Abstract
Glial cells release gliotransmitters which signal to adjacent neurons and glial cells. Previous studies showed that in response to stimulation with bradykinin, glutamate is released from rat astrocytes and causes NMDA receptor-mediated elevation of intracellular Ca2+ in adjacent neurons. Here, we investigate how bradykinin-induced glutamate release from mouse astrocytes signals to neighbouring neurons in co-cultures. Astrocyte-to-neuron signalling and bradykinin-induced glutamate release from mouse astrocytes were both inhibited by the anion channel blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and phloretin. Glutamate release was also sensitive to 4-(2-Butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxybutyric acid (DCPIB), a specific blocker of the volume-sensitive outwardly rectifying anion channel (VSOR). Astrocytes, but not neurons, responded to bradykinin with activation of whole-cell Cl− currents. Although astrocytes stimulated with bradykinin did not undergo cell swelling, the bradykinin-activated current exhibited properties typical of VSOR: outward rectification, inhibition by osmotic shrinkage, sensitivity to DIDS, phloretin and DCPIB, dependence on intracellular ATP, and permeability to glutamate. Bradykinin increased intracellular reactive oxygen species (ROS) in mouse astrocytes. Pretreatment of mouse astrocytes with either a ROS scavenger or an NAD(P)H oxidase inhibitor blocked bradykinin-induced activation of VSOR, glutamate release and astrocyte-to-neuron signalling. By contrast, pretreatment with BAPTA-AM or tetanus neurotoxin A failed to suppress bradykinin-induced glutamate release. Thus, VSOR activated by ROS in mouse astrocytes in response to stimulation with bradykinin, serves as the pathway for glutamate release to mediate astrocyte-to-neuron signalling. Since bradykinin is an initial mediator of inflammation, VSOR might play a role in glia–neuron communication in the brain during inflammation.
There is accumulating evidence for the existence of bidirectional communication between astrocytes and neurons under physiological/pathophysiological conditions (Haydon 2001; Parri et al. 2001; Hansson & Ronnback, 2003; Hama et al. 2004). Astrocytes respond to neurotransmitters released from neurons by releasing a number of gliotransmitters, such as glutamate, ATP and D-serine, and signalling back to neurons. Astrocytes also release glutamate in response to a variety of stimuli including osmotic swelling (Kimelberg et al. 1990), receptor activation and electrical, mechanical and photo-stimulation (Ni et al. 2007). The most intensively studied gliotransmitter is glutamate, which causes an NMDA receptor-mediated increase in the cytosolic Ca2+ level in neighbouring neurons (Parpura et al. 1994; Araque et al. 1998; Bezzi et al. 1998; Parri et al. 2001; Angulo et al. 2004; Fellin et al. 2004). In response to stimulation with bradykinin, an initial mediator of inflammation, rat astrocytes release glutamate (Parpura et al. 1994; 1995a,b; Jeftinija et al. 1996, 1997), causing an increase in the intracellular Ca2+ concentration of neurons in contact with the astrocytes (Parpura et al. 1994).
A number of putative pathways for astrocytic glutamate release have been proposed (Malarkey & Parpura, 2008), including Ca2+-dependent exocytotic vesicular transport and Ca2+-independent transport via transporters or channels. Exocytosis may be involved in the release of glutamate, which takes place in response to mechanical stimulation (Araque et al. 2000; Montana et al. 2004; Chen et al. 2005), electrical stimulation (Jourdain et al. 2007), and chemical stimulation with glutamate (Bezzi et al. 1998; Pasti et al. 2001), NO (Bal-Price et al. 2002) and extracellular ATP (Zhang et al. 2004a,b; Höltje et al. 2008). It has also been suggested that astrocytes release glutamate in a Ca2+-independent manner by operating glutamate transporters in reverse mode under high K+ conditions (Szatkowski et al. 1990) and in brain anoxia (Nicholls & Attwell, 1990), or by operating cystine/glutamate exchangers under basal in situ conditions (Baker et al. 2002) and upon increasing the extracellular cystine concentration in rat hippocampal slices (Cavelier & Attwell, 2005). Ca2+-independent glutamate release from astrocytes has also been reported to involve ion channels. These include gap junction hemi-channels in divalent cation-free conditions (Ye et al. 2003), P2X7 receptor cation channels stimulated with BzATP (Duan et al. 2003; Fellin et al. 2006), some swelling-activated anion channels upon stimulation with ATP or UTP (Takano et al. 2005), volume-sensitive outwardly rectifying anion channels (VSORs) during osmotic swelling (Abdullaev et al. 2006; Liu et al. 2006) and under ischaemic stress (Liu et al. 2006), and maxi-anion channels under osmotic or ischaemic stress (Liu et al. 2006).
