tests, the middle-most PF-eating group always consumed an amount of PF between that of BEPs and BERs (not shown).
Figure 1.
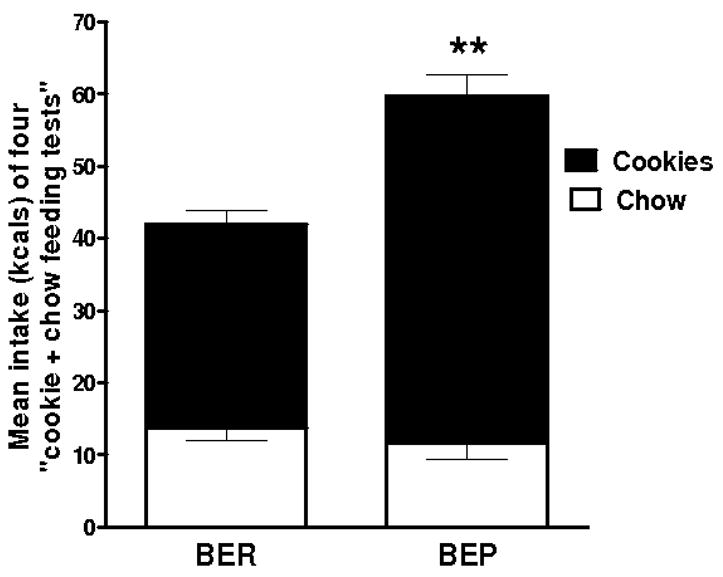
The mean amount of chow and Oreo cookie kcals ingested in the first 4 hrs of dark across four feeding tests used to determine binge-eating prone (BEP) from binge-eating resistant (BER) rats. BEPs ate more of the highly palatable food (cookies) than did BERs; **p<0.01 (and at each feeding test, **p<0.01 per test).
Experiment 2. Initial Test for Context-Cued Overeating With and Without a PF-Trigger
During the pre-test conditioning phase, BEPs ate significantly more PF in the Cookie Cage than BERs, as expected (p<0.05; not shown). We observed no trend for the rats to eat more cookie kcals in the Cookie Cages than they did previously in their home cages during the BEP/BER assignment tests nor a trend for cookie consumption to escalate or decrease with repeated time in the Cookie Cages. For the actual tests, when only chow was made available in the Cookie Cages, we observed no differences in intake when rats were placed in each cage during the first 4 hrs of feeding (Figure 2A). However, by 24 hrs, there was a significant cage effect so that rats ate more chow when in the Cookie Cage vs. when in the home cage (Fig. 2B, p<0.01). When the rats were allowed a 2 g morsel of Oreo cookie as a PF-trigger, being in the Cookie Cage increased the rats’ intake of chow significantly compared to their intake in the home cage (Fig. 2C, p<0.001). This overeating lasted 24 hrs (Fig. 2D, p<0.001). There was no main effect of BEP/BER status. That is, BEP, BER, and middle most rats were equally as likely to overeat when placed in the Cookie Cage with or without a PF-trigger.
Figure 2.
