Abstract
Ca2+-activated Cl− channels (CaCCs) perform many important functions in cell physiology including secretion of fluids from acinar cells of secretory glands, amplification of olfactory transduction, regulation of cardiac and neuronal excitability, mediation of the fast block to polyspermy in amphibian oocytes, and regulation of vascular tone. Although a number of proteins have been proposed to be responsible for CaCC currents, the anoctamin family (ANO, also known as TMEM16) exhibits characteristics most similar to those expected for the classical CaCC. Interestingly, this family of proteins has previously attracted the interest of both developmental and cancer biologists. Some members of this family are up-regulated in a number of tumours and functional deficiency in others is linked to developmental defects.
Ca2+-activated Cl− currents (CaCCs) were first described in the 1980s in Xenopus oocytes (Miledi, 1982; Barish, 1983) and salamander photoreceptor inner segments (Bader et al. 1982). In Xenopus oocytes these channels play a role in the fast block to polyspermy. They are activated by increases in [Ca2+]i that occur upon fertilization, depolarize the membrane, and prevent additional sperm entry. In vertebrate photoreceptors, CaCCs are thought to play an important role in transmitter release (MacLeish & Nurse, 2007). It is now known that similar channels are expressed in many cell types. CaCCs play key roles in epithelial secretion (Kunzelmann et al. 2007), membrane excitability in cardiac muscle and neurons (Andre et al. 2003; Guo et al. 2008), olfactory transduction (Matthews & Reisert, 2003), regulation of vascular tone (Angermann et al. 2006), and photoreception (Lalonde et al. 2008). Although ICl.Ca has been studied for almost 30 years, its molecular identity has been controversial.
Knowing the molecular identity of CaCCs is an important goal in understanding how these channels work in normal physiology as well as in disease. The search for the molecular counterparts for CaCCs has been arduous for several reasons. First, expression cloning has drawbacks, namely, that expression of a variety of membrane proteins often seems to result in up-regulation of endogenous Cl− channels, resulting in false positives. Moreover, a favourite system for expression cloning of ion channels, the Xenopus oocyte, is not suitable for expression cloning this channel, precisely because this cell expresses large endogenous CaCCs. Second, the problem is compounded by the fact that, until very recently, drugs to differentiate CaCCs from other Cl− channels lack specificity (De La Fuente et al. 2008). The paucity of specific drugs for CaCCs has also hampered cloning approaches that require purification of the CaCC protein. Finally, homology cloning has not been fruitful because none of the known cloned Cl− channels including CFTR, GABAA and glycine receptors, and the ClCs have properties that suggest clear structural relationships to CaCCs.
There may be several different kinds of Ca2+-activated Cl− channels. In this review, the term CaCC refers specifically to the kind of Ca2+-activated Cl− current seen in Xenopus oocytes and acinar cells of secretory glands like pancreas and salivary gland. Several molecular candidates have been proposed as CaCCs, but none of them fit the bill as well as the very recently discovered anoctamin (also known as TMEM16) family. Below, we briefly summarize the genes that have been suggested to encode CaCCs, but the review is focused on the newly described anoctamin family.
Proposed candidate proteins for CaCCs
CLCA
The Ca2+-activated Cl− channel (CLCA) family was cloned from a bovine tracheal cDNA expression library and the library was screened with an antibody generated against a purified protein that behaved as a CaCC when incorporated into artificial lipid bilayers (Cunningham et al. 1995). Transfection of various cell types with cDNAs encoding various CLCAs induces Ca2+-dependent currents. However, for a number of reasons CLCAs are no longer seriously considered as contenders for the title of the CaCC (Jentsch et al. 2002; Eggermont, 2004). CLCAs have very high homology to known cell adhesion proteins and some are soluble, secreted proteins (Loewen & Forsyth, 2005). Furthermore, despite the fact that it has been nearly 20 years since the first CLCA was cloned, structure–function analysis has not provided any clear evidence that a CLCA is actually a channel. In addition, there are differences in Ca2+ sensitivity, voltage sensitivity, and pharmacology between CLCA currents and native CaCCs. Another argument against CLCAs being CaCCs is that a number of cell types that express native CaCCs do not express CLCAs (Papassotiriou et al. 2001). Some investigators have suggested that CLCAs modulate endogenous Cl− channels (Loewen & Forsyth, 2005).
Tweety
Recently, two human genes (hTTHY2 and hTTYH3) with homology to a gene in the flightless locus of Drosophila called tweety have been suggested as the molecular basis for a Ca2+-regulated maxi-Cl− channel (260 pS) (Suzuki & Mizuno, 2004; Suzuki, 2006). This channel might correspond to the maxi-Cl− channel found in spinal neurons (Hussy, 1992) and skeletal muscle (Fahmi et al. 1995). However, it is unlikely that this protein is responsible for the classical CaCC currents such as those in salivary glands and Xenopus oocytes. hTTYH3 is not expressed in acinar cells of secretory glands. Furthermore, it is generally thought that CaCC single channels are small (see below), whereas this channel is clearly much larger. A related gene, hTTYH1, encodes a channel that is not regulated by Ca2+.
Bestrophins
It has recently been proposed that bestrophins are Cl− channels (Sun et al. 2002). Mutations in human bestrophin-1 (hBest-1) are associated with several kinds of retinopathy (Hartzell et al. 2008). One type of retinopathy, Best vitelliform macular dystrophy, is characterized by an abnormal electro-oculogram that is consistent with a loss of a Ca2+-activated Cl− channel in the basolateral membrane of retinal pigment epithelial cells. Bestrophins function as Cl− channels when expressed heterologously. Many disease-causing mutations of hBest1 produce defective Cl− currents. The bestrophins that have been most thoroughly studied are stimulated by Ca2+ with a Kd of ∼200 nm (Sun et al. 2002; Tsunenari et al. 2003; Qu et al. 2003b). It has now been shown in several cell types that RNAi can knock down endogenous Cl− currents that are stimulated by Ca2+ (Barro Soria et al. 2006; Chien et al. 2006; Chien & Hartzell, 2007; Matchkov et al. 2008), but are bestrophins the molecular counterpart for classical CaCCs? Although this seemed a hopeful prospect several years ago, reservations have been voiced often (Pusch, 2004; Hartzell et al. 2005, 2008; Marmorstein et al. 2006). Although both expressed bestrophin channels and classical CaCCs are gated directly by Ca2+ without the involvement of kinases and both exhibit the generic lyotropic anion selectivity sequence, there are important differences. Heterologously expressed hBest1 and mBest2 have an apparent affinity for Ca2+ that is 10 times higher than that of CaCCs. Furthermore, classical CaCCs exhibit voltage-dependent kinetics and outward rectification that is not seen with hBest1 or mBest2 (Qu et al. 2003b; Qu et al. 2004). This difference could be explained if native CaCCs have another subunit not present in the expressed homomeric channels. But, perhaps more important, it seems that the bestrophins are regulated in ways that classical CaCCs are not. For example, hBest1, mBest2, and dBest1 can also be activated by osmotic cell swelling in the absence of Ca2+, a property that is not shared by classical CaCCs (Fischmeister & Hartzell, 2005; Chien & Hartzell, 2007). Also, Best3, expressed in vascular smooth muscle, is regulated by Ca2+ and by cGMP (Matchkov et al. 2008). Thus, bestrophins may be a special type of Ca2+-activated Cl− channel and not the classical CaCC. Heterologously expressed bestrophins have never been shown to be activated by receptors that elevate cytosolic Ca2+.