Ca2+-dependent vesicular exocytosis has been considered to serve as the pathway for bradykinin-induced glutamate release from rat astrocytes. Parpura et al. (1994) reported that bradykinin-induced glutamate release from rat astrocytes was dependent on intracellular Ca2+ and sensitive to furosemide. They also reported that rat astrocytes express some proteins belonging to the machinery of neurotransmitter release from nerve terminals, such as synaptobrevin II and syntaxin (Parpura et al. 1995a). Jeftinija et al. (1996, 1997) found that glutamate release from rat astrocytes was sensitive to botulinus toxin-B and tetanus toxin, two blockers of exocytosis. Interestingly, in mouse astrocytes VSORs and maxi-anion channels rather than other mechanisms mediate the release of glutamate in response to hypotonic and ischaemic stimuli (Liu et al. 2006). Based on these data, we investigated the possibility that anion channels might mediate glutamate release from mouse astrocytes stimulated with bradykinin. In contrast to studies using rats, we found that bradykinin-induced astrocyte-to-neuron signalling in the mouse is mediated by the tetanus toxin-insensitive release of glutamate via VSOR which was activated by reactive oxygen species (ROS) produced upon stimulation with bradykinin in mouse astrocytes without the cells undergoing sizable cell swelling.
A brief account of this work has been reported previously (Liu, 2007).
Methods
Animal experiments
The experiments were done using cultured cells derived from ddY mice (Japan SLC, Inc., Hamamatsu, Japan). All procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals of the Physiological Society of Japan. Experimental protocols were reviewed and approved in advance by the Ethics Review Committee for Animal Care and Experimentation of the National Institutes of Natural Sciences. Adult and neonatal or fetal mice were killed by cervical dislocation and decapitation, respectively, under anaesthesia (for around 3 min) with halothane (1 ml added to air of 1000 cm3), and then their uteri and brains were dissected from them, respectively.
Cell cultures
Astrocytes in primary culture at a density of 1 × 105 cells cm−2 (or 5 × 105 cells cm−2 when indicated) were prepared from newborn mice (2–3 days old, n= 288), as described previously (Liu et al. 2006). Briefly, the cerebral cortex was dissected, minced, digested with enzymes, and cultured. After becoming confluent, the cells were trypsinized and transferred to new plastic dishes or bottles for further culturing for 2–3 weeks. For Ca2+ imaging, patch-clamp experiments and single cell size measurements, the cells were transferred to polyethyleneimine-coated glass coverslips and cultured for 1–2 days before use. Neurons in primary culture at a density of 1 × 105 cells cm−2 were prepared from mouse embryos (day 16, n= 174), as described previously (Inoue et al. 2005). Briefly, brain cells dissociated from the cerebral cortex were plated on coverslips and cultured for 5–7 days before use. To prevent growth of non-neuronal cells, cytosine arabinoside (10 μm) was added to the cultures 24 h after plating. To obtain co-cultures of astrocytes and neurons, astrocytes were plated onto cultured neurons and cultured with the neurons for a further 2–3 days. In co-cultures, neurons and astrocytes could be distinguished from each other by their morphologically distinct features. This was verified by immunostaining for microtubule-associated protein 2 (MAP-2) and glial fibrillary acidic protein (GFAP), as described previously (Nedergaard et al. 1991).
