Abstract
Postmortem research has revealed that there is a lower density of glial cells in regions of the prefrontal cortex (PFC) of uncomplicated alcoholics when compared with control subjects. Impairment of astrocyte function in the PFC may contribute to malfunction in circuits involved in emotion- and reward-related subcortical centers, heavily connected with the PFC and directly involved in the pathophysiology of addictive behaviours. The hypothesis was tested that infusion of gliotoxins known to injure astrocytes or of a gap junction blocker into the prelimbic area of the rat PFC results in increased preference for ethanol in rats exposed to free choice between water and 10% ethanol. Fluorocitric acid, L-α-aminoadipic acid (AAD) or the gap junction blocker 18-α-glycyrrhetinic acid (AGA) were bilaterally infused once into the rat prelimbic cortex and alcohol preference (ratio of 10% ethanol consumed to total liquid ingested) was measured before and after infusion. Infusion of AAD or AGA dissolved in their vehicles, but not of their vehicles alone, resulted in significant transient increase of preference for 10% ethanol. The present data suggest that impaired integrity of glial cells or the gap junctional communication between them in the rat PFC may contribute to changes in ethanol preference.
Keywords: alcoholism, astrocytes, glia, prefrontal cortex, rat model
Introduction
Recent human postmortem research has revealed that in the dorsolateral and orbitofrontal regions of the prefrontal cortex (PFC) and in the hippocampus of ‘uncomplicated’ alcoholics, the density of glial cells is significantly lower than in healthy controls (Korbo, 1999; Miguel Hidalgo, et al., 2006; Miguel-Hidalgo, et al., 2002). In addition, research in the prelimbic region of the rat PFC [prelimbic cortex (PLC)] has revealed that the density of astrocytes immunoreactive for glial fibrillary acidic protein (GFAP) is significantly lower in alcohol preferring rats than in Wistar or alcohol nonpreferring rats (Miguel-Hidalgo, 2005, 2006). Although various regions of the PFC are heavily involved in the expression of behaviours of addiction (Volkow and Fowler, 2000; Volkow, et al., 1993, 1997), the lower densities of glial cells suggest that deficient or compromised astrocytic function in the PFC may contribute to an enhancement of alcohol intake or preference.
Significant contribution of astrocytes to the prefrontal physiopathology of alcoholism is mechanistically plausible, because astrocytes are essential for the support of neuronal metabolism, neurotransmission and synaptic plasticity (Ullian, et al., 2004; Anderson and Swanson, 2000; Haydon and Carmignoto, 2006; Qutub and Hunt, 2005). In addition, astrocytes are directly affected by exposure to ethanol. Ethanol has been shown to inhibit proliferation of cultured astrocytes (Davies and Vernadakis, 1984; Costa and Guizzetti, 2002; Miller and Luo, 2002; Kane, et al., 1996), and astrocytes in the brains of alcoholics might respond to chronic alcohol misuse with increased cell death in the form of apoptosis (Ikegami, et al., 2003). Either by reduction in the density of astrocytes or due to altered communication between astrocytes, neuronal function in the PFC may be impaired and lead to alterations in alcohol intake and preference.
One possibility for an astrocyte-mediated mechanism affecting alcohol preference and intake is an alteration in glutamate reuptake, which is overwhelmingly performed by glutamate transporters located in the cell membrane of astrocyte processes. For instance, recent research has shown that increased extracellular glutamate caused by deficient glutamate transport in transgenic mice with the Per2Brdm1 mutation results in increased alcohol intake (Spanagel, et al., 2005), whereas some single nucleotide polymorphisms in the human Per2 gene are associated to alcoholism (Spanagel, et al., 2005). Repeated administration of ethanol to rats also results in a decrease of glutamate transport (Melendez, et al., 2005). Likewise involvement of astrocytes may play a role in addiction to other substances of abuse. MS-153, an activator of glutamate transport, when co-administered with morphine attenuates the development of morphine tolerance and physical dependence (Nakagawa, et al., 2001). MS-153 reduced conditioned place preference (CPP) caused by methamphetamine. Morphine-induced CPP was also reduced in mice that received adenoviral transfer of the gene for the glutamate transporter GLT-1 into the Nucleus accumbens shell (Fujio, et al., 2005). Given the direct involvement of astrocytes in glutamate transport, reduced numbers or function of astrocytes in other brain areas involved in addiction may be also reflected in altered alcohol preference or intake.