Anoctamins
Very recently, it has been proposed that the anoctamin/TMEM16 family of membrane proteins are CaCCs (Caputo et al. 2008; Yang et al. 2008; Schroeder et al. 2008). The term ‘anoctamin’ was coined because these channels are ANion selective and have eight (OCT) transmembrane segments (Yang et al. 2008). For the first time, these channels look and sound like the right thing. If they are, this is very exciting because this family of proteins has previously attracted significant interest from other fields, particularly cancer and developmental biology. The first member of the family, called GDD1 and now known as ANO5, was found as the gene responsible for gnathodiaphyseal dysplasia (Tsutsumi et al. 2004). The family was then assembled from bioinformatic analyses (Katoh & Katoh, 2003; Katoh & Katoh, 2005). ANO1 has a number of different names, the most common being TMEM16A, but others include TAOS2 (tumour amplified and overexpressed sequence) (Huang et al. 2002), ORAOV2 (oral cancer overexpressed) (Mammalian Gene Collection Program Team, 2002) and DOG-1 (discovered on GIST-1) (West et al. 2004). As these names suggest, ANO1 is highly over-expressed in some cancers. ANO7 (also called D-TMPP and NGEP) is specifically expressed in prostate and is up-regulated in a number of prostate tumours (Kiessling et al. 2005). ANO family members have a conserved C-terminal domain of unknown function (DUF590; Interpro IPR007632).
Anoctamins (TMEM16s) as CaCCs
Discovery of ANO1 as a CaCC
Yang et al. (2008) selected ANO1 from a bioinformatic search of channel- or transporter-like genes with multiple transmembrane domains and multiple isoforms. They found that transfection of HEK-293 cells with endothelin receptor (ETA) and ANO1 resulted in endothelin-induced Cl− currents. ANO1 is the first CaCC candidate that has been shown to be activated by receptors that elevate intracellular Ca2+ (Yang et al. 2008). The authors show that the current has the appropriate reversal potential for a Cl− current and that replacement of extracellular Cl− with gluconate abolishes the current. Furthermore, the current is blocked by low concentrations of classical Cl− channel blockers. However, the pharmacology of over-expressed ANO1 is not quantitatively the same as reported for native CaCCs. For example, ANO1 currents are blocked nearly completely by 10 μm 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), tamoxifen and niflumic acid (NFA). In native tissues the IC50 values are 16–250 μm for DIDS, 22–68 μm for NPPB and 2–44 μm for NFA, and in many tissues tamoxifen does not block (Hartzell et al. 2005). This may suggest that the native currents are composed of other ANO family members or have accessory subunits that alter the pharmacology. The ANO1 current is activated by cytosolic Ca2+ with an EC50 of 2.6 μm at −60 mV, which is very close to that reported for classical CaCCs. Yang et al. (2008) showed that RNAi injection into mouse salivary gland reduced pilocarpine-induced salivary secretion by ∼35%. Whether this rather modest effect of RNAi is attributable to incomplete knockdown of ANO1 or whether there are additional Cl− conductances involved is not known. In addition, Yang et al. also show that the acetylcholine-induced Cl− currents in primary cultures from submandibular gland of mice injected with ANO1 RNAi were greatly reduced. These findings are precisely what one would expect if ANO1 was the CACC in submandibular gland responsible for salivary secretion.
Caputo et al. (2008) became interested in CaCCs when they noted that long-term stimulation of airway epithelial cells with IL-4 caused a marked increase in CaCC current. Microarray analysis identified several membrane proteins that were up-regulated. Among these, ANO1 mRNA was increased 7-fold. They then used an interfering RNA strategy in monolayers of two cell lines (CFPAC-1 and CFBe41o–) that express large CaCC currents. Treatment with siRNA to ANO1 caused a reduction in purinergic receptor stimulated I− flux and transepithelial short circuit currents. Currents stimulated by ionomycin were also greatly reduced, as were CaCC currents in patch clamped isolated cells. Expression of ANO1 in HEK293, COS7, or FRT cells induced Cl− currents that were stimulated by intracellular Ca2+. I− flux in FRT cells was blocked by NFA and NPPB, which typically block CaCCs, but was not blocked by inhibitors of CFTR such as CFTRinh-172 and diphenylamine-2-carboxylate (DPC) at concentrations up to 100 μm. GlyH-101, a drug often considered as CFTR selective, blocks ANO1 with high potency (> 50% block at 20 μm), suggesting that this drug should not be considered selective for CFTR.
Schroeder et al. (2008) identified the ANO/TMEM16 family by expression cloning using a novel expression system, the axolotl oocyte. Photolysis of caged IP3 to release intracellular Ca2+ did not activate CaCCs in native axolotl oocytes. This permitted the use of these cells to screen a cDNA library prepared from size-fractionated RNA isolated from Xenopus oocytes, which express very robust endogenous CaCC currents. After subdivision of pools, a cDNA clone was found that induced currents activated by caged IP3 photolysis. The cDNA clone was an orthologue of mouse and human ANO1. The xANO1 current was outwardly rectifying at low intracellular Ca2+ concentrations, but exhibited a linear current–voltage relationship at higher intracellular Ca2+, just as has been seen for the native Xenopus oocyte CaCC (Kuruma & Hartzell, 2000). The current could be activated by CCh when xANO1 was coexpressed with the m1 muscarinic ACh receptor. Furthermore, the current exhibited a pharmacological profile very similar to the native Xenopus oocyte CaCC (NFA > DIDS > NPPB > DPC). In contrast to the results of Yang et al. (2008) on mANO1, the xANO1 current was insensitive to tamoxifen, as has been found for native CaCCs. Schroeder et al. (2008) also expressed mouse ANO1 in HEK cells and found an outwardly rectifying Cl− current with 500 nm intracellular free Ca2+. The currents generated by expression of xANO1, mANO1 and mANO2 in axolotl oocytes have different kinetics, suggesting that different ANOs are likely to have different gating characteristics. Also, Schroeder et al. showed that mANO1 is expressed in mammary and salivary glands by in situ hybridization, as expected for CaCCs.