Solutions
Standard Ringer solution contained (in mm): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 Na-Hepes, 6 Hepes and 5 glucose (pH 7.4, 290 mosmol (kg H2O)−1). Hypertonic (350 mosmol (kg H2O)−1) and hypotonic (210 mosmol (kg H2O)−1) Ringer solutions were prepared by adding mannitol and by reducing NaCl, respectively. In some experiments, we used NMDG-rich isotonic (280 mosmol (kg H2O)−1) solution containing (mm): 140 NMDG, 135 HCl, 2 CaCl2, 1 MgCl2, 5 glucose, 10 Hepes (pH 7.4). The standard pipette solution employed for whole-cell patch-clamp studies contained (mm): 125 CsCl, 2 CaCl2, 1 MgCl2, 3 Na2ATP, 5 Hepes (pH 7.4 adjusted with CsOH) and 10 EGTA (pCa 7.15, 275 mosmol (kg H2O)−1). For glutamate permeability measurements, 100 mm CsCl was replaced with 100 mm caesium glutamate in the pipette solution. When required, ATP was removed from the pipette solution. In some experiments, we used NMDG-rich pipette solution containing (mm): 160 NMDG, 140 HCl, 2 CaCl2, 1 MgCl2, 3 Na2ATP, 5 Hepes (pH 7.4) and 10 EGTA (277 mosmol (kg H2O)−1).
Calcium imaging
Intracellular Ca2+ signals in cultured cells loaded with fluo-4 AM (Molecular Probes, Eugene, OR, USA) were measured using a MetaFluor imaging system (Universal Imaging Co., West Chester, PA, USA) and an IX81-ZDCF inverted microscope (Olympus, Tokyo, Japan) equipped with a cooled CCD camera (NTE/CCD-512-EBFT, GR-1, Roper Scientific, Trenton, NJ, USA). Cells were loaded with fluo-4 AM (5 μm) for 20 min at 37°C in 5% CO2, washed with dye-free medium and cultured for 30 min before imaging. Ca2+ imaging was performed at wavelengths of 490 nm (bandwidth, 14 nm) for excitation and 535 nm (bandwidth, 25 nm) for emission. The experimental data were analysed using the MetaFluor software and Origin 7.0. The Ca2+ response was evaluated as the percentage increase in fluorescence intensity, 100(F–F0)/F0, where F0 and F represent the fluorescence intensity before and after application of bradykinin, respectively.
Assays of glutamate and ATP released from astrocytes
For assays of either glutamate or ATP release, astrocytes cultured at a density of 5 × 105 cells cm−2 were prepared. The samples were collected from the bulk solution of each well before and after stimulation with 1 μm bradykinin in the absence or presence of given drugs.
The bulk extracellular concentration of glutamate released from astrocytes cultured in 24-well plates was measured by the fluorometric NADH assay, as described previously (Liu et al. 2006) with slight modification. The fluorescence from each sample (0.2 ml) was measured with an MTP-100F microplate reader (Corona Electric Co., Katsuta, Japan) using a 360 nm filter for excitation and a 460 nm filter for emission (bandwidth of both filters, 70 nm).
The concentration of ATP released from astrocytes cultured in 24-well plates was measured by a luciferin–luciferase assay using an ATP analyser (AF-100, DKK-TOA Co., Tokyo, Japan), as described previously (Dutta et al. 2004). Each sample of 50 μl was used in the luminometric ATP measurements after it was mixed with 500 μl of standard Ringer solution and 50 μl of luciferin–luciferase reagent.
Astrocyte volume or size measurements
Changes in the size of single astrocytes adhered to coverslips were measured by monitoring changes in the intracellular concentration of calcein, as described previously (Inoue et al. 2005), after loading the cells with 5 μm calcein-AM (Molecular Probes) for 30 min. Calcein fluorescence in response to excitation at 390 nm (bandwidth, 14 nm) was measured at 535 nm (bandwidth, 25 nm) using a MetaFluor imaging system.
The mean cell volume of astrocytes was measured using a Coulter-type cell size analyser (CDA-500, Sysmex, Kobe, Japan), after suspending the cells in Ringer solution, as reported previously (Hazama & Okada, 1988).
Measurements of astrocyte ROS production
The intracellular ROS level was measured using 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Molecular Probes), as described previously (Shimizu et al. 2004), in astrocytes preincubated with 10 μm carboxy-H2DCFDA for 30 min. A rise in the intracellular ROS level was evaluated as the percentage increase in the fluorescence intensity, 100(F–F0)/F0, where F0 and F represent the fluorescence intensity at time 0 and a given time period after incubation, respectively.
