Abstract
The solute carrier 26 (SLC26) transporters are anion transporters with diverse substrate specificity. Several members are ubiquitous while others show limited tissue distribution. They are expressed in many epithelia and to the extent known, play a central role in anion secretion and absorption. Members of the family are primarily Cl− transporters, although some members transport mainly SO42−, Cl−, HCO3− or I−. A defining feature of the family is their functional diversity. Slc26a1 and Slc26a2 function as specific SO42− transporters while Slc26a4 functions as an electroneutral Cl−/I−/HCO3− exchanger. Slc26a3 and Slc26a6 function as coupled electrogenic Cl−/HCO3− exchangers or as bona fide anion channels. SLC26A7 and SLC26A9 function exclusively as Cl− channels. This short review discusses the functional diversity of the SLC26 transporters.
A crucial function of most epithelia is Cl− absorption and HCO3− secretion that is coupled to fluid secretion. This controls the electrolyte composition and pH of the fluid secreted by exocrine cells (Melvin et al. 2005; Steward et al. 2005; Blouquit-Laye & Chinet, 2007). Vectorial Cl− absorption and HCO3− secretion are mediated by HCO3− entry at the basolateral membrane that is mostly mediated by the pNBC1 (NBCe1-B) isoform of the Na+–HCO3− cotransporter family and to a lesser extent by the combined action of the Na+/H+ exchanger NHE1 and the Cl−/HCO3− exchanger AE2 (Zhao et al. 1994; Melvin et al. 2005; Steward et al. 2005; Bachmann et al. 2006). The nature of the transporters mediating HCO3− exit at the luminal membrane remained elusive until the discovery of the SLC26 family of anion transporters (Dorwart et al. 2008). Although the first member of the family to be identified was the liver SO42− transporter SLC26a1 (Bissig et al. 1994), the breakthrough in appreciating the function of the family in Cl− absorption and HCO3− secretion was made with the discovery that congenital Cl− diarrhoea is caused by mutations in SLC26A3 (Höglund et al. 1996) and that SLC26a3 is expressed in the luminal membrane of colonic epithelium where it functions as a Cl− and HCO3− transporter (Moseley et al. 1999) that can mediate Cl−/HCO3− exchange activity (Melvin et al. 1999). It then became clear that the previously identified SO42− transporter SLC26A2, which is mutated in dystrophic dysplasia (DTDST) (Hastbacka et al. 1994), belongs to the same family. Subsequent identification of SLC26A4 as the transporter mutated in Pendred syndrome (Everett et al. 1997) and of SLC26A5 (Prestin) as the protein mediating electromotility of outer hair cells in the cochlea (Zheng et al. 2000) was followed by identification of the remaining members of the family, mostly by database searches (Dorwart et al. 2008).
By convention, upper and lower case letters are used to, respectively, refer to the human and mouse transporters. The human family of SLC26 transporters is coded by 11 genes, although SLC26A10 is probably a pseudogene. Homologues of the family are found in many species, from the human to Drosophila to Arabidopsis (Dorwart et al. 2008). The family of SLC26 transporters is relatively new and many structural and functional features of all members of the family are still not well understood. The current knowledge of the general features of the transporters and their potential cellular function has been summarized in several recent reviews (Markovich & Aronson, 2007; Sindićet al. 2007; Dorwart et al. 2008). Here, we will only highlight the intriguingly diverse transport modes of members of the family. Members of the family can be grouped into three general categories: the SO42− transporters SLC26A1 and SLC26A2; the Cl−/HCO3− exchangers SLC26A3, SLC26A4 and SLC26A6; and the ion channels SLC26A7 and SLC26A9 (Dorwart et al. 2008). The mammalian SLC26A5 was reported to not function as a transporter, although the invertebrate Slc26a5 does (Detro-Dassen et al. 2007; Schaechinger & Oliver, 2007). However, recent study suggests that SLC26A5 may mediate Cl−/formate exchange (J. Santos-Sacchi, personal communication). The transport function of SLC26A8 and SLC26A11 is not known.