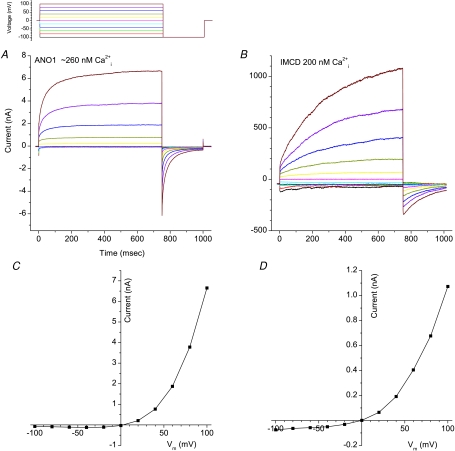
Figure 1 compares Ca2+-activated Cl− currents in HEK cells transfected with ANO1 and native IMCD (inner medullary collecting duct) cells. Intracellular free Ca2+ was controlled by EGTA buffer to approximately 200 nm in both cases. Both currents activate slowly with time, are strongly outwardly rectifying, and exhibit de-activating tail currents upon repolarization. However the time course of activation of the currents in IMCD cells is significantly slower than the ANO1-transfected cells. This suggests that another ANO subfamily or other subunits may contribute to the current in IMCD cells. Nevertheless, the current induced by ANO1 is much more similar in appearance to native CaCCs than any other CaCC candidate previously examined.
Figure 1. Ca2+-activated Cl− currents.
Comparison of Ca2+-activated Cl− currents induced by ANO1 transfected in HEK293 cells and in native IMCD cells. Recordings were performed as previously described (Qu et al. 2003a). The voltage clamp protocol is shown above A. A and B, currents from HEK-cells transfected with ANO1 cDNA (provided by Uhtaek Oh, Seoul National University) (A) and native IMCD cells (B). C and D, I–V curves of currents measured at the end of the test pulse in A and B, respectively.
Phylogeny of the ANO family
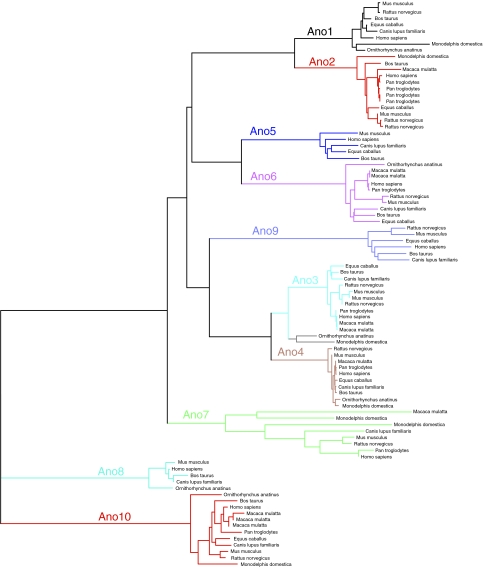
Members of the ANO family are found throughout the eukaryotes, including mammals, flies, worms, plants, protozoa and yeast. However, the ANOs seem best represented in the higher vertebrates. Mammals have 10 gene members (Fig. 2), whereas invertebrates and plants have distinctly fewer. C. elegans, for example, has only two ANO genes and Drosophila has six paralogues. Mammalian subfamilies 1 and 2 are closely related, as are 3 and 4, and 8 and 10. The ANO family is now the second largest of the five known Cl− channel families. In mammals, ligand gated anion channels (e.g. GABA/glycine receptors) include hundreds of members (the CLCs have nine members, bestrophins have four, and CFTR is apparently a loner; Jentsch et al. 2002; Hartzell et al. 2008).
Figure 2. Phylogeny of the ANO family.
Human ANO1 was subjected at NCBI to position-specific iterated BLAST (2.2.18+) (http://www.ncbi.nlm.nih.gov/blast) (Altschul et al. 1990) with a threshold of 0.005 against all mammalian sequences in the reference proteins database (ref_seq_protein) through 6 iterations to find all of the mammalian members of the family. The tree was constructed using the fast minimal evolution method (Desper & Gascuel, 2004) using distances computed according to Grishin (1995).
This molecular diversity of the ANO family is reflected in the phenotypic diversity of CaCC currents. Although whole-cell CaCC currents are superficially similar in different cell types including Xenopus oocytes, various secretory epithelial cells, hepatocytes, vascular, airway and gut smooth muscle cells, Jurkat T-cells and pulmonary artery endothelial cells, there are significant differences (Hartzell et al. 2005). Some CaCCs are activated directly by Ca2+ binding and others by Ca2+-dependent phosphorylation (Arreola et al. 1998). Also, the voltage dependence, Ca2+ sensitivity, and pharmacology of these channels are different in different cells. At this point in time, it has not been shown experimentally that every ANO family member is a Cl− channel, and it remains to be seen whether they are all activated by Ca2+ or if there are other regulators. Also, it will be interesting to learn whether different members of the family form heteromers with other members.
Single channel properties
Although whole-cell CaCC currents are similar in different cell types, there is considerable diversity in the properties of the single channels. There appear to be at least four different types of CaCCs in different cell types and the channels may have multiple conductance states (Piper & Large, 2003). Low conductance CaCCs (1–3 pS) have been described in cardiac myocytes (Collier et al. 1996), arterial smooth muscle (Klockner, 1993; Hirakawa et al. 1999; Piper & Large, 2003), A6 kidney cells (Marunaka & Eaton, 1990), endocrine cells from pituitary (Taleb et al. 1988), Xenopus oocytes (Takahashi et al. 1987), and the Drosophila S2 cell line (Chien et al. 2006). Within this class of channels, there is considerable diversity in properties. Depending on the study (or on the conditions), the channels can exhibit either linear or outwardly rectifying I–V curves, Kd values for Ca2+ over a ∼500-fold range, variable voltage sensitivity, and different susceptibility to rundown after excision of the patch. Rundown after patch excision has been interpreted to suggest that the channels are regulated by a factor which is lost upon excision.
The second class of CaCC channels are 8 pS with linear I–V relationships, found in endothelial cells (Nilius et al. 1997b) and hepatocytes (Koumi et al. 1994). The ANO1 channels expressed in HEK cells have a conductance of 8 pS (Yang et al. 2008). CaCCs with a 15 pS single channel conductance have been described in colon (Morris & Frizzell, 1993) and a biliary cell line (Schlenker & Fitz, 1996) and have a linear I–V relationships but are blocked by CaM antagonists. The highest conductance channels (40–50 pS), described in Jurkat T-cells (Nishimoto et al. 1991), Xenopus spinal neurons (Hussy, 1992) and airway epithelial cells (Frizzell et al. 1986), are outwardly rectifying. At least some of these channels are activated by CaMKII. Several other single channel conductances have also been described (Hartzell et al. 2005). These different single channel conductances suggest a molecular diversity. Whether this will be explained by different ANO members or different heteromeric combinations of ANOs remains to be seen.