Patch-clamp studies
Whole-cell currents were measured by the whole-cell patch-clamp recording technique in mouse primary astrocytes or neurons, as described previously (Liu et al. 2006), before and after stimulation with 1 μm bradykinin in the absence or presence of given drugs. Briefly, patch pipettes were fabricated from borosilicate glass capillaries and had a resistance of 2–3 MΩ when filled with pipette solution. Series resistance was compensated for by 80%. Current signals were filtered at 1 kHz and sampled at 5 kHz. The time courses of current activation, inhibition and recovery were monitored by repetitively applying alternating step pulses (0.5 s duration, every 5 or 15 s) of ±40 mV from a holding potential of 0 mV. To examine the voltage dependence of whole-cell currents, step pulses were applied from the holding potential to test pulses (0.5 or 1 s duration) of −100 mV to +100 mV in 20 mV increments with a pre-pulse (0.1 or 0.4 s duration) to −100 mV and a post-pulse (0.1 or 0.2 s duration) to −100 or −60 mV.
All the experiments were performed at room temperature (23–26°C).
Statistical analysis
All the data were analysed using OriginPro7.0 (OriginLab Corp., Northampton, MA, USA) and are given as the mean ±s.e.m. of observations (n). Statistical differences of the data were evaluated by ANOVA and Student's t test for paired or unpaired data where appropriate, and considered significant at P < 0.05.
Results
Bradykinin-induced mouse astrocyte–neuron signalling was sensitive to anion channel blockers
In rat astrocyte–neuron co-cultures, bradykinin, an initial mediator of inflammation, increased intracellular Ca2+ in astrocytes and adjacent neurons (Parpura et al. 1994). To investigate the effects of bradykinin in co-cultures of mouse astrocytes and neurons, we first made fluo-4 fluorescence measurements. Application of 1 μm bradykinin induced a slow increase in the cytosolic Ca2+ level in mouse cortical astrocytes in primary culture, and the mean peak Ca2+ response evaluated as the percentage increase in the fluorescence intensity was 52.6 ± 6.8% (n= 28). However, bradykinin induced little Ca2+ response in cultured mouse cortical neurons (n= 26). By contrast, in co-cultures of astrocytes and neurons, both astrocytes and neurons responded to bradykinin with a Ca2+ response (Fig. 1A). The mean peak Ca2+ response in neurons (16.2 ± 7.3%, n= 39) was smaller than that in astrocytes (50.8 ± 7.8%, n= 22: P < 0.001). Virtually all the astrocytes (Fig. 1Aa, squares) responded to bradykinin with Ca2+ increases. In contrast, brief Ca2+ responses were elicited by bradykinin only in neurons in contact with astrocytes (Fig. 1Aa, circles) and not in neurons remote from, or not in contact with, astrocytes (inverted triangles). The neuronal Ca2+ response followed the glial Ca2+ response with a delay of around 10 s, suggesting the involvement of a transmitter released from astrocytes in the generation of the Ca2+ responses in neighbouring neurons. These observations agree completely with previous observations using rat astrocytes (Parpura et al. 1994).
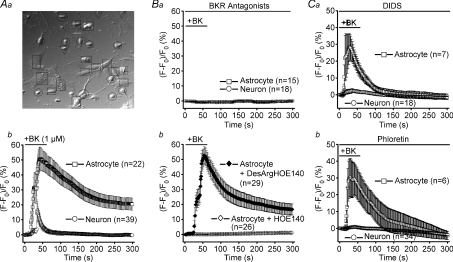
Figure 1. Sensitivity of mouse astrocyte–neuron signalling induced by bradykinin to a B2 receptor antagonist and anion channel blockers.