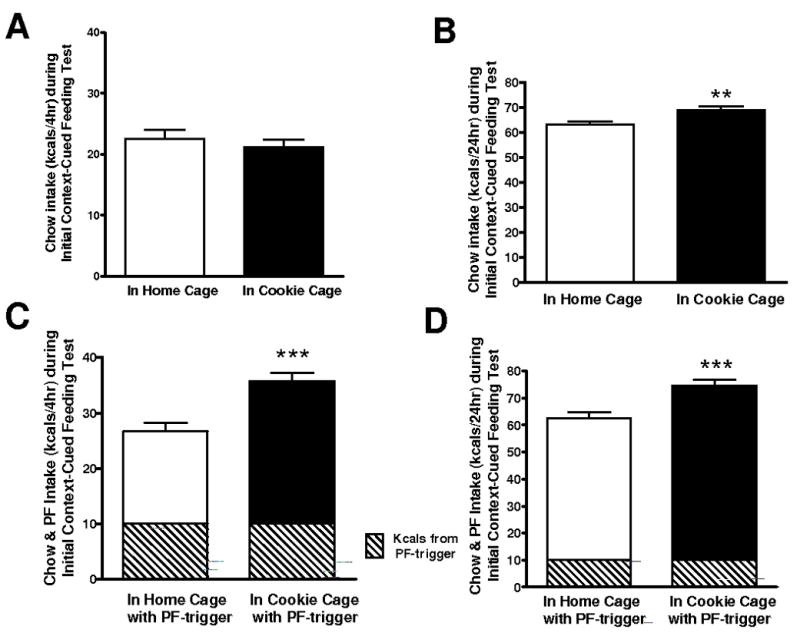
Amount of chow consumed when rats were placed in their home cage with only chow vs. in a cage previously associated with cookies in addition to chow (Cookie Cage) without counterbalancing context elements for each food condition. A) Intake of chow in each cage in the first 4 hrs; ns. B) Intake of chow in each cage over a 24 hr period; **p<0.01 chow intake in Cookie Cage vs. home cage. C) Intake of chow in each cage following a 2 g (10 kcal) preload of cookie as the palatable food (PF) “trigger” over 4 hrs; ***p<0.001 in Cookie vs. home cage. D) PF-triggered intake of chow in each cage over 24 hrs; ***p<0.001 in Cookie vs. home cage. Because there was no effect of group status (BEP vs. BER), both groups are represented in the bar graphs.
Experiment 3. Tests for Context-Cued Overeating, With and Without a PF-Trigger, Controlling for Cues
When the context cues were counterbalanced across the Chow Cage and the Cookie Cage, rats still overate when subjected to the cues associated only with the Cookie Cage. As shown in Figure 3A, as early as in the first 4 hrs of feeding, the rats consumed more kcals of chow when in the Cookie Cage whether they received a PF-trigger or not (p<0.001). Inspection of only the chow intake (Fig. 3B) reveals that in the Chow Cage, rats were able to compensate for the additional kcals of the PF-trigger by eating significantly less chow (p<0.01) than they would without the PF-trigger. However, this ability to compensate for the extra calories of the PF-trigger was abolished if the rats were in the Cookie Cage. I.e., the cues associated with the Cookie Cage caused rats to eat as much chow as they ate without the PF-trigger preload (ns chow intake in Cookie Cage with vs. without PF-trigger; Fig. 3B). After being placed back into their home cages for 2 days then re-tested with a PF-trigger test, they again consumed more chow in the Cookie Cage vs. than in the Chow Cage (Figure 4A). However the difference in mean intake was not as large as in Fig. 3, likely due to more unreinforced experience (no ad lib cookies in the Cookie Cage). By the third PF-trigger test, the differences in chow intake between Cages were not observed (not shown) indicating conditioning extinction. However, once they were re-exposed to cookies in the Cookie Cage and only Chow in the Chow cage, the rats again, under a PF-trigger test, consumed significantly more chow in the Cookie Cage when only chow was available there. This is evident in Figure 4B (p<0.01).
Figure 3.