Structure
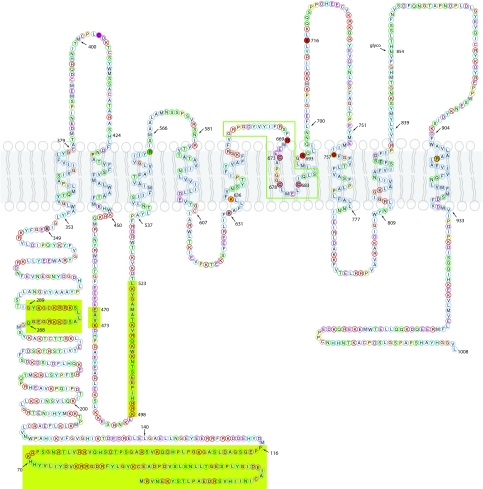
ANO proteins exhibit multiple alternatively spliced forms. For example, ANO1 has at least four alternatively spliced exons (named a, b, c and d) (Caputo et al. 2008) resulting in proteins having between 712 and 1006 amino acids. The alternatively spliced exons include two at the cytoplasmic N-terminus (a and b) and two in the first cytoplasmic loop between transmembrane segments 2 and 3 (c and d). Figure 3 shows the sequence and topology of ANO1 containing all of the alternatively spliced exons, referred to as ANO1(abcd) by Caputo et al. (2008) They found that splice variants a, ac, abc and abcd produced CaCC currents when expressed in HEK cells, but the variant without any of these segments (ANO1(0)) was not functional. In the text below, amino acid positions refer to positions in the ANO1 abcd reference sequence shown in Fig. 3.
Figure 3. Topology of ANO1.The human ANO1 protein containing all of its potential alternatively spliced exons (the ‘abcd’ form, 1008 amino acids, Caputo et al. 2008).
The ANO1 protein sequence was aligned with the ANO7 sequence and then ANO1 was mapped onto the topology of the ANO7 sequence determined by Das et al. (2008). Alternatively spliced segments a, b, c and d are shown with a chartreuse background. Single amino acid codes are in circles coloured according to the physical properties of the amino acid (green, hydrophilic; pale blue, hydrophobic; red, acidic; magenta, basic; cyan, other ionizable (tyrosine and histidine); yellow, proline; gold, glycine; pink, cysteine). Amino acids shown as filled circles have been mutated (Caputo et al. 2008; Yang et al. 2008): filled red amino acids have altered ionic permeability or gating, grey are wild-type, pink have altered sensitivity to MTSET. Glyco is a conserved N-linked glycosylation site. Purple: C404 in this sequence corresponds to C356 in ANO5 that is mutated to cause GDD. The green box shows the region including the re-entrant loop that is not conserved in ANO8 and ANO10.
One of the transcripts of ANO7 has been shown to encode a 179-amino acid cytosolic protein (Bera et al. 2004). A search of GenBank reveals that many of the ANOs have short transcripts, suggesting the possibility that ANOs could have additional non-channel functions.
Hydropathy analysis predicts that ANO1 has eight to nine transmembrane domains. The topology of hANO7 has been analysed extensively by incorporating epitope tags into the protein in all predicted extracellular and intracellular regions (Das et al. 2008). The accessibility of the epitope tags to antibodies was measured in permeabilized and intact cells to determine their orientations. These studies show that ANO7 consists of eight transmembrane regions with both the NH2 and COOH termini intracellular. This predicted topology was then confirmed with monoclonal antibodies and N-linked glycosylation. The N-linked glycosylation site in the last extracellular loop of ANO7 (854 in the reference sequence of Fig. 3; 809 in ANO7) is conserved among all members of the human ANO family except ANO10. There is another site at 824 in ANO7 that is glycosylated in ANO7 that is not present in any of the others. In addition, there is a hydrophobic region between TM5 and TM6 that protrudes into the membrane and is predicted to form a re-entrant loop. Interestingly, re-entrant loops are a common feature of ion channel pores. Because the predicted transmembrane domains of the ANO family members are highly conserved, it seems reasonable to expect that all of them share this basic topology.
Ca2+ and voltage dependence
Yang et al. (2008) observed that the current activated in HEK cells coexpressing the ETA receptor and ANO1 was initially strongly outwardly rectifying but with time gradually adopted a linear current–voltage relationship. They also observed that the Ca2+ sensitivity of the channel in excised inside-out patches was voltage dependent. At −60 mV the EC50 for Ca2+ was 2.6 μm, whereas it decreased to 0.4 μm at +60 mV. Schroeder et al. (2008) reported very similar findings. This is very similar to what is seen with native CaCC currents in Xenopus oocytes (Kuruma & Hartzell, 2000) and parotid gland (Arreola et al. 1996). For example, the CaCC current is outwardly rectifying and exhibits time-dependent activation and deactivation at Ca2+ concentrations below ∼1 μm, but a linear I–V and time-independent kinetics at higher Ca2+ concentrations. The data were interpreted in terms of a voltage-dependent Ca2+ affinity due to a kinetic gating scheme in which channel activation was Ca2+ dependent and channel closing was voltage sensitive (Arreola et al. 1996; Kuruma & Hartzell, 2000). The ANO1 currents, like native Xenopus CaCCs, are inhibited by high Ca2+.
In Xenopus oocytes, it has been suggested that different components of the CaCC current exhibit different ionic selectivities (Kuruma & Hartzell, 1999). These observations have led to discussion whether the Xenopus oocyte CaCC is mediated by one or multiple channel types (see detailed discussion in Kuruma & Hartzell, 1999). Schroeder et al. (2008) show that expression of xANO1 in Xenopus oocytes produces currents with multiple components with different reversal potentials. These findings suggest that xANO1 has multiple open states that differ in their gating kinetics and ionic selectivity.
Expression
ANO1 is expressed in many of the tissues that are known to express CaCCs: bronchiolar epithelial cells, pancreatic acinar cells, proximal kidney tubule epithelium, retina, dorsal root ganglion sensory neurons, and submandibular gland (Caputo et al. 2008; Yang et al. 2008; Schroeder et al. 2008). In submandibular gland, ANO1 and aquaporin-5 are on the apical membrane and the sodium–potassium–chloride cotransporter NKCC is on the basolateral membrane (Yang et al. 2008).
Pore
Mutational analysis of ANO1 suggests that the re-entrant membrane loop between TMD5 and TMD6 is important in ion selectivity. Yang et al. (2008) found that replacing the positively charged Arg at position 669 of the ‘abcd’ variant (position 621 of the ‘a’ variant that Yang et al. used) with a negatively charged Glu increased the cation permeability of the channel dramatically (the PNa/PCl ratio increased from 0.03 to 0.83). The K716E mutation also changed the ionic selectivity, although the effect was smaller than with the K669E mutation. The K693E mutation produced channels that were not functional. This suggests that positively charged amino acids in the re-entrant loop form part of the ion conduction pathway. To test this further, Yang et al. (2008) found that replacing C673, C678, and C683 in the re-entrant loop with alanines abolished inhibition of the current by extracellular MTSET, a membrane-impermeant sulfhydryl-reactive reagent. This result is consistent with the interpretation that these cysteines are accessible to the extracellular space and participate in ion conduction. Mutation of a few amino acids in transmembrane domains 1 and 7 had no effect (K349A, K631A and T830A), whereas neutralization of basic amino acids in transmembrane domains 5 and 6, flanking the re-entrant loop affected channel gating and voltage dependence (Caputo et al. 2008).