The same particular metabolic and neurotransmitter-processing properties of astrocytes that are important in supporting neuronal function have facilitated the discovery of gliotoxins that have been reported to preferentially damage astrocytes when used at relatively low concentrations, both in vivo and in vitro. Two of these gliotoxic compounds are fluorocitric acid (FC) and L-α-aminoadipic acid (AAD). Fluorocitric acid inhibits aconitase, a TCA cycle enzyme, enriched in astrocytes, that transforms citrate into isocitrate (Peters, 1957). Thus, FC application results in interruption of ATP production and damage to astrocytes (Paulsen, et al., 1987; Hosoi, et al., 2004; Peters, 1957). On the other hand, glutamate reuptake via glutamate transporters and the cystine-glutamate antiporter is heavily dependent on astrocytes (Anderson and Swanson, 2000). These transport features have been exploited to damage astrocytes using AAD, a structural homologue of glutamate. At low concentrations AAD is transported into astrocytes, but not neurons, through glutamate transporters, and the cystine-glutamate antiporter, and causes intracellular toxicity (Brown and Kretzschmar, 1998; Huck, et al., 1984a,b; Sagara, et al., 1993). Both FC and AAD have been shown to severely impair astrocytes in vitro and after infusion into the striatum, with little or no damage to neurons, although the sparing of neurons is better documented in the case of AAD (Khurgel, et al., 1996; Takada, et al., 1990; Takada and Hattori, 1986; Paulsen, et al., 1987). Thus, these compounds can be used to test whether injury to astrocytes results in particular neurophysiological or behavioural deficits.
Another main determinant of astrocytic function is the communication between adjacent astrocytes through gap junctions (Larrosa, et al., 2006; Rouach, et al., 2002). In the neocortex, most of gap junctional communication occurs between astrocytes or is mediated by them (Nagy and Rash, 2000; Rash, et al., 2001). Waves of transient increases of calcium concentration in the cytoplasm of astrocytes spread from astrocytes to astrocyte and coordinate responses between them depend largely (although not exclusively) on communication through gap junctions (Venance, et al., 1997; Giaume and Venance, 1998). Reduced inter-astrocyte communication due to lower glial densities or inhibition of gap junction communication may result in altered astrocytic function in the PFC, and contribute to increased alcohol preference in at risk subjects. Some compounds like 18-α-glycyrrhetinic acid (AGA) are relatively good specific inhibitors of gap junction communication (Davidson and Baumgarten, 1988; Giaume and McCarthy, 1996; Goldberg, et al., 1996) and their application to the PFC might result in changed alcohol-drinking behaviour due to impairment of astrocytic function even in the absence of overt toxic damage.
The PLC is a component of rat PFC that has been implicated in the control of addictive behaviours (Zavala, et al., 2003; Miller and Marshall, 2004). Thus, we started testing the hypothesis that reduced astrocytic function in the rat PLC may increase alcohol preference by infusing gliotoxins AAD and FC, or gap junction blocker AGA into the PLC and measuring preference for an ethanol-containing solution over water.
Materials and methods
Animals
Male outbred Wistar rats weighing between 300 and 400 g were used in the present study. Animals were kept on a 12/12-h light/dark cycle and fed ad libitum standard chow. During the first week at our facilities, there were three animals per cage. One week after their arrival animals were singly caged and each cage supplied with two bottles, one bottle with tap water and the other with 10% ethanol (EtOH) in tap water. One week later, the bottle with water alone was removed and animals remained only with the 10% EtOH water for 4 days, after which the water-only bottle was reintroduced again, and the animals continued drinking from two bottles for an additional 7-day period. At the end of this period animals received a single infusion of fluorocitrate (FC) (8 mM), AAD (125 mM), gap junction blocker AGA (100 μM) or vehicle into the PLC of each cerebral hemisphere. The vehicle control group for FCand AAD-treated groups was one and the same, and these three groups were all infused in the same session. The experiment with AGA was conducted at a different time and involved its own vehicle control group.