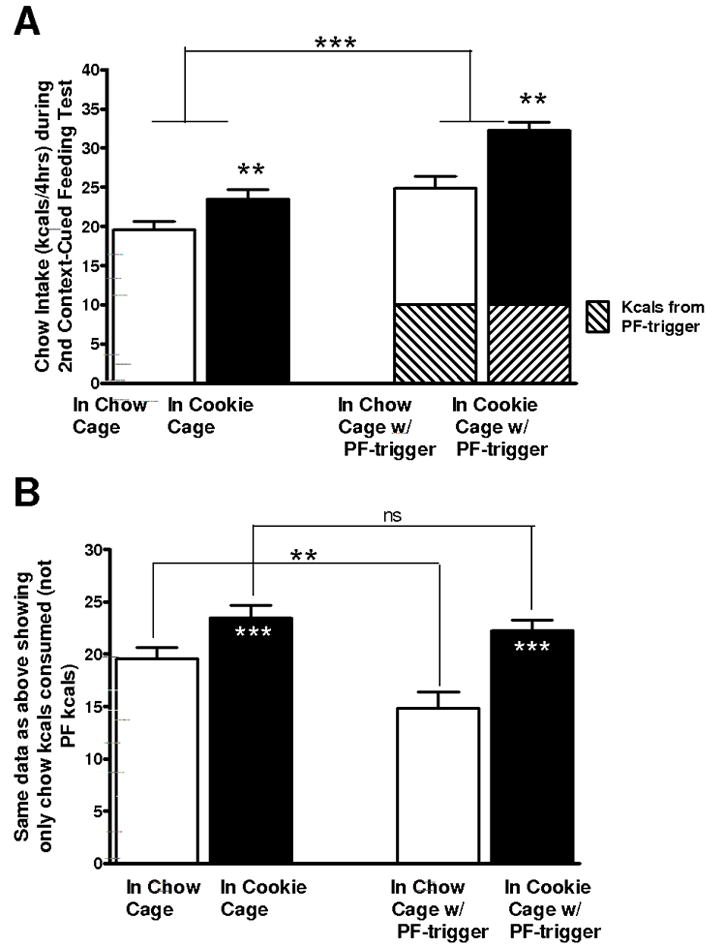
Amount of chow consumed over 4 hrs when rats were placed in a cage previously associated only with chow (Chow Cage) vs. in a cage previously associated with cookies in addition to chow (Cookie Cage) with context elements counterbalanced for each food condition. A) Intake of chow in each cage with and without a 2 g (10 kcal) piece of cookie as the palatable food (PF) “trigger”; ***p<0.001 main effect of PF-trigger to increase chow intake; **p<0.01 main effect of Cookie Cage to increase chow intake. B) Duplication of graph A but without the PF kcals shown to highlight the compensatory decrease in chow intake after a PF preload but only when rats were in the Chow Cage (**p<0.01). In the Cookie Cage, chow intake was greater than in the Chow Cage (***p<0.001) and there was no compensatory decrease in intake following the PF-trigger preload (ns).
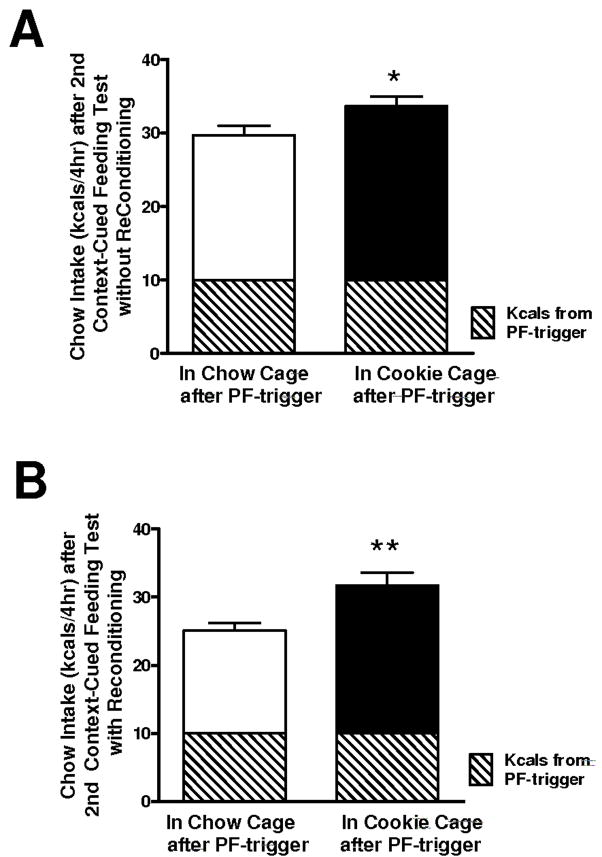
Figure 4.