The SO42− transporters
SLC26A1 is a basolateral membrane SO42− (Karniski et al. 1998; Regeer et al. 2003) and oxalate (Xie et al. 2002) transporter and does not transport Cl−, OH− or HCO3− (Karniski et al. 1998; Regeer et al. 2003). The mechanism of SO42− and oxalate transport by SLC26A1 is not known. SLC26A1-mediated SO42− uptake is enhanced by extracellular halides and acidic extracellular pH (Xie et al. 2002), suggesting that SLC26A1 does not function as a SO42−/Cl− exchanger. The physiological role for SLC26A1 is not well understood, but it has been suggested to play a role in SO42− homeostasis and sulphation of proteoglycans in the liver (Quondamatteo et al. 2006) and in oxalate homeostasis in the kidney (Pritchard & Renfro, 1983; Kuo & Aronson, 1988).
SLC26A2 is ubiquitous and is expressed at high level in all epithelia examined and in connective tissues (Hastbacka et al. 1994; Haila et al. 2001). SLC26A2 functions as a SO42− transporter and provides SO42− for proteoglycan sulphation, which is needed for cartilage development (Hastbacka et al. 1996; Forlino et al. 2005). Accordingly, mutations in the SLC26A2 gene cause diastrophic dysplasia (Hastbacka et al. 1994). It was proposed that SLC26A2 functions as a SO42−/Cl− exchanger, but not as a SO42−/HCO3− exchanger (Satoh et al. 1998). However, preliminary results from our laboratory suggest that SO42− transport by SLC26A2 may not be coupled to Cl− transport and that SLC26A2 may function as an electroneutral SO42−–2H+ cotransporter (or SO42−/2OH− exchanger). In addition, SLC26A2 did not appear to generate ionic current in the presence of any of the anions tested, including NO3− and SCN− (N. Shcheynikov & S Muallem, unpublished observations).
The anion exchangers
The most intriguing members of the family are the anion exchangers SLC26A3, SLC26A4 and SLC26A6. SLC26A3 and SLC26A6 are expressed in the luminal membrane of many epithelia (Höglund et al. 1996, 2001; Haila et al. 2000; Lohi et al. 2002, 2003) and play a central role in Cl− absorption and HCO3− secretion in several epithelia, including that of the intestine (Jacob et al. 2002; Simpson et al. 2007) and of the pancreas (Wang et al. 2006). SLC26A4 is expressed at high levels in the luminal membrane of follicular cells in the thyroid, in the inner ear (Everett et al. 1997; Royaux et al. 2000), in the renal cortical collecting duct (Royaux et al. 2001; Soleimani et al. 2001) and in the salivary gland ducts (Shcheynikov et al. 2008), where it participates in transcellular I− transport and in Cl−/HCO3− exchange.
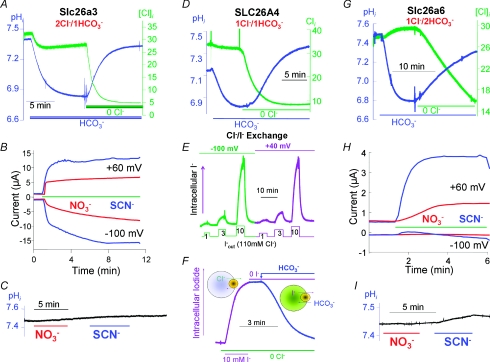
Slc26a3, Slc26a4 and Slc26a6 function as obligatory Cl−/HCO3− exchangers, but with different stoichiometries (Shcheynikov et al. 2006a,b, 2008). The coupled exchange is evident from the stimulation of Cl− fluxes by HCO3− and stimulation of HCO3− fluxes by Cl−. However, Slc26a3 functions as a 2Cl−/1HCO3− exchanger (Ko et al. 2002; Shcheynikov et al. 2006b), and Slc26A4 as a 1Cl−/1HCO3− exchanger (Shcheynikov et al. 2008), while Slc26a6 functions as a 1Cl−/2HCO3− exchanger (Ko et al. 2002; Shcheynikov et al. 2006b). This is illustrated in Fig. 1A, D and G, which shows the mode of Cl−/HCO3− exchange of the respective transporters, as determined by simultaneous measurement of Cl− and HCO3− fluxes in Xenopus oocytes.