A, control experiments. a, differential interference-contrast micrograph. Squares, circles and inverted triangles designate responder astrocytes, responder neurons and non-responder neurons, respectively. b, intracellular Ca2+ changes, monitored by the percentage increase in the fluo-4 fluorescence intensity, during and after stimulation with 1 μm bradykinin (BK). The mean peak Ca2+ response in co-cultured astrocytes is not significantly different (P > 0.05) from that in astrocytes cultured alone, whereas that in co-cultured neurons is significantly different (P < 0.005) from that in neurons cultured alone. B, experiments with antagonists of bradykinin receptors (BKR). a, effects of combined application of antagonists of B1 and B2 subtypes of BKR on Ca2+ responses to 1 μm bradykinin (BK) in both astrocytes and neurons in the same co-culture. b, effects of an antagonist of the B2 receptor, 5 μm HOE140, and an antagonist of the B1 receptor, 5 μm[des-Arg10]-HOE140 (DesArgHOE140), on BK-induced Ca2+ responses in astrocytes. The mean peak Ca2+ response in the presence of DesArgHOE140 is not significantly different (P > 0.1) from that in its absence (Ab). C, experiments with blockers of VSOR (a: 200 μm DIDS, b: 100 μm phloretin). The astrocytic Ca2+ responses in the presence of DIDS (a) and phloretin (b) are not significantly different (P > 0.05) from that in the absence of drugs (Ab), whereas the neuronal Ca2+ responses are significantly different (P < 0.005) from that in Ab.
When antagonists of bradykinin B1 and B2 receptors (5 μm[des-Arg10]-HOE140 and 5 μm HOE140, respectively) were applied together, the bradykinin-induced Ca2+ rises in both co-cultured astrocytes and neurons were abolished (Fig. 1Ba). Moreover, bradykinin-induced Ca2+ responses in astrocytes were abolished by the B2 receptor antagonist, but not by the B1 receptor antagonist (Fig. 1Bb). Thus, it appears that the Ca2+ response induced by bradykinin in astrocytes is mediated by the B2 receptor. These observations are in good accord with previous observations in rat Schwann cells (Parpura et al. 1995c) and in rat cortical astrocytes (Jeftinija et al. 1996).
Consistent with data from rat astrocyte–neuron co-cultures (Parpura et al. 1994), an antagonist of the NMDA receptor, d(–)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μm), abolished the bradykinin-induced Ca2+ response in neurons co-cultured with astrocytes without affecting the astrocytic Ca2+ response, whereas an antagonist of the AMPA/kainate receptor, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm), failed to abolish the Ca2+ responses both in neurons and astrocytes in co-cultures (n= 7–9; data not shown). Extracellular application of an ATP-hydrolysing enzyme, apyrase (0.1 mg ml−1), was without effect on the bradykinin-induced Ca2+ responses in astrocytes or neurons in co-cultures (n= 6; data not shown). These data indicate that bradykinin-induced astrocyte-to-neuron signalling is mediated by glutamate, but not ATP. They also argue that the glutamate is released from astrocytes and activates neuronal NMDA receptors.
Application of a non-specific blocker of anion channels, 4,4′-diisothiocyanato-2,2′-stilbenedisulphonate (DIDS; 200 μm), significantly suppressed the bradykinin-induced Ca2+ response in neurons co-cultured with astrocytes, largely without affecting the response in astrocytes (Fig. 1Ca). As shown in Fig. 1Cb, bradykinin-induced Ca2+ responses in neurons, but not co-cultured astrocytes, were markedly inhibited by 100 μm phloretin, which blocks VSOR more potently than other anion channels (Fan et al. 2001). These results suggest an involvement of a pathway sensitive to both DIDS and phloretin, presumably the VSOR-type anion channel, in the glutamate-mediated astrocyte-to-neuron signalling that occurs upon stimulation with bradykinin.
Bradykinin-induced glutamate release from mouse astrocytes was sensitive to anion channel blockers and osmotic shrinkage
In order to examine whether bradykinin-induced glutamate release from mouse astrocytes is actually sensitive to anion channel blockers, we next evaluated glutamate release with an NADH-fluorometric glutamate assay. Mouse primary astrocytes responded to bradykinin (1 μm) with a substantial release of glutamate (Fig. 2A). In contrast, little or no ATP was released from mouse astrocytes when stimulated with 1 to 5 μm bradykinin (n= 12; data not shown).
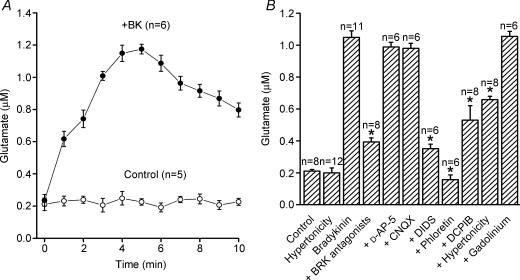
Figure 2. Bradykinin-induced glutamate release from mouse astrocytes and its pharmacological properties.