A) Amount of PF-triggered chow consumed when rats were retested for context-cued overeating after Exp. 3 (Fig. 3) with no additional re-exposure to PF in the Cookie Cage and chow in the Chow Cage (reconditioning; instead rats spent 2 days in home cages prior to this retest); *p<0.05 greater chow intake in the Cookie Cage vs. Chow Cage. B) Amount of PF-triggered chow consumed when rats were retested for context-cued overeating after a short period of re-conditioning to PF in the Cookie cage and chow in the Chow Cage (reconditioning); **p<0.01 greater chow intake in the Cookie Cage vs. Chow Cage.
Experiment 4. Verification of the Stability of BEP/BER Status
At the end of the context-cued feeding tests, we determined that BEP and BER rats still did not differ in body weight (BER: 316.8 ± 13.7 vs. BEP: 318.2 ± 8.1 g, ns; not shown). Their BEP/BER status was also intact as BEPs consumed significantly more PF kcals than BERS in a 4hr period of time (BEP: 46.3 ±2.7 kcals of PF vs. BER: 29.47 ± 1.6 kcals of PF; p<0.01; not shown). As expected, their chow intake did not differ. Therefore, any lack of BEP/BER effects could not be ascribed to a loss of their characteristic difference in amount of PF consumption after exposure to the cue-conditioning manipulations.
DISCUSSION
The present animal model utilized classic Pavlovian conditioning to establish, in rats, an association between context cues and PF intake. This was accomplished by repeatedly pairing PF-intake with distinct context cues and chow-only intake with other cues. The results confirm our hypothesis that in sated rats, context-cues paired with PF intake are powerful enough to elicit overeating of chow, a less preferred food and a food that normally does not stimulate overeating under sated conditions. Further, this cue-conditioned overeating is strengthened with just a taste of the PF that was initially used to make the cue-associations. Additionally, we learned that overeating to context cues is not influenced by a baseline penchant to eat more PF than other rats. Both BEP and BER rats were equally likely to fail to limit their normal intake of chow when exposed to cues previously paired with PF.
We are not the first to find that non-food cues can stimulate spontaneous eating in rats. Rats are able to discriminate and learn context cues associated with PF as evident by conditioned place preference studies [30]. Weingarten found that sated rats will initiate liquid meals to non-food cues under classic Pavlovian procedures [31]. Petrovich and colleagues found that rats consume more training pellets when placed in locations previously paired with those pellets when hungry (under 20-hr food deprived conditions [32]). This effect, however, was only observed when the same training pellets, but not a different and familiar food, rat chow, was available with the cues. In contrast, our protocol produced a conditioned overeating response on regular rat chow, a different but familiar food, and under sated, not hungry learning conditions. In addition, the rats made the association between PF and the context cues quickly and were as quickly able to relearn the association when different cues were paired with the PF. We were surprised that manipulating just one cue element (type of bedding) was enough to establish conditioning to PF-paired cues. Importantly, priming the rats with a morsel of PF caused the most potent dysregulation of normal chow intake. This is reminiscent of the priming effects of small doses of electrical stimulation or drugs to reinstate addictive drug use [33], and of sucrose to reinstate PF seeking [33–35] in rats and to induce craving in humans [36]. Nonetheless, it is important to note that the same PF-trigger did not cause the rats to overeat on chow when they were exposed to chow-paired cues. It was the PF-cues that were ultimately responsible for caloric dysregulation of chow intake.