Figure 1. Cl−/HCO3− exchange and channel function of slc26a3, SLC26A4 and slc26a6.
The transporters were expressed in Xenopus oocytes and Cl−/HCO3− exchange or anion current were measured as described in the respective publications. The results in A and B were modified from Shcheynikov et al. (2006b) and show Cl−/HCO3− exchange (A) and NO3− and SCN− current (B) in the absence of pHi changes (C) by Slc26a3. The results in D–F were modified mostly from Shcheynikov et al. (2008) and show Cl−/HCO3− exchange (D), I−/Cl− exchange at two membrane potentials (E) and I−/HCO3− exchange (F) by SLC26A4. The results in panels G–I were modified from Shcheynikov et al. (2006b) and show Cl−/HCO3− exchange (G), NO3− and SCN− current (H) in the absence of pHi changes (I) by Slc26a6.
The SLC26 exchangers can also transport other anions of physiological relevance. A special case is SLC26A4, which functions as a Cl−/HCO3−, Cl−/I− and I−/HCO3− exchanger (Fig. 1D and F; Shcheynikov et al. 2008). SLC26A4 has a relatively high affinity for I− and prefers I− over Cl− and HCO3− as the transported ion. This is illustrated in Fig. 1E, which shows that SLC26A4 can transport I− in media containing 110 mm Cl− and 1 mm I−. Cl−, HCO3− and I− transport by SLC26A4 is electroneutral, as is apparent from the 1Cl−/1HCO3− exchange stoichiometry and the same rate of I−/Cl− exchange at membrane potentials of −100 and +40 mV (Fig. 1D and E). All modes of transport are relevant physiologically. Mutations in SLC26A4 cause Pendred syndrome, which is associated with goitre as a result of impaired I− organification in the thyroid (Everett et al. 1997; Campbell et al. 2001). This is probably due to impaired HCO3−/I− and Cl−/I− exchange, and consequently limited I− secretion into the follicular space. Pendred syndrome is also associated with hearing loss (Coyle et al. 1996; Everett et al. 1997; Campbell et al. 2001), and deletion of Slc26a4 in mice revealed that the renal Slc26a4 modulates vascular volume and arterial pH (Wall et al. 2004; Wall, 2005). These functions are probably mediated by the Cl−/HCO3− exchange function of SLC26A4. Accordingly, Slc26a4 mediates HCO3− secretion by epithelial cells of the inner ear to alkalinize the pH of the endolymphatic fluid (Wangemann et al. 2007).
Measurement of Cl−, NO3− and SCN− transport in the absence of HCO3− revealed an unexpected function of Slc26a3 and Slc26a6. Both transporters showed a channel-like activity and generated large currents (Shcheynikov et al. 2006b). The Cl− current by Slc26a3 and Slc26a6 expressed in oocytes was about 0.5 μA and was associated with small or no Cl−/OH− exchange with Slc26a3 and Slc26a6, respectively (Ko et al. 2002). Strikingly, Slc26a3 and Slc26a6 mediate large NO3− and SCN− currents that are not coupled to OH− or HCO3− transport (Ko et al. 2002; Shcheynikov et al. 2006b). Several features of the currents are illustrated in Fig. 1B, C, H and I, which shows the large NO3− and very large SCN− currents mediated by Slc26a3 and Slc26a6 with minimal changes in pHi. The outward current appeared on addition of NO3− and SCN− to the extracellular media, while the inward currents developed more slowly, reflecting the rate of entry of the conducted anions into the oocytes. The slow rates of current development and extent of the inward NO3− and SCN− currents by Slc26a6 relative to that mediated by Slc26a3, indicate lower permeability of Slc26a6 to these anions. These findings suggest that the same transporter (Slc26a3 and Slc26a6) can function either as an obligatory coupled exchanger or as an ion channel, depending on the transported ion. This property is reminiscent of the behaviour of several neurotransmitter transporters (Fairman & Amara, 1999; Torres & Amara, 2007) and of Na+ transport by the Na+–HCO3− cotransporter NBCe1 (Choi et al. 2000).