It is interesting to note that ANO8 and 10, which are most distant from ANO1, are poorly conserved in the region of the re-entrant loop. Clustal-W alignments show that a total of ∼25 amino acids (including R669) are missing from this region of ANO10. Likewise, in ANO8 the re-entrant loop appears to be missing but, in addition, there are two ∼60 amino acid insertions. One of the insertions contains a stretch of 20 consecutive acidic amino acids!
Ca2+ binding
ANO1 does not have any obvious E-F hand-like Ca2+-binding sites or IQ-domain CaM binding sites. This could mean that either the protein requires another subunit to confer Ca2+ sensitivity or that the Ca2+ binding site is a novel type that is not easily recognized. If another subunit is required for Ca2+ sensitivity, this subunit must be expressed endogenously in the expression systems (HEK293 cells) used. The N- and C-termini and the first cytoplasmic loop of ANO1 have several regions rich in acidic amino acids that could possibly be involved in coordinating Ca2+. However, at present there are no data about the Ca2+ binding site.
Yeast ANO
Yeast has one ANO homologue, called Ist2p. This protein was identified during sequencing of S. cerevisiae chromosome II as having homology to Na+ and Ca2+ channels (Mannhaupt et al. 1994). Although it was suggested that Ist2p plays a role in Na+ tolerance (Entian et al. 1999), recent data show that Ist2p deletion mutants grow normally on high NaCl (Kim et al. 2005). Nevertheless, Ist2p does play a role in salt balance and transport through interactions with other proteins, notably Btn2p (Kim et al. 2005). Although deletion of Ist2 or Btn2 separately has no effect on salt tolerance, the double mutant is unable to grow in high salt (Kim et al. 2005). Btn2p is a coiled-coil protein that seems to be a scaffold protein that interacts with several proteins besides Ist2p, including Rhb1p, a negative regulator of a plasma membrane arginine and lysine permease, and Ypt1p, a Golgi protein involved in membrane trafficking (Chattopadhyay et al. 2003; Kim et al. 2005). Deletion of Btn2 results in mislocalization of these three proteins. Intriguingly, another Cl− channel, Btn1p, which is a yeast homologue of ClC-3, also interacts with Ist2p. The double Ist2–Btn1 deletion mutant is unable to grow in high NaCl, like the Ist2–Btn2 knockout (Kim et al. 2005). Furthermore, there is a complex and incompletely understood relationship between Ist2p, Btn2p and Btn1p in regulating arginine uptake (Chattopadhyay & Pearce, 2002; Kim et al. 2005). Deletion of Btn1 decreases arginine uptake into the vacuole and deletion of Ist2 suppresses this effect. The mechanisms involved in these intriguing interactions remain to be determined.
Interestingly, Ist2p is localized preferentially to the bud in budding yeast (Takizawa et al. 2000). It turns out that Ist2p is trafficked to the plasma membrane by a novel pathway that bypasses the traditional Golgi secretory pathway (Juschke et al. 2004, 2005). Ist2p is encoded by an mRNA that is localized to the cortex of daughter cells and is translated locally at the cortical ER and is trafficked and inserted into the plasma membrane by a novel mechanism, possibly involving the fusion of cortical ER with the plasma membrane. Whether mammalian ANO proteins are trafficked by similarly novel mechanisms remains to be determined, but if this does occur, it might have implications in processes such as local protein synthesis in axons and dendrites (for example Lin & Holt, 2008).
Implications in cancer and development
Link to cancer
The ANOs attracted the interest of cancer biologists for several years before their identification as ion channels because they are highly up-regulated in cancers (Galindo & Vacquier, 2005). Because they are accessible cell surface proteins and are up-regulated in cancer, they are viewed as potential targets for therapeutic antibodies and as biomarkers for tumours (Espinosa et al. 2008; Das et al. 2008). The idea that ion channels may have functions in cancer is not new (Sontheimer, 2008; Shimizu et al. 2008; Pardo & Stuhmer, 2008), but the addition of this well-studied channel family to the cancer proteome foreshadows new mechanistic insights.
Gastrointestinal stromal tumour (GIST) is the most common kind of mesenchymal tumour found in the gastrointestinal tract. Most GISTs contain mutations in either KIT or PDGFRA receptor tyrosine kinases that result in their constitutive, ligand-independent activation. An inhibitor of KIT and PDGFRA is the primary therapy for metastatic or unresectable GIST. ANO1 is highly up-regulated in these tumours (Espinosa et al. 2008). Although ANO1 may not be the cause of the tumour, its function may support tumour progression. ANO1 is located on chromosome 11q13 and it appears that amplification of this chromosomal region occurs in many tumours including almost half of oral squamous cell carcinomas (OSCC), where it has been correlated with a poor outcome (Huang et al. 2006), and human neck squamous cell carcinomas (Carles et al. 2006). It is suggested that 11q13 amplification may be driven by a cassette of genes that provide growth or metastatic advantage to cancer cells.
ANO7 (also called NGEP) is expressed on the apical and lateral membranes of normal prostate and prostate cancer cells and at cell–cell junctions of the LNCaP prostate cancer cell line (Das et al. 2007). This localization has suggested it may play a role in cell adhesion, especially since LNCaP cells expressing ANO7 form aggregates. This aggregation property was lost when ANO7 expression was decreased by RNAi. Because of its prostate-specific expression, ANO7 is considered a potential immunotherapeutic target.
Although ANOs are up-regulated in tumours, it does not seem that mutations in ANO1 are linked to carcinogenesis (Miwa et al. 2008). Rather, ANOs may participate in cell proliferation or tumour progression. The activities of several anion channels correlate with the cell cycle (Villaz et al. 1995; Machaca & Haun, 2002; Klausen et al. 2007). And, intriguingly, a dominant mutant of a Drosophila ANO, called Axs (aberrant x-segregation), is linked to chromosomal nondisjunction and progression of the meiotic cycle (Kramer & Hawley, 2003). Axs is ∼35% identical to ANO8 and 10. Like them, Axs lacks the re-entrant loop. Whether this means that Axs is not a Cl− channel or that this part of the protein is not essential for Cl− channel function remains to be seen. Axs is localized to the ER in early embryos and to membranes associated with the meiotic spindle. A dominant mutation, Axs[D], increases achiasmate-specific chromosome non-disjunction and exhibits defects in spindle formation and cell cycle progression. Interestingly, Axs knock-outs do not mimic the phenotype of the Axs[D] mutant (Flynn et al. 2007). One possible explanation is that the Axs[D] mutation may produce a protein that has acquired a new function that disrupts meiosis. Alternatively, other ANO family members may be redundant and replace Axs function when it is knocked out. However, it appears that at least three of the other ANO members in flies do not function in meiosis.