Infusion of compounds and preference estimates
Animals were anaesthetized using isoflurane (induction of anaesthesia at 5% and maintenance at 1%) and attached to a stereotaxic apparatus. The 33-gauge, beveled needle of a 2.5-μl Hamilton syringe was lowered once at each cerebral hemisphere into the PLC aiming to these coordinates (mm): anterior 3.8 (rostrally from Bregma), lateral 0.4 (from the midline to each side), vertical 3.7 (ventrally from the brain surface) (Paxinos and Watson, 1998). Two minutes after needle placement, 0.5 μl of a solution containing AAD (125 mM, n = 4), FC (0.8 mM, n = 4), AGA (100 μM, n = 5) or vehicle were infused at a continuous rate of 0.25 μl/min. Phosphate-buffered saline (pH 7.4) (PBS) was the vehicle in the control group (n = 4) for AAD- and FC-infused animals, while 0.6% chloroform + 0.4% methanol in PBS was the vehicle in the control group (n = 6) for AGA. After the infusion, the needle was slowly retracted over 2 min. The incision in the scalp was then sutured, antibiotic cream applied and animals were returned to their individual cages. Animals typically recovered from the isoflurane anaesthesia in <3 min. After surgery, the cages were still provided with solid food ad libitum and with two bottles (one with water the other one with 10% EtOH). The position of the bottles was changed every day to rule out preference solely based on the position of the bottle.
The amount of liquid consumed from each bottle in the week before and 4 days after surgery was recorded daily. A preference ratio was calculated dividing the volume drunk from the 10% ethanol bottle into the total volume of liquid consumed; a ratio of ‘1’ indicating maximum preference for alcohol (all liquid consumption would be from the bottle with 10% ethanol) and a ratio of ‘0’ indicating maximum preference for water.
Histology and immunohistochemistry
Four days after surgery, animals were anaesthetized and perfused through the ascending aorta with 50 ml of PBS followed by 250 ml of 4% paraformaldehyde in PBS. Brains were removed from the skull, postfixed overnight in 4% paraformaldehyde and coronal sections through the PLC at a thickness of 30 μm were obtained on a vibratome. Alternating series of sections were stained with cresyl violet or processed for immunohistochemistry of GFAP (a specific marker of astrocytes) or AGA. GFAP was probed with a primary mouse monoclonal antibody obtained commercially (Chemicon, Temecula, California, USA), used at a 1:1000 dilution. After the primary antibody, sections were washed with Tris–HCl buffer (pH 7.6) and incubated with a biotinylated secondary antibody, washed again and the antibody complexes further bound to the ABC complex (ABC peroxidase kit; Vector Laboratories, Burlingame, California, USA). The antibody complexes were finally observed by incubating the sections with a solution containing 0.05% 3-3′-diaminobenzidine, 0.6% (NH4)2Ni(SO4)2·6H2O and 0.005% H2O2 in TBS. Animals in which the track of the infusion needle missed the PLC were excluded from the statistical analysis.
To estimate the extent of diffusion of injected compounds, some animals that had AGA infused were killed two days after the infusion. Sections through the PLC were processed for immunohistochemistry as above, using a primary antibody that recognizes AGA. This antibody had been prepared by one of the authors (Shoyama) and shown to specifically bind to glycyrrhizic acid and AGA (Shan, et al., 1999, 2001; Tanaka and Shoyama, 1998). The staining with this antibody was limited to the neuropil in the PLC mainly in the direction to the medial cortical surface (Figure 1) and was not seen to pass into other adjacent areas, such as the infralimbic cortex. Sections from some of the animals with four days of survival were also immunostained for AGA. In this case, the AGA immunolabelling was considerably reduced and restricted to the track of the infusion needle within the PLC (Figure 1).
Figure 1.