How can the same transporter function as a coupled transporter and as an ion channel? A clue might be provided by the function of the bacterial ClC-ec1. ClC-ec1 functions as a 2Cl−/H+ exchanger, but mutating Glu148 or Tyr445 in the ion-conducting pathway converts it to a Cl− channel (Accardi et al. 2004). By analogy, it is possible that a similar mechanism regulates the Slc26a3 and Slc26a6 pores to hinder the movement of Cl− through the transporters with HCO3− facilitating the movement of Cl− through the pore to generate coupled transport. In this case, Cl− may be occluded in the pore with HCO3− releasing the occluded state while itself entering the pore and being transported, as occurs with other coupled transporters. The residues that hinder the movement of Cl− may not interact with NO3− and SCN−, allowing their flow through the pore to result in an uncoupled conductive transport. Unlike Cl−, NO3− and SCN− do not become occluded and flow freely through the pore to generate the current. Another scenario is a change of the pore conformation by NO3− and SCN− to convert Slc26a3 and Slc26a6 from coupled transporters to channels.
The anion channels
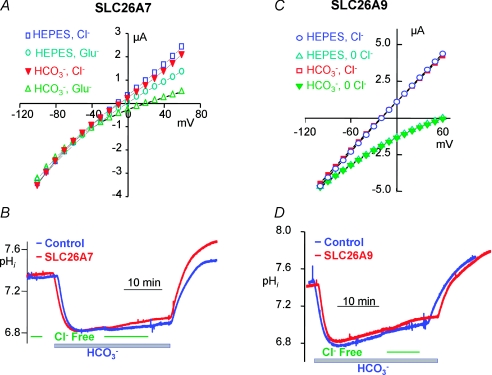
The two established SLC26 transporters that function exclusively as channels are SLC26A7 and SLC26A9. Current measurement in Xenopus oocytes and HEK cells transfected with SLC26A7 showed that SLC26A7 functions as a Cl− channel (Kim et al. 2005). As illustrated in Fig. 2A, the extent of the SLC26A7 current is not affected by HCO3−, indicating that SLC26A7 is not permeable to HCO3− and thus does not function as a Cl−/HCO3− exchanger. Accordingly, no Cl−/HCO3− exchange activity could be measured with SLC26A7 (Fig. 2B). However, HCO3− increases the selectivity of SLC26A7 for Cl− (Fig. 2A) due to regulation of the channel function of SLC26A7 by intracellular H+, raising the possibility that SLC26A7 may function as a pHi sensor (Kim et al. 2005).
Figure 2. Cl− channel activity and lack of Cl−/HCO3− exchange by SLC26A7 and SLC26A9.
The results in A and B were modified from Kim et al. (2005) and show Cl− channel activity in Hepes- and in HCO3−-buffered media (A) and the minimal Cl−/HCO3− exchange activity (B) by SLC26A7. The results in C and D were modified from Dorwart et al. (2007) and show Cl− channel activity in Hepes- and in HCO3−-buffered media (C) and the minimal Cl−/HCO3− exchange activity (D) by SLC26A9.