A, the time courses of changes in the bulk extracellular concentration of glutamate released from astrocytes in the absence (Control) and presence (+BK) of 1 μm bradykinin (BK). B, pharmacological characterisation of glutamate release from astrocytes stimulated with 1 μm BK for 5 min. *P < 0.05. The drugs used are: 5 μm[des-Arg10]-HOE140 plus 5 μm HOE140 as BKR antagonists, 50 μm D-AP-5 as an antagonist of the NMDA receptor, 10 μm CNQX as an antagonist of the AMPA/kainate receptor as well as 200 μm DIDS, 100 μm phloretin, 10 μm DCPIB and 50 μm Gd3+ as anion channel blockers. The effect of extracellular hypertonicity was observed by applying hypertonic Ringer solution (350 mosmol (kg H2O)−1).
The bradykinin-induced glutamate release was markedly suppressed by the combined application of B1 and B2 receptor antagonists (5 μm[des-Arg10]-HOE140 and 5 μm HOE140), but was not affected by glutamate receptor antagonists (50 μm D-AP-5 and 10 μm CNQX: Fig. 2B). Application of the B2 receptor antagonist (5 μm HOE140) alone also effectively suppressed the bradykinin-induced glutamate release (data not shown). This release of glutamate was markedly inhibited by 200 μm DIDS and almost completely eliminated by 100 μm phloretin (Fig. 2B). Cell shrinkage induced by a hypertonic challenge (350 mosmol (kg H2O)−1) significantly suppressed bradykinin-induced glutamate release without affecting the basal release of glutamate in the absence of bradykinin (Fig. 2B). DCPIB (10 μm), a specific blocker of VSOR (Decher et al. 2001), also significantly suppressed bradykinin-induced glutamate release (Fig. 2B). However, gadolinium (Gd3+ 50 μm), a non-specific blocker of the maxi-anion channel (Sabirov et al. 2001; Liu et al. 2006), failed to affect glutamate release (Fig. 2B). These results suggest that VSOR, but not the maxi-anion channel, serves as the pathway for release of anionic glutamate from astrocytes stimulated with bradykinin.
Bradykinin activated VSOR currents in mouse astrocytes without inducing detectable cell swelling
We then performed whole-cell patch-clamp experiments in mouse astrocytes in order to examine whether VSOR currents are actually activated by bradykinin. Mouse cortical neurons never responded to bradykinin (1 μm) with activation of whole-cell anion currents (n= 12; data not shown). In contrast, stimulation of mouse astrocytes with bradykinin (1 μm) induced activation of whole-cell anion currents (Fig. 3A and B). The current response was eliminated by the combined application of B1 and B2 receptor antagonists (5 μm[des-Arg10]-HOE140 and 5 μm HOE140) or using the B2 receptor antagonist alone (Fig. 3C and D and data not shown). When the intracellular Cl− concentration was reduced from 131 to 31 mm by replacing CsCl with caesium glutamate in the pipette solution, the reversal potential was shifted to a negative potential of −28.6 ± 1.1 mV (n= 6), indicating that the bradykinin-induced current exhibited a significant permeability to glutamate (Pglutamate/PCl= 0.17 ± 0.02), albeit less than that of Cl−. The bradykinin-induced Cl− current exhibited inactivation kinetics at large positive voltages (Fig. 3Aa) and outward rectification (Fig. 3B).
Figure 3. Bradykinin-induced anion currents in mouse astrocytes.