Images of the PLC in AGA-immunostained sections of AGA-infused animals two (top panels) or four (bottom panel) after the infusion of AGA. WM, white matter; PLC, prelimbic cortex.
Statistical analysis was performed using repeated measures (preference levels at different days) ANOVA and post hoc pairwise comparisons were performed using the Tukey–Kramer test to account for multiple comparisons.
Results
Histology
Infusion of vehicle in the control experiments for gliotoxins or AGA resulted in small lesions in the PLC when observed four days after the surgery (Figure 2). In these sites infused with vehicle, neurons appeared intact around the needle track (Figure 2a) and GFAP-immunostained astrocytes were slightly enlarged without obvious damage (Figure 2b). In animals infused with AAD also neurons appeared intact, although a region with reduced GFAP immunoreactive astrocytes was present around the infusion (Figure 3a). With fluorocitrate the damage was greater than with AAD, including necrotic-like damage to neurons around the infusion site. After infusion of AGA, GFAP immunoreactive astrocytes appeared very similar to those in vehicle-infused animals (Figure 3b), also without apparent damage to surrounding neurons.
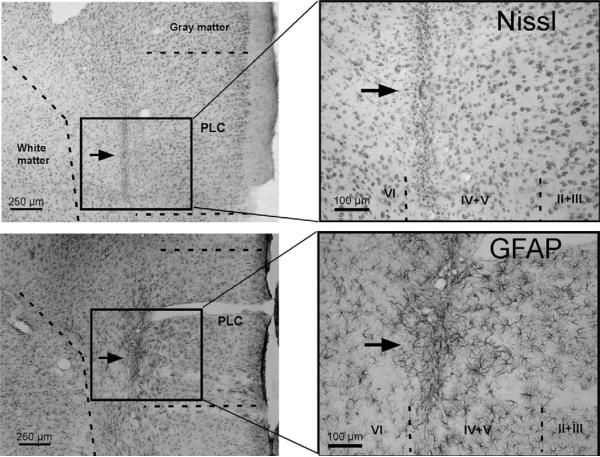
Figure 2.
Micrographs sections of the prelimbic cortex (PLC) stained with cresyl violet (a Nissl-type staining; top panels) or immunostained for GFAP (bottom panels) in animals receiving infusion of vehicle into the PLC. Panels at the right are magnified images of the rectangles in the left panels. Arrows point to the track of the inserted needles. Roman numerals on the right panels denote the cortical layers.
Figure 3.
Images of the infusion site 4 days after infusion of either L-α-aminoadipic acid (L-AAD) or 18-α-glycyrrhetinic acid (AGA) into the PLC. Sections were immunostained with and antibody to GFAP.
Alcohol preference and liquid intake
The preference ratio for 10% EtOH over water stabilized after the first 3 days of the week preceding the infusion to reach an average level between 0.25 and 0.3 in all groups of animals, indicating a preference for the water bottle over the 10% ethanol bottle during the preinfusion period (Figures 4a and 5a). After the infusion, preference for the 10% EtOH-containing bottle was not changed in the animals treated with vehicle, either in the controls for infusions of FC and AAD (F(3,15) = 0.60, P = 0.70) or in the controls for AGA infusion (F(5,25) = 0.36, P = 0.87) (Figures 4a and 5a). In animals that received infusion of FC, there was no difference in preference between the days before and after the infusion (F(3,15) = 1.36, P = 0.29), although the highest values for preference occurred in the second day after the infusion, with a ratio of 0.40 ± 0.1 (mean ± standard error of the mean) when compared with the day before infusion (0.22 ± 0.1) (Figure 4b). In animals infused with AAD, there was a significant difference between days in the preference ratio (Figure 4c) (F = 8.0, P < 0.001). The preference in the second day after infusion (0.63 ± 0.12) was significantly larger than the preference measured in the 2 days before the infusion (q = 7.1, P < 0.01 and q = 8.1, P = 0.001). In animals treated with AGA, there was also a significant difference between days in the preference for the 10% EtOH bottle (F(4,20) = 10.99, P < 0.0001). The preference in the first (0.44 ± 0.05) and second days (0.55 ± 0.06) after infusion was significantly higher (q = 4.8, P < 0.05 and q = 7.34, P < 0.001 respectively) than the preference 1 day before the infusion (0.23 ± 0.05) (Figure 5b). Both in animals treated with AAD and those treated with AGA the increase in preference was transient, so that on the third and fourth days after the infusion the preference ratio was not significantly different from the ratio on the day before the infusion, and (with the exception of the third day after infusion in AAD infused animals) was significantly lower than the value of preference at 2 days after the infusion (q = 4.28, P > 0.05 and q = 5.61, P < 0.05 for AAD-treated animals and q = 7.10, P < 0.001 and q = 8.23, P < 0.001 for AGA-treated animals) (Figures 4c and 5b).