A question raised by our protocol is whether it is PF intake itself or overeating in general that becomes conditioned to the context cues. This could be answered by matching the amount of PF kcals the rats are allowed to eat in the cued environment (the Cookie Cage) to the amount of chow kcals they normally consume in the same period of time. If the cues still evoke overeating, the effect is contingent on the hedonic properties of PF intake and not on overeating per se. Future experiments with this model will test for this. In real life, however, palatability and overeating are highly correlated and highly correlated to increased BMI [18,37]. Because our protocol called for intermittent exposure to PF and tested overeating on chow which is low in fat and calories, obesity was not an expected outcome. However, in humans, cued-overeating is likely to occur on high calorie foods and over extended periods of time. Given that as small a reduction as 50 kcal/day could offset weight gain in about 90%of the population [3], any excess calories consumed from cued-overeating may significantly contribute to the maintenance of overweight and obesity.
In drug addiction, physiological effects that are opposite of the initial effects of the drug develop after repeated drug use. These effects become conditioned to surrounding non-drug cues so that eventually just the cues instate a craving for the drug and allow the body to safely tolerate incrementing doses of the drug [26]. We believe that similar changes might be taking place in conditioned overeating to explain the greater intake of chow by PF-paired cues. The increased sugar and fat ingestion that occurs with PF intake and any changes induced by repeated overeating that comes naturally with PFs may induce opposite physiological effects such as hypoglycemia and increased insulin secretion. Such responses are in fact known to occur in conditioned meal anticipation. They would favor increased appetite and allow the organism to digest greater quantities of food [38]. In individuals trying to limit their caloric intake, such conditioned physiological changes would make it difficult to adhere to lower calorie regimens. According to our results with rats, where just a morsel of PF strengthened cue-conditioned overeating, it would be doubly difficult if these individuals were to allow themselves just a bite of a PF. Our observations in the rats further predict that in humans, PF triggers may be less potent saboteurs if the PF is eaten in a new environment (e.g., dessert at a new restaurant). This is because prior overeating has not been paired with the novel context cues. However, a PF trigger might be much more likely to sabotage a reduced calorie regimen if eaten in a location previously and repeatedly paired with overeating (e.g., dessert at home).
In Petrovich and colleagues’ model of hunger-paired cue conditioned eating, the fact that rats only overate the food initially paired with hunger was explained as being driven by a more specific motivation, one resembling craving, as opposed to a general motivation to eat such as that induced by hunger [32]. Then what motivation is behind the overeating in the present model? We believe that the motivation includes a specific “Oreo” craving at first, but without hope of obtaining the craved food, the rats’ physiology, now conditioned to tolerate extra calories when exposed to PF-paired cues, may have worked to decrease their satiety response. Therefore, greater intake of chow was necessary to meet this altered satiety set-point. Certainly any specific craving for Oreos could not be assuaged by overeating chow. However, a motivation to reach satiety could be. Davidson & Swithers found that in rat pups, the taste of a novel sweet food was able to disrupt their normal caloric regulation of chow [39] but only if they had prior experience with sweet foods. Ferriday & Brunstom recently published that, in humans, not taste but the sight and smell of pizza was associated with greater intake not only of pizza but other foods [40]. Our results take these studies further by showing that not only taste and orosensory properties of food can dysregulate normal caloric intake of other foods; non-food context cues can also disrupt normal caloric regulation of other foods. A recent study in preschool children found that they begin to eat when presented with a light and musical tune that was previously and repeatedly paired with snacks intake, even after they consumed a snack preload [41]. The extent to which PF-paired context cues are able to reset satiety thresholds to explain our animal model and these other findings in humans merits further research. Since all humans partake in PF intake but not all become obese, the more important question may not be what individual characteristics predispose cue-induced overeating [42,43] but what predicts the ability to compensate for calories ingested under PF-cued conditions.