A, representative whole-cell anion currents activated by 1 μm bradykinin (BK) before and during application of 100 μm phloretin. Alternating step pulses of ±40 mV (0.5 s duration, every 5 s) or step pulses from −100 to +100 mV in 20 mV increments with a pre-pulse (0.1 s duration) to −100 mV and a post-pulse (0.1 s duration) to −100 mV (at a and b) were applied from a holding potential of 0 mV. a and b, expanded traces of current responses to step pulses before (a) and after (b) application of phloretin. Arrowheads: the zero-current level. B, instantaneous current–voltage relationships measured during BK application in the absence (Control, open circles) and presence of 100 μm phloretin (filled circles) or 10 μm DCPIB (filled squares). Instantaneous currents were measured 25–30 ms after the onset of the test pulses. The current density was evaluated by dividing the current by the cell capacitance (59.2 ± 3.8 pF, n= 33). Phloretin and DCPIB significantly inhibited bradykinin-activated anion currents at all the voltages tested (except for 0 mV). C and D, sensitivity of bradykinin-induced currents recorded at +100 mV (C) and −100 mV (D) to bradykinin receptor antagonists, anion channel blockers (phloretin, DCPIB and DIDS), cytosolic ATP removal (>15 min equilibration with ATP-free pipette solution) and osmotic cell shrinkage induced by a hypertonic challenge (350 mosmol (kg H2O)−1, P < 0.001), but not to a maxi-anion channel blocker (gadolinium, P > 0.1). The percentage inhibition was calculated from the bradykinin-induced peak currents recorded at ±100 mV from astrocytes under control (isotonic, ATP-containing, and blocker-free) conditions (n= 5) and the cells under test conditions. The concentrations of drugs are the same as those in Fig. 2.
The pharmacological profile of the bradykinin-induced Cl− current was characterized using a variety of Cl− channel blockers. Phloretin (100 μm) almost completely inhibited the current and block was voltage independent (Fig. 3). By contrast, DCPIB (10 μm) and DIDS (200 μm) caused a weakly voltage-dependent block of the bradykinin-induced Cl− current that was strongest at positive voltages (Fig. 3B, C and D). Figure 1 of the online supplemental material demonstrates that DCPIB (10 μm) blocked VSOR currents in mouse astrocytes activated by hypotonicity to a similar extent (VSOR, ∼85% at +100 mV; bradykinin-induced Cl− current, 89% at +100 mV). When astrocytes were equilibrated with an ATP-free intracellular (pipette) solution for over 15 min, bradykinin failed to activate the anion current (Fig. 3C and D). The current response to bradykinin was markedly inhibited by osmotic cell shrinkage induced by exposure to a hypertonic solution, but was insensitive to gadolinium (Gd3+ 50 μm), a blocker of the maxi-anion channel which is also known to be activated by cell swelling and to be permeable to anionic glutamate in mouse astrocytes (Liu et al. 2006). These current properties are identical to those of VSOR currents in mouse astrocytes (Liu et al. 2006) and in many other cell types (Strange et al. 1996; Nilius et al. 1997; Okada, 1997).
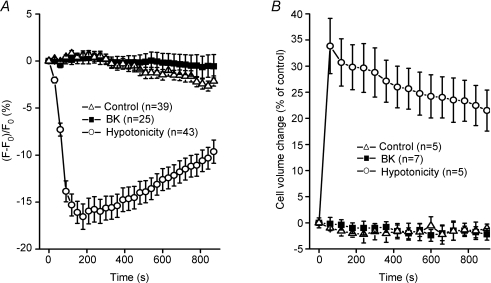
The most effective stimulus for activating the VSOR current is cell swelling (Okada, 1997). Kimelberg et al. (1990) suggested that swelling-induced glutamate release from astrocytes is mediated by VSOR. Thus, we examined whether the bradykinin-induced current activation is associated with cell swelling in cultured mouse astrocytes. When the size of mouse astrocytes was monitored by observing the dilution of cytosolic calcein fluorescence, cells attached to coverslips never responded to bradykinin by swelling (Fig. 4A, squares), whereas a hypotonic challenge induced osmotic cell swelling (Fig. 4A, circles). The mean cell volume measurements using a Coulter-type cell volume analyser showed that, while isolated mouse astrocytes in suspension responded to a hypotonic challenge (210 mosmol (kg H2O)−1) with rapid swelling followed by a slow regulatory volume decrease (RVD) (Fig. 4B, circles), they never underwent a significant volume change in response to stimulation with 1 μm bradykinin under isotonic conditions (Fig. 4B, squares). Consistent with these data, bradykinin induced no change in the cell volume of rat astrocytes (Parpura et al. 1995b).
Figure 4. Lack of cell volume change in mouse astrocytes after stimulation with bradykinin.