Figure 4.
Graphs of alcohol preference ratio in the days before and after the infusion of vehicle (PBS) (n = 4) (a), fluorocitric acid (n = 4) (b) or L-α-aminoadipic acid (n = 4) (c) into the PLC of Wistar rats. Whiskers represent standard errors. At the bottom of the graph, day 1 is the day preceding the infusion and day 2 is the day of the infusion. Consequently, day 4 represents 2 days after the infusion. **Significantly different from day 1 at P < 0.001.
Figure 5.
Graphs of alcohol preference ratio in the days before and after the infusion of vehicle (0.6% chloroform + 0.4% methanol in PBS, n = 6) (a) or 18-α-glycyrrhetinic acid (n = 5) (b) into the PLC of Wistar rats. Whiskers represent standard errors. *Significantly different from day 1 at P < 0.05; **Significantly different from day 1 at P < 0.001.
The total daily amount of liquid ingested by each animal was also calculated. There was no difference between groups in the amount of total liquid ingested (Figure 6). When compared with the days before infusion, the total liquid intake did not significantly change in the days with increased preference for alcohol either in the animals injected with gliotoxins or in those infused with only vehicle, although at 4 days postsurgery all groups appeared to have increased their total liquid intake (Figure 6).
Figure 6.
Graph showing the liquid consumption for the days before and after the infusion into the PLC for the vehicle and compound infused animals.
Discussion
The results above suggest that damage to astrocytes or impairment of gap junction communication in the PFC of Wistar rats results in increased preference for 10% EtOH. One parsimonious explanation would attribute the changed preference to altered neuronal function in the PFC contingent upon temporary disturbance of astrocyte function. However, a simple cessation of function in prelimbic neurons might not be sufficient to explain the change in preference. After excitotoxic lesions to the medial PFC directly damaging neurons, alcohol drinking was not altered (Hansen, et al., 1995). In addition, the extent of the ibotenic acid lesions in Hansen's study (1995) included both infralimbic and prelimbic cortex, clearly an area of damage larger than the area affected in our experiments. The possibility that damage to neurons or larger PFC lesions do not necessarily result in changed alcohol preference is also supported by the lack of significant change in preference with FC in our experiments, because FC produced larger lesions than AAD or AGA. Thus damage circumscribed to the PLC or alteration of synaptic regulation within the PLC may be crucial to the increased alcohol preference observed in the present experiments. In fact, one study, although addressing cocaine rather than alcohol addiction, found that lesions well-circumscribed to the PLC induce deficits of some aspects of working memory and reduced sensitization to cocaine treatment (Heidbreder and Groenewegen, 2003).
Other studies have examined neuronal activation related to alcohol- or other drug-induced changes in preference. In the rat PLC, alcohol, but not sucrose deprivation, produces specific c-fos activation upon re-exposure to the environment of alcohol self-administration (Wedzony, et al., 2003). Cocaine-induced place preference is also associated with activation of c-fos expression in GABAergic neurons in the PLC, while cocaine also reduces c-fos expression in putative PLC glutamatergic neurons with projections to the nucleus accumbens (Miller and Marshall, 2004; Ciccocioppo, et al., 2001). If damage or reduced function of glial cells similarly increases the inhibitory tone (for example by impaired glutamate uptake by astrocytes, which is affected by L-AAD) upon projecting neurons, this inhibition might contribute to the increased alcohol preference seen in our experiments.