Initial characterization of SLC26A9 transport properties by measurement of pHi suggested that SLC26A9 functions as a Cl−/HCO3− exchanger (Xu et al. 2005). On the other hand, current measurement in Xenopus oocytes and HEK or COS-7 cells expressing SLC26A9 revealed that SLC26A9 functions as a Cl− channel with minimal permeability to HCO3− (Dorwart et al. 2007; Loriol et al. 2008). Figure 2C illustrates the Cl− channel function of SLC26A9 and the lack of effect of HCO3− on the current, and Fig. 2D shows that SLC26A9 does not function as a Cl−/HCO3− exchanger. However, a recent study reported that HCO3− slightly stimulated Cl− channel activity by SLC26A9 (Loriol et al. 2008). How HCO3− may influence channel activity is not known at present, although it does not appear to be mediated by changes in pHi.
This brief review emphasizes the remarkable functional diversity of the SLC26 transporters, suggesting diverse physiological roles for these transporters that has yet to be fully understood. The basis for the functional diversity in terms of substrate specificity and transport modes is not known at present. The SLC26 transporters show only limited sequence similarity among members of the family, including those with similar transport modes, which is not sufficient to deduce functional specificity. However, the functional diversity of the SLC26 transporters, from coupled electroneutral exchangers to electrogenic exchangers to ion channels, offers a good model to study the structural basis of different transport functions. Within the family, the most intriguing are Slc26a3 and Slc26a6 that can function both as coupled transporters and as anion channels. Deciphering the underlying structural basis for these functions should also impact our understanding of the function of other anion transporters like the CLC family, members of which can function as electrogenic Cl−/H+ exchangers and as Cl− channels (Miller, 2006).
References
- Accardi A, Kolmakova-Partensky L, Williams C, Miller C. Ionic currents mediated by a prokaryotic homologue of CLC Cl- channels. J Gen Physiol. 2004;123:109–119. doi: 10.1085/jgp.200308935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+-HCO3− cotransport activity in murine colonic crypts in a M3-, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2006;291:G650–G657. doi: 10.1152/ajpgi.00376.2005. [DOI] [PubMed] [Google Scholar]
- Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem. 1994;269:3017–3021. [PubMed] [Google Scholar]
- Blouquit-Laye S, Chinet T. Ion and liquid transport across the bronchiolar epithelium. Respir Physiol Neurobiol. 2007;159:278–282. doi: 10.1016/j.resp.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Campbell C, Cucci RA, Prasad S, Green GE, Edeal JB, Galer CE, Karniski LP, Sheffield VC, Smith RJ. Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum Mutat. 2001;17:403–411. doi: 10.1002/humu.1116. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Coyle B, Coffey R, Armour JA, Gausden E, Hochberg Z, Grossman A, Britton K, Pembrey M, Reardon W, Trembath R. Pendred syndrome (goitre and sensorineural hearing loss) maps to chromosome 7 in the region containing the nonsyndromic deafness gene DFNB4. Nat Genet. 1996;12:421–423. doi: 10.1038/ng0496-421. [DOI] [PubMed] [Google Scholar]
- Detro-Dassen S, Schänzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J Biol Chem. 2007;283:4177–4188. doi: 10.1074/jbc.M704924200. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Amara SG. Functional diversity of excitatory amino acid transporters: ion channel and transport modes. Am J Physiol Renal Physiol. 1999;277:F481–F486. doi: 10.1152/ajprenal.1999.277.4.F481. [DOI] [PubMed] [Google Scholar]
- Forlino A, Piazza R, Tiveron C, Della Torre S, Tatangelo L, Bonafe L, Gualeni B, Romano A, Pecora F, Superti-Furga A, Cetta G, Rossi A. A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum Mol Genet. 2005;14:859–871. doi: 10.1093/hmg/ddi079. [DOI] [PubMed] [Google Scholar]
- Haila S, Hastbacka J, Bohling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U. SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem Cytochem. 2001;49:973–982. doi: 10.1177/002215540104900805. [DOI] [PubMed] [Google Scholar]
- Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, Lohi H, Airola K, Holmberg C, Hastbacka J, Kere J, Hoglund P. The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol. 2000;113:279–286. doi: 10.1007/s004180000131. [DOI] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Hastbacka J, Superti-Furga A, Wilcox WR, Rimoin DL, Cohn DH, Lander ES. Sulfate transport in chondrodysplasia. Ann N Y Acad Sci. 1996;785:131–136. doi: 10.1111/j.1749-6632.1996.tb56251.x. [DOI] [PubMed] [Google Scholar]
- Höglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- Höglund P, Sormaala M, Haila S, Socha J, Rajaram U, Scheurlen W, Sinaasappel M, de Jonge H, Holmberg C, Yoshikawa H, Kere J. Identification of seven novel mutations including the first two genomic rearrangements in SLC26A3 mutated in congenital chloride diarrhea. Hum Mutat. 2001;18:233–242. doi: 10.1002/humu.1179. [DOI] [PubMed] [Google Scholar]
- Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology. 2002;122:709–724. doi: 10.1053/gast.2002.31875. [DOI] [PubMed] [Google Scholar]
- Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber J, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol. 1998;275:F79–F87. doi: 10.1152/ajprenal.1998.275.1.F79. [DOI] [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SM, Aronson PS. Oxalate transport via the sulfate/HCO3 exchanger in rabbit renal basolateral membrane vesicles. J Biol Chem. 1988;263:9710–9717. [PubMed] [Google Scholar]
- Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7-A8, and -A9. J Biol Chem. 2002;277:14246–14254. doi: 10.1074/jbc.M111802200. [DOI] [PubMed] [Google Scholar]
- Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol. 2003;284:C769–C779. doi: 10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J. Characterization of SLC26A9, facilitation of Cl− transport by bicarbonate. Cell Physiol Biochem. 2008;22:15–30. doi: 10.1159/000149780. [DOI] [PubMed] [Google Scholar]
- Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol. 2007;69:361–375. doi: 10.1146/annurev.physiol.69.040705.141319. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse Down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol. 1999;276:G185–G192. doi: 10.1152/ajpgi.1999.276.1.G185. [DOI] [PubMed] [Google Scholar]
- Pritchard JB, Renfro JL. Renal sulfate transport at the basolateral membrane is mediated by anion exchange. Proc Natl Acad Sci U S A. 1983;80:2603–2607. doi: 10.1073/pnas.80.9.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quondamatteo F, Krick W, Hagos Y, Kruger MH, Neubauer-Saile K, Herken R, Ramadori G, Burckhardt G, Burckhardt BC. Localization of the sulfate/anion exchanger in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1075–G1081. doi: 10.1152/ajpgi.00492.2005. [DOI] [PubMed] [Google Scholar]
- Regeer RR, Lee A, Markovich D. Characterization of the human sulfate anion transporter (hsat-1) protein and gene (SAT1; SLC26A1) DNA Cell Biol. 2003;22:107–117. doi: 10.1089/104454903321515913. [DOI] [PubMed] [Google Scholar]
- Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y. Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem. 1998;273:12307–12315. doi: 10.1074/jbc.273.20.12307. [DOI] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci U S A. 2007;104:7693–7698. doi: 10.1073/pnas.0608583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006a;273:177–186. Discussion pp. 186–192 and 261–174. [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006b;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1079–G1088. doi: 10.1152/ajpgi.00354.2006. [DOI] [PubMed] [Google Scholar]
- Sindić A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens. 2007;16:484–490. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens. 2005;14:480–484. doi: 10.1097/01.mnh.0000168390.04520.06. [DOI] [PubMed] [Google Scholar]
- Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension. 2004;44:982–987. doi: 10.1161/01.HYP.0000145863.96091.89. [DOI] [PubMed] [Google Scholar]
- Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25:5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- Xu J, Henriksnas J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange, and is inhibited by NH4+ Am J Physiol Cell Physiol. 2005;289:C493–C505. doi: 10.1152/ajpcell.00030.2005. [DOI] [PubMed] [Google Scholar]
- Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]