Roles in development
Although the exact functions of ANOs in cancer are speculative, mutations in ANO1 and 5 clearly produce developmental abnormalities.
ANO5 (also known as GDD1)
Dominant mutations in a conserved cysteine (C356) in the first extracellular loop of ANO5 produce a bone fragility syndrome called gnathodiaphyseal dysplasia (GDD, MIM 166260) caused by chondrocyte and osteoblast dysfunction. C356 (C404 in the reference sequence in Fig. 1) is conserved in ANO1–7, but absent in ANO8-10.
Interestingly, ANO5 is highly expressed in cardiac and skeletal muscle as well as bone and is greatly up-regulated during myocyte differentiation (Tsutsumi et al. 2005; Mizuta et al. 2007). It seems that ANO5 is expressed on intracellular membranes in muscle. There is extensive alternative splicing generating 14 different isoforms (Mizuta et al. 2007). Interestingly, ANO5 is greatly up-regulated in muscles of dystrophin-deficient MDX mice (Mizuta et al. 2007).
ANO1
ANO1 dysfunction also causes a type of endoskeletal defect, but by a different mechanism. ANO1 knockout mice die within 1–2 months after birth apparently because of tracheal cartilage malformation (Rock & Harfe, 2008; Rock et al. 2008). Surprisingly, the chondrogenic mesenchymal cells do not express ANO1 at any time, but the tracheal epithelium does express it. This suggests that the cartilage ring defect observed in ANO1 mutants may be secondary to expansion of the embryonic tracheal epithelium.
Summary
In the long search for the molecule responsible for classical CaCCs, it seems that ANO1 is the best candidate. The existence of 10 mammalian genes with multiple splice variants, some of them apparently encoding soluble proteins, raises intriguing questions about the function of all these different proteins. Are all anoctamins, including ANO8 and ANO10, Cl− channels? What are the functions of the ANO5 isoforms that apparently have altered transmembrane topology and the ANO7 cytoplasmic variant? How does Ca2+ activate the channel? Are there other mechanisms of activation? For example, is it possible that some anoctamin is the long sought-after volume-regulated anion channel (Nilius et al. 1997a)? Links between anoctamin, cancer and development are intriguing and are sure to open exciting new avenues of research.
Acknowledgments
The authors thank Dr Uhtaek Oh for the generous gift of the Ano1 cDNA. The research in the authors' lab is supported by NIH grants EY014852 and GM60448. Q.X. is supported by an American Heart Association postdoctoral fellowship.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andre S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J Neurophysiol. 2003;90:3764–3773. doi: 10.1152/jn.00449.2003. [DOI] [PubMed] [Google Scholar]
- Angermann JE, Sanguinetti AR, Kenyon JL, Leblanc N, Greenwood IA. Mechanism of the inhibition of Ca2+-activated Cl− currents by phosphorylation in pulmonary arterial smooth muscle cells. J Gen Physiol. 2006;128:73–87. doi: 10.1085/jgp.200609507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Differences in regulation of Ca2+-activated Cl− channels in colonic and parotid secretory cells. Am J Physiol Cell Physiol. 1998;274:C161–C166. doi: 10.1152/ajpcell.1998.274.1.C161. [DOI] [PubMed] [Google Scholar]
- Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barro Soria R, Spitzner M, Schreiber R, Kunzelmann K. Bestrophin 1 enables Ca2+ activated Cl− conductance in epithelia. J Biol Chem. 2006;281:17460–17467. doi: 10.1074/jbc.M605716200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera TK, Das S, Maeda H, Beers R, Wolfgang CD, Kumar V, Hahn Y, Lee B, Pastan I. NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc Natl Acad Sci U S A. 2004;101:3059–3064. doi: 10.1073/pnas.0308746101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJV. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Carles A, Millon R, Cromer A, Ganguli G, Lemaire F, Young J, Wasylyk C, Muller D, Schultz I, Rabouel Y, Dembele D, Zhao C, Marchal P, Ducray C, Bracco L, Abecassis J, Poch O, Wasylyk B. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene. 2006;25:1821–1831. doi: 10.1038/sj.onc.1209203. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Pearce DA. Interaction with Btn2p is required for localization of Rsglp: Btn2p-mediated changes in arginine uptake in Saccharomyces cerevisiae. Eukaryot Cell. 2002;1:606–612. doi: 10.1128/EC.1.4.606-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Roberts PM, Pearce DA. The yeast model for Batten disease: a role for Btn2p in the trafficking of the Golgi-associated vesicular targeting protein, Yif1p. Biochem Biophys Res Commun. 2003;302:534–538. doi: 10.1016/s0006-291x(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Chien LT, Hartzell HC. Drosophila bestrophin-1 chloride current is dually regulated by calcium and cell volume. J General Physiol. 2007;130:513–534. doi: 10.1085/jgp.200709795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LT, Zhang ZR, Hartzell HC. Single Cl− channels activated by Ca2+ in Drosophila S2 cells are mediated by bestrophins. J Gen Physiol. 2006;128:247–259. doi: 10.1085/jgp.200609581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier ML, Levesque PC, Kenyon JL, Hume JR. Unitary Cl− channels activated by cytoplasmic Ca2+ in canine ventricular myocytes. Circ Res. 1996;78:936–944. doi: 10.1161/01.res.78.5.936. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Awayda MS, Bubien JK, Ismailov II, Arrate MP, Berdiev BK, Benos DJ, Fuller CM. Cloning of an epithelial chloride channel from bovine trachea. J Biol Chem. 1995;270:31016–31026. doi: 10.1074/jbc.270.52.31016. [DOI] [PubMed] [Google Scholar]
- Das S, Hahn Y, Nagata S, Willingham MC, Bera TK, Lee B, Pastan I. NGEP, a prostate-specific plasma membrane protein that promotes the association of LNCaP cells. Cancer Res. 2007;67:1594–1601. doi: 10.1158/0008-5472.CAN-06-2673. [DOI] [PubMed] [Google Scholar]
- Das S, Hahn Y, Walker DA, Nagata S, Willingham MC, Peehl DM, Bera TK, Lee B, Pastan I. Topology of NGEP, a prostate-specific cell: cell junction protein widely expressed in many cancers of different grade level. Cancer Res. 2008;68:6306–6312. doi: 10.1158/0008-5472.CAN-08-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- Desper R, Gascuel O. Theoretical foundation of the balanced minimum evolution method of phylogenetic inference and its relationship to weighted least-squares tree fitting. Mol Biol Evol. 2004;21:587–598. doi: 10.1093/molbev/msh049. [DOI] [PubMed] [Google Scholar]
- Eggermont J. Calcium-activated chloride channels (Un)known, (Un)loved? Proc Am Thorac Soc. 2004;1:22–27. doi: 10.1513/pats.2306010. [DOI] [PubMed] [Google Scholar]