A reduction in the plasticity of cholinergic prefrontal neurons as a consequence of alcohol drinking in alcohol-preferring rats has also been proposed to affect alcohol intake. This reduction consists of lower numbers of Cdk5-immunoreactive cholinergic neurons in the PLC of those rats (Camp, et al., 2006). The lower numbers of PLC Cdk5-immunoreactive cholinergic neurons are concomitant with an increase in Cdk5-immunoreactive cells in the nucleus accumbens and amygdala. Cholinergic muscarinic receptors mediate calcium elevations in astrocytes (Shelton and McCarthy, 2000; Araque, et al., 2002), changes in astrocyte proliferation (Guizzetti, et al., 1996) and expression of neurotrophic factors (Wu, et al., 2004). Furthermore, ethanol itself is capable of inhibiting muscarinic receptor-mediated proliferation of astrocytes (Guizzetti and Costa, 1996). Thus changes in prefrontal cholinergic neuro-transmission in alcoholism, combined with direct actions of alcohol on astrocytes in the PFC, may also contribute to behavioural alterations in alcohol preference.
In summary, the present results are consistent with astrocytic dysfunction in the PLC contributing to a transient increase in preference for alcohol. Future experiments should determine whether the possible glial contribution to alcohol preference is specific to the PFC and how mechanisms involving inter-astrocyte communication, defective glutamate transport or cholinergic responsiveness of astrocytes may alter neuronal function in reward-related cortical and subcortical centers.
Acknowledgements
This work was supported by the Center for Psychiatric Neuroscience at the University of Mississippi Medical Center (grant RR017701).
Contributor Information
J Miguel-Hidalgo, Psychiatry and Human Behaviour, University of Mississippi Medical Center, Jackson, Mississippi, USA..
Y Shoyama, Department of Chemo-Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan..
V Wanzo, Psychiatry and Human Behaviour, University of Mississippi Medical Center, Jackson, Mississippi, USA..
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Araque A, Martin ED, Perea G, Arellano JI, Buno W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci. 2002;22:2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Kretzschmar HA. The glio-toxic mechanism of alpha-aminoadipic acid on cultured astrocytes. J Neurocytol. 1998;27:109–118. doi: 10.1023/a:1006947322342. [DOI] [PubMed] [Google Scholar]
- Camp MC, Mayfield RD, Mccracken M, Mccracken L, Alcantara A. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res. 2006;30:1322–1335. doi: 10.1111/j.1530-0277.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D (1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Guizzetti M. Inhibition of muscarinic receptor-induced proliferation of astroglial cells by ethanol: mechanisms and implications for the fetal alcohol syndrome. Neurotoxicology. 2002;23:685–691. doi: 10.1016/S0161-813X(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- Davies DL, Vernadakis A. Effects of ethanol on cultured glial cells: proliferation and glutamine synthetase activity. Brain Res. 1984;318:27–35. doi: 10.1016/0165-3806(84)90059-2. [DOI] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, et al. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Giaume C, Mccarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Bechberger JF, Hearn SS, Shivers RR, Macphee DJ, et al. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp Cell Res. 1996;222:48–53. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Hansen S, Fahlke C, Hard E, Thomasson R. Effects of ibotenic acid lesions of the ventral striatum and the medial prefrontal cortex on ethanol consumption in the rat. Alcohol. 1995;12:397–402. doi: 10.1016/0741-8329(95)00008-f. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Okada M, Hatazawa J, Gee A, Inoue O. Effect of astrocytic energy metabolism depressant on 14C-acetate uptake in intact rat brain. J Cereb Blood Flow Metab. 2004;24:188–190. doi: 10.1097/01.WCB.0000098606.42140.02. [DOI] [PubMed] [Google Scholar]
- Huck S, Grass F, Hatten ME. Gliotoxic effects of alpha-aminoadipic acid on monolayer cultures of dissociated postnatal mouse cerebellum. Neuroscience. 1984a;12:783–791. doi: 10.1016/0306-4522(84)90170-2. [DOI] [PubMed] [Google Scholar]