Despite the need for further experiments to fully understand PF-cued overeating, the present model has immediate and valuable implications. The model should be easily replicated. We predict that various context cues beyond the ones utilized here and other sweet or high-fat primers will elicit the effect. It is also possible that shorter conditioning periods are sufficient to establish dysregulation of chow intake. The model can be used to identify the physiological mediators that drive cue-induced caloric dysregulation. Pharmacologic targeting of these substrates should help prevent relapse in millions of persons trying to break out of chronic weight cycling or binge-eating patterns. Candidate substrates worth targeting include β-adrenergic-[44] and opioid-receptors [35]. The medial prefrontal cortex and connections between the basolateral amygdala and lateral hypothalamus are emerging as key neural sites in the ability of conditioned cues to override satiety [45]. The model highlights the power that non-food cues can evoke on the control of food intake. Observing this effect in rats is evidence that higher cognitive processing is not necessary to disinhibit food intake. While humans may be acutely aware of internal sensations such as hunger and food cravings, they tend not to be aware of external cues [46,47]. They are probably even less likely to attribute cravings to context cues. If they fail to adhere to a weight-reducing calorie regimen they are likely to attribute this to personal weakness, not conditioned cues.
In sum, this rodent model suggests that context cues in the environment may have a more prominent role in sabotaging the regulation of caloric intake than is commonly believed. Some have proposed that strategies aimed at extinguishing learned food-associated cues may be useful in the management of overeating and obesity [8,10,36]. It is our hope that the present animal model will increase interest in moving such strategies forward. Drug-related cues are given very serious consideration in relapse-prevention strategies against drug addiction [26,27]. We believe that the success of programs designed to promote weight loss or abstinence from binge-eating might be significantly increased if a similar, more serious and explicitly consideration is given to the “cued-overeating” factor.
Acknowledgments
We thank Dr. Paul D. Blanton for his editorial assistance. This work was funded in part by NIH grant DK066007 and UAB Support for Development and Application of Research Using Animal Models (SDARAM).
References
- 1.Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, Pietrobelli A. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85:346–354. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 2.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 4.Schlundt DG, Hill JO, Pope-Cordle J, Arnold D, Virts KL, Katahn M. Randomized evaluation of a low fat ad libitum carbohydrate diet for weight reduction. Int J Obes Relat Metab Disord. 1993;17:623–629. [PubMed] [Google Scholar]
- 5.de Castro JM, Brewer EM. The amount eaten in meals by humans is a power function of the number of people present. Physiol Behav. 1992;51:121–151. doi: 10.1016/0031-9384(92)90212-k. [DOI] [PubMed] [Google Scholar]
- 6.Hetherington MM. Cues to overeat: psychological factors influencing overconsumption. Proc Nutr Soc. 2007;66:113–123. doi: 10.1017/S0029665107005344. [DOI] [PubMed] [Google Scholar]
- 7.Raynor DA, Phelan S, Hill JO, Wing RR. Television viewing and long-term weight maintenance: results from the National Weight Control Registry. Obesity. 2006;14:1816–1824. doi: 10.1038/oby.2006.209. [DOI] [PubMed] [Google Scholar]
- 8.Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R. Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite. 2008;51:556–562. doi: 10.1016/j.appet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Palfai TP, Macdonald A. Effects of temptations on the affective salience of weight control goals. Behav Res Ther. 2007;45:449–458. doi: 10.1016/j.brat.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Wardle J. Conditioning processes and cue exposure in the modification of excessive eating. Addict Behav. 1990;15:387–393. doi: 10.1016/0306-4603(90)90047-2. [DOI] [PubMed] [Google Scholar]
- 11.Adam TC, Epel ES. Stress, eating, and the reward system. Physiol Behav. 2007;91:441–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Thomas SL, Hyde J, Karunaratne A, Kausman R, Komesaroff PA. “They all work…when you stick to them”: A qualitative investigation of dieting, weight loss, and physical exercise in obese individuals. Nutr J. 2008;7:34. doi: 10.1186/1475-2891-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe MG. The role of anticipated deprivation in overeating. Addict Behav. 1982:103–112. doi: 10.1016/0306-4603(82)90035-1. [DOI] [PubMed] [Google Scholar]