- Entian KD, Schuster T, Hegemann JH, Becher D, Feldmann H, Guldener U, Gotz R, Hansen M, Hollenberg CP, Jansen G, Kramer W, Klein S, Kotter P, Kricke J, Launhardt H, Mannhaupt G, Maierl A, Meyer P, Mewes W, Munder T, Niedenthal RK, Ramezani RM, Rohmer A, Romer A, Hinnen A. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol Gen Genet. 1999;262:683–702. doi: 10.1007/pl00013817. [DOI] [PubMed] [Google Scholar]
- Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- Fahmi M, Garcia L, Taupignon A, Dufy B, Sartor P. Recording of a large-conductance chloride channel in normal rat lactotrophs. Am J Physiol Endocrinol Metab. 1995;269:E969–E976. doi: 10.1152/ajpendo.1995.269.5.E969. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Hartzell C. Volume-sensitivity of the bestrophin family of chloride channels. J Physiol. 2005;552:477–491. doi: 10.1113/jphysiol.2004.075622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn SM, Hughes SE, Hawley RS. Why don't null alleles of axs have a phenotype? A Dros Res Conf. 2007;48:183C. [Google Scholar]
- Frizzell RA, Rechkemmer G, Shoemaker RL. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986;233:558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Galindo BE, Vacquier VD. Phylogeny of the TMEM16 protein family: some members are overexpressed in cancer. Int J Mol Med. 2005;16:919–924. [PubMed] [Google Scholar]
- Grishin NV. Estimation of the number of amino acid substitutions per site when the substitution rate varies among sites. J Mol Evol. 1995;41:675–679. doi: 10.1007/BF00175826. [DOI] [PubMed] [Google Scholar]
- Guo D, Young L, Patel C, Jiao Z, Wu Y, Liu T, Kowey PR, Yan GX. Calcium-activated chloride current contributes to action potential alternations in left ventricular hypertrophy rabbit. Am J Physiol Heart Circ Physiol. 2008;295:H97–H104. doi: 10.1152/ajpheart.01032.2007. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Gericke M, Cohen RA, Bolotina VM. Ca2+-dependent Cl− channels in mouse and rabbit aortic smooth muscle cells: regulation by intracellular Ca2+ and NO. Am J Physiol Heart Circ Physiol. 1999;277:H1732–H1744. doi: 10.1152/ajpheart.1999.277.5.H1732. [DOI] [PubMed] [Google Scholar]
- Huang X, Godfrey TE, Gooding WE, McCarty KS, Jr, Gollin SM. Comprehensive genome and transcriptome analysis of the 11q13 amplicon in human oral cancer and synteny to the 7F5 amplicon in murine oral carcinoma. Genes Chromosomes Cancer. 2006;45:1058–1069. doi: 10.1002/gcc.20371. [DOI] [PubMed] [Google Scholar]
- Huang X, Gollin SM, Raja S, Godfrey TE. High-resolution mapping of the 11q13 amplicon and identification of a gene, TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci U S A. 2002;99:11369–11374. doi: 10.1073/pnas.172285799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy N. Calcium-activated chloride channels in cultured embryonic Xenopus spinal neurons. J Neurophysiol. 1992;68:2042–2050. doi: 10.1152/jn.1992.68.6.2042. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. [erratum appears in Physiol Rev 2003 83 (2): following table of contents] Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Juschke C, Ferring D, Jansen RP, Seedorf M. A novel transport pathway for a yeast plasma membrane protein encoded by a localized mRNA. Curr Biol. 2004;14:406–411. doi: 10.1016/j.cub.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Juschke C, Wachter A, Schwappach B, Seedorf M. SEC18/NSF-independent, protein-sorting pathway from the yeast cortical ER to the plasma membrane. J Cell Biol. 2005;169:613–622. doi: 10.1083/jcb.200503033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 2003;22:1375–1381. [PubMed] [Google Scholar]
- Katoh M, Katoh M. Identification and characterization of TMEM16H gene in silico. Int J Mol Med. 2005;15:353–358. [PubMed] [Google Scholar]
- Kiessling A, Weigle B, Fuessel S, Ebner R, Meye A, Rieger MA, Schmitz M, Temme A, Bachmann M, Wirth MP, Rieber EP. D-TMPP: a novel androgen-regulated gene preferentially expressed in prostate and prostate cancer that is the first characterized member of an eukaryotic gene family. Prostate. 2005;64:387–400. doi: 10.1002/pros.20250. [DOI] [PubMed] [Google Scholar]
- Kim Y, Chattopadhyay S, Locke S, Pearce DA. Interaction among Btn1p, Btn2p, and Ist2p reveals potential interplay among the vacuole, amino acid levels, and ion homeostasis in the yeast Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:281–288. doi: 10.1128/EC.4.2.281-288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen TK, Bergdahl A, Hougaard C, Christophersen P, Pedersen SF, Hoffmann EK. Cell cycle-dependent activity of the volume- and Ca2+-activated anion currents in Ehrlich lettre ascites cells. J Cell Physiol. 2007;210:831–842. doi: 10.1002/jcp.20918. [DOI] [PubMed] [Google Scholar]
- Klockner U. Intracellular calcium ions activate a low-conductance chloride channel in smooth-muscle cells isolated from human mesenteric artery. Pflugers Arch. 1993;424:231–237. doi: 10.1007/BF00384347. [DOI] [PubMed] [Google Scholar]
- Koumi S, Sato R, Aramaki T. Characterization of the calcium-activated chloride channel in isolated guinea-pig hepatocytes. J Gen Physiol. 1994;104:357–373. doi: 10.1085/jgp.104.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J, Hawley RS. The spindle-associated transmembrane protein Axs identifies a new family of transmembrane proteins in eukaryotes. Cell Cycle. 2003;2:174–176. [PubMed] [Google Scholar]
- Kunzelmann K, Milenkovic VM, Spitzner M, Soria RB, Schreiber R. Calcium-dependent chloride conductance in epithelia: is there a contribution by bestrophin? Pflugers Arch. 2007;454:879–889. doi: 10.1007/s00424-007-0245-z. [DOI] [PubMed] [Google Scholar]
- Kuruma A, Hartzell HC. Dynamics of calcium regulation of chloride currents in Xenopus oocytes. Am J Physiol Cell Physiol. 1999;276:C161–C175. doi: 10.1152/ajpcell.1999.276.1.C161. [DOI] [PubMed] [Google Scholar]
- Kuruma A, Hartzell HC. Bimodal control of a Ca2+-activated Cl− channel by different Ca2+ signals. J Gen Physiol. 2000;115:59–80. doi: 10.1085/jgp.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde MR, Kelly ME, Barnes S. Calcium-activated chloride channels in the retina. Channels (Austin) 2008;2:252–260. doi: 10.4161/chan.2.4.6704. [DOI] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen ME, Forsyth GW. Structure and function of CLCA proteins. Physiol Rev. 2005;85:1061–1092. doi: 10.1152/physrev.00016.2004. [DOI] [PubMed] [Google Scholar]
- Machaca K, Haun S. Induction of maturation- promoting factor during Xenopus oocyte maturation uncouples Ca2+ store depletion from store- operated Ca2+ entry. J Cell Biol. 2002;156:75–85. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeish PR, Nurse CA. Ion channel compartments in photoreceptors: evidence from salamander rods with intact and ablated terminals. J Neurophysiol. 2007;98:86–95. doi: 10.1152/jn.00775.2006. [DOI] [PubMed] [Google Scholar]