- Huck S, Grass F, Hortnagl H. The glutamate analogue alpha-aminoadipic acid is taken up by astrocytes before exerting its gliotoxic effect in vitro. J Neurosci. 1984b;4:2650–2657. doi: 10.1523/JNEUROSCI.04-10-02650.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami Y, Goodenough S, Inoue Y, Dodd PR, Wilce PA, Matsumoto I. Increased TUNEL positive cells in human alcoholic brains. Neurosci Lett. 2003;349:201–205. doi: 10.1016/s0304-3940(03)00826-7. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Berry A, Boop FA, Davies DL. Proliferation of astroglia from the adult human cerebrum is inhibited by ethanol in vitro. Brain Res. 1996;731:39–44. doi: 10.1016/0006-8993(96)00456-8. [DOI] [PubMed] [Google Scholar]
- Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia. 1996;16:351–358. doi: 10.1002/(SICI)1098-1136(199604)16:4<351::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–168. [PubMed] [Google Scholar]
- Larrosa B, Pastor J, Lopez-Aguado L, Herreras O. A role for glutamate and glia in the fast network oscillations preceding spreading depression. Neuroscience. 2006;141:1057–1068. doi: 10.1016/j.neuroscience.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miguel Hidalgo JJ, Overholser J, Meltzer H, Stockmeier C, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship to suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clin Exp Res. 2005;29:766–772. doi: 10.1097/01.alc.0000164378.92680.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, et al. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Luo J. Effects of ethanol and basic fibroblast growth factor on the transforming growth factor beta1 regulated proliferation of cortical astrocytes and C6 astrocytoma cells. Alcohol Clin Exp Res. 2002;26:671–676. [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Peters RA. Mechanism of the toxicity of the active constituent of Dichapetalum cymosum and related compounds. Adv Enzymol Relat Subj Biochem. 1957;18:113–159. doi: 10.1002/9780470122631.ch3. [DOI] [PubMed] [Google Scholar]
- Qutub AA, Hunt CA. Glucose transport to the brain: a systems model. Brain Res Brain Res Rev. 2005;49:595–617. doi: 10.1016/j.brainresrev.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S. Maintenance of neuronal glutathione by glial cells. J Neurochem. 1993;61:1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- Shan S, Tanaka H, Shoyama Y. Western blotting method for the immunostaining detection of glucuronides of glycyrrhetic acid using anti-glycyrrhizin monoclonal antibody. Biol Pharm Bull. 1999;22:221–223. doi: 10.1248/bpb.22.221. [DOI] [PubMed] [Google Scholar]
- Shan S, Tanaka H, Shoyama Y. Enzyme-linked immunosorbent assay for glycyrrhizin using anti-glycyrrhizin monoclonal antibody and an eastern blotting technique for glucuronides of glycyrrhetic acid. Anal Chem. 2001;73:5784–5790. doi: 10.1021/ac0106997. [DOI] [PubMed] [Google Scholar]
- Shelton MK, Mccarthy KD. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem. 2000;74:555–563. doi: 10.1046/j.1471-4159.2000.740555.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Takada M, Hattori T. Fine structural changes in the rat brain after local injections of gliotoxin, alpha-aminoadipic acid. Histol Histopathol. 1986;1:271–275. [PubMed] [Google Scholar]
- Takada M, Li ZK, Hattori T. Astroglial ablation prevents MPTP-induced nigrostriatal neuronal death. Brain Res. 1990;509:55–61. doi: 10.1016/0006-8993(90)90308-x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shoyama Y. Formation of a monoclonal antibody against glycyrrhizin and development of an ELISA. Biol Pharm Bull. 1998;21:1391–1393. doi: 10.1248/bpb.21.1391. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, et al. Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry. 1993;150:417–422. doi: 10.1176/ajp.150.3.417. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Overall JE, Hitzemann R, Fowler JS, Pappas N, et al. Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin Exp Res. 1997;21:1278–1284. [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, et al. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:331–341. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- Wu H, Friedman WJ, Dreyfus CF. Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. J Neurosci Res. 2004;76:76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]