- 14.McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999;67:177–185. doi: 10.1037//0022-006x.67.2.177. [DOI] [PubMed] [Google Scholar]
- 15.Niemeier HM, Phelan S, Fava JL, Wing RR. Internal disinhibition predicts weight regain following weight loss and weight loss maintenance. Obesity. 2007;15:2485–2494. doi: 10.1038/oby.2007.295. [DOI] [PubMed] [Google Scholar]
- 16.Bond DS, Phelan S, Leahey TM, Hill JO, Wing RR. Weight-loss maintenance in successful weight losers: surgical vs non-surgical methods. Int J Obes. 2008 doi: 10.1038/ijo.2008.256. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA. Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutrition. 2004;79:372–378. doi: 10.1093/ajcn/79.3.372. [DOI] [PubMed] [Google Scholar]
- 18.Nasser J. Taste, food intake and obesity. Obes Rev. 2001;2:213–218. doi: 10.1046/j.1467-789x.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge-eating: key synergistic role of past caloric restriction and stress. Physiol & Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 21.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- 22.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge-eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Dunn-Meynel lAA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol. 2002;282:R46–54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- 24.Boggiano MM, Artiga AI, Pritchett CE, Chandler PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating characteristics independent of susceptibility to obesity: An animal model of lean vs. obese binge eating and obesity with and without binge-eating. Int J Obesity. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 25.Siegel S, Hinson RE, Krank MD, McCully J. Heroin “overdose” death: contribution of drug-associated environmental cues. Science. 1982;216:436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- 26.Siegel S, Ramos BM. Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp Clin Psychopharmacol. 2002;10:162–183. doi: 10.1037//1064-1297.10.3.162. [DOI] [PubMed] [Google Scholar]
- 27.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.027. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boggiano MM, Cavigelli SA, Dorsey JR, Pritchett-Kelley CE, Ragan CM, Chandler-Laney PC. Effect of a cage divider permitting social stimuli on stress and food intake in rats. Physiol Behav. 2008;95:222–228. doi: 10.1016/j.physbeh.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarosz PA, Kessler JT, Sekhon P, Coscina DV. Conditioned place preferences (CPPs) to high-caloric “snack foods” in rat strains genetically prone vs. resistant to diet-induced obesity: Resistance to naltrexone blockade. Pharmacol Biochem Behav. 2007;86:699–704. doi: 10.1016/j.pbb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 32.Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharm. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hood S, Sorge RE, Stewart J. Chronic buprenorphine reduces the response to sucrose-associated cues in non food-deprived rats. Pharmacol Biochem Behav. 2007;86:566–575. doi: 10.1016/j.pbb.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res. 2005;13:797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- 38.Woods SC. Conditioned hypoglycemia. J Comp Physiol Psychol. 1976;90:1164–1168. doi: 10.1037/h0077292. [DOI] [PubMed] [Google Scholar]
- 39.Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. Int J Obes Relat Metab Disord. 2004;28:933–935. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- 40.Ferriday D, Brunstrom JM. How does food-cue exposure lead to larger meal sizes? Br J Nutr. 2008;100:1325–1332. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- 41.Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- 42.Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161:751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- 43.Werrij MQ, Roefs A, Janssen I, Stapert D, Wolters G, Mulkens S, Hospers HJ, Jansen A. Early associations with palatable foods in overweight and obesity are not disinhibition related but restraint related. J Behav Ther Exp Psychiatry. 2008 doi: 10.1016/j.jbtep.2008.07.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learning & Memory. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 45.Petrovich GD, Gallagher M. Control of food consumption by learned cues: a forebrain-hypothalamic network. Physiol Behav. 2007;24:397–403. doi: 10.1016/j.physbeh.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen DA. Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes. 2008;57:1768–1773. doi: 10.2337/db08-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vartanian LR, Herman CP, Wansink B. Are we aware of the external factors that influence our food intake? Health Psychol. 2008;27:553–538. doi: 10.1037/0278-6133.27.5.533. [DOI] [PubMed] [Google Scholar]