- Mammalian Gene Collection Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhaupt G, Stucka R, Ehnle S, Vetter I, Feldmann H. Analysis of a 70 kb region on the right arm of yeast chromosome II. Yeast. 1994;10:1363–1381. doi: 10.1002/yea.320101014. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (Best-1) J Gen Physiol. 2006;127:577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y, Eaton DC. Effects of insulin and phosphatase on a Ca2+-dependent Cl− channel in a distal nephron cell line (A6) J Gen Physiol. 1990;95:773–789. doi: 10.1085/jgp.95.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchkov VV, Larsen P, Bouzinova EV, Rojek A, Briggs Boedtkjer DM, Golubinskaya V, Pedersen FS, Aalkjar C, Nilsson H. Bestrophin-3 (vitelliform macular dystrophy 2-like 3 protein) is essential for the cGMP- dependent calcium-activated chloride conductance in vascular smooth muscle cells. Circ Res. 2008;103:864–872. doi: 10.1161/CIRCRESAHA.108.178517. [DOI] [PubMed] [Google Scholar]
- Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr Opin Neurobiol. 2003;13:469–475. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miwa S, Nakajima T, Murai Y, Takano Y, Sugiyama T. Mutation assay of the novel gene DOG1 in gastrointestinal stromal tumors (GISTs) J Gastroenterol. 2008;43:531–537. doi: 10.1007/s00535-008-2195-4. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Tsutsumi S, Inoue H, Sakamoto Y, Miyatake K, Miyawaki K, Noji S, Kamata N, Itakura M. Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochem Biophys Res Commun. 2007;357:126–132. doi: 10.1016/j.bbrc.2007.03.108. [DOI] [PubMed] [Google Scholar]
- Morris AP, Frizzell RA. Ca2+-dependent Cl− channels in undifferentiated human colonic cells (HT-29). II. Regulation and rundown. Am J Physiol Cell Physiol. 1993;264:C977–C985. doi: 10.1152/ajpcell.1993.264.4.C977. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997a;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Szucs G, Wei L, Tanzi F, Voets T, Droogmans G. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. J Physiol. 1997b;498:381–396. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I, Wagner JA, Schulman H, Gardner P. Regulation of Cl- channels by multifunctional CaM kinase. Neuron. 1991;6:547–555. doi: 10.1016/0896-6273(91)90057-7. [DOI] [PubMed] [Google Scholar]
- Papassotiriou J, Eggermont J, Droogmans G, Nilius B. Ca2+-activated Cl− channels in Ehrlich ascites tumor cells are distinct from mCLCA1, 2 and 3. Pflugers Arch. 2001;442:273–279. doi: 10.1007/s004240100526. [DOI] [PubMed] [Google Scholar]
- Pardo LA, Stuhmer W. Eag1: an emerging oncological target. Cancer Res. 2008;68:1611–1613. doi: 10.1158/0008-5472.CAN-07-5710. [DOI] [PubMed] [Google Scholar]
- Piper AS, Large WA. Multiple conductance states of single Ca2+-activated Cl− channels in rabbit pulmonary artery smooth muscle cells. J Physiol. 2003;547:181–196. doi: 10.1113/jphysiol.2002.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M. Ca2+-activated chloride channels go molecular. J Gen Physiol. 2004;123:323–325. doi: 10.1085/jgp.200409053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Fischmeister R, Hartzell HC. Mouse bestrophin-2 is a bona fide Cl− channel: identification of a residue important in anion binding and conduction. J Gen Physiol. 2004;123:327–340. doi: 10.1085/jgp.200409031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Wei RW, Hartzell HC. Characterization of Ca2+-activated Cl− currents in mouse kidney inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2003a;285:F326–F335. doi: 10.1152/ajprenal.00034.2003. [DOI] [PubMed] [Google Scholar]
- Qu Z, Wei RW, Mann W, Hartzell HC. Two bestrophins cloned from Xenopus laevis oocytes express Ca-activated Cl currents. J Biol Chem. 2003b;278:49563–49572. doi: 10.1074/jbc.M308414200. [DOI] [PubMed] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rock JR, Harfe BD. Expression of TMEM16 paralogs during murine embryogenesis. Dev Dyn. 2008;237:2566–2574. doi: 10.1002/dvdy.21676. [DOI] [PubMed] [Google Scholar]
- Schlenker T, Fitz JG. Ca2+-activated C1− channels in a human biliary cell line: regulation by Ca2+/calmodulin- dependent protein kinase. Am J Physiol Cell Physiol. 1996;271:G304–G310. doi: 10.1152/ajpgi.1996.271.2.G304. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Lee EL, Ise T, Okada Y. Volume-sensitive Cl− channel as a regulator of acquired cisplatin resistance. Anticancer Res. 2008;28:75–83. [PubMed] [Google Scholar]
- Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med. 2008;233:779–791. doi: 10.3181/0711-MR-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tsunenari T, Yau K-W, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. The Drosophila tweety family: molecular candidates for large-conductance Ca2+-activated Cl− channels. Exp Physiol. 2006;91:141–147. doi: 10.1113/expphysiol.2005.031773. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A. A novel human Cl− channel family related to Drosophila flightless locus. J Biol Chem. 2004;279:22461–22468. doi: 10.1074/jbc.M313813200. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Neher E, Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987;84:5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Taleb O, Feltz P, Bossu JL, Feltz A. Small-conductance chloride channels activated by calcium on cultured endocrine cells from mammalian pars intermedia. Pflugers Arch. 1988;412:641–646. doi: 10.1007/BF00583766. [DOI] [PubMed] [Google Scholar]
- Tsunenari T, Sun H, Williams J, Cahill H, Smallwood P, Yau K-W, Nathans J. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Inoue H, Sakamoto Y, Mizuta K, Kamata N, Itakura M. Molecular cloning and characterization of the murine gnathodiaphyseal dysplasia gene GDD1. Biochem Biophys Res Commun. 2005;331:1099–1106. doi: 10.1016/j.bbrc.2005.03.226. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Kamata N, Vokes TJ, Maruoka Y, Nakakuki K, Enomoto S, Omura K, Amagasa T, Nagayama M, Saito-Ohara F, Inazawa J, Moritani M, Yamaoka T, Inoue H, Itakura M. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD) Am J Hum Genet. 2004;74:1255–1261. doi: 10.1086/421527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaz M, Cinniger JC, Moody WJ. A voltage-gated chloride channel in ascidian embryos modulated by both the cell cycle clock and cell volume. J Physiol. 1995;488:689–699. doi: 10.1113/jphysiol.1995.sp021000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]