Effects of stimulation with 1 μm bradykinin (BK) and a hypotonic challenge (Hypotonicity, 210 mosmol (kg H2O)−1) on cell volume monitored by changes in the intracellular concentration of calcein loaded in astrocytes adhered to coverslips (A) or monitored with a Coulter-type cell size analyser in isolated astrocytes in suspension (B).
ROS production is involved in bradykinin-induced mouse astrocyte responses and astrocyte–neuron signalling
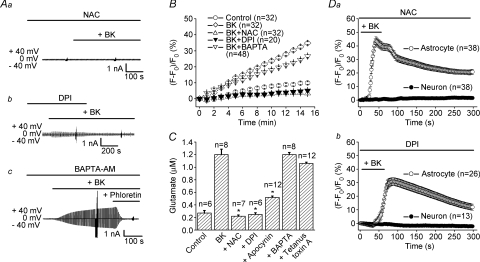
Activation of the B2 receptor induces the production and release of ROS in the brain (Rosenblum, 1987; Sobey et al. 1997). Moreover, ROS activates VSOR in a number of cell types (Browe & Baumgarten, 2004; Shimizu et al. 2004; Varela et al. 2004) without inducing cell swelling (Shimizu et al. 2004). Therefore, we tested the possibility that bradykinin-induced activation of VSOR current in mouse astrocytes involves ROS. First, a ROS scavenger, N-acetylcysteine (NAC, 10 mm), applied continuously after 5 min pretreatment, completely abolished the bradykinin-induced activation of whole-cell VSOR current (Fig. 5Aa). Second, a general inhibitor of NAD(P)H oxidases (NOXs), diphenylene-iodonium chloride (DPI, 10 μm), similarly applied after 10 min pretreatment, suppressed the bradykinin-induced activation of VSOR current (Fig. 5Ab). However, a Ca2+ chelator, BAPTA-AM (50 μm), failed to prevent the activation of the phloretin-sensitive anion current, even when it was continuously present in the bath solution during and after 30 min pretreatment (Fig. 5Ac). These data suggest that in mouse astrocytes bradykinin activates VSOR through generation of ROS by NAD(P)H oxidases. Indeed, when we investigated the intracellular level of ROS in mouse astrocytes using a membrane-permeant fluorescent probe, carboxy-H2DCFDA, we found that stimulation with bradykinin increased ROS levels and that, while this increase was highly sensitive to NAC and DPI, it was much less sensitive to BAPTA-AM (Fig. 5B).
Figure 5. Role of ROS production by mouse astrocytes in bradykinin-induced anion currents, glutamate release and astrocyte–neuron signalling.
A, effects of application (at horizontal bars) of a ROS scavenger (10 mm NAC, after 5 min pretreatment) (a), an NAD(P)H oxidase inhibitor (10 μm DPI, after 10 min pretreatment) (b), and a Ca2+ chelator (50 μm BAPTA-AM, after 30 min pretreatment) (c) on anion currents before and during application of 1 μm bradykinin (BK). Each trace is representative of 4 or 5 whole-cell experiments made as in Fig. 3A. B, effects of pretreatment with NAC (10 mm, 5 min), DPI (10 μm, 10 min) and BAPTA-AM (50 μm, 30 min) on BK-induced ROS production. The level of intracellular ROS was monitored by DCF fluorescence. NAC and DPI significantly inhibited this increase observed ≥2 min after stimulation, but BAPTA-AM never inhibited this increase observed ≤6 min after stimulation. C, effects of pretreatment with NAC (10 mm, 5 min), DPI (10 μm, 10 min), BAPTA-AM (50 μm, 30 min), apocynin (2 mm, 10 min) and tetanus toxin A (1 μg ml−1 for 24 h) on BK-induced glutamate release. *P < 0.05. D, effects of application (at horizontal bars) of 10 mm NAC after 5 min pretreatment (a) or 10 μm DPI after 10 min pretreatment (b) on BK-induced Ca2+ responses in astrocytes (open circles) and neurons (filled circles).
Bradykinin-induced glutamate release from mouse astrocytes was also blocked by pretreatment with the ROS scavenger NAC (10 mm) or the NOX inhibitor DPI (10 μm), as summarized in Fig. 5C. Another NOX inhibitor, apocynin (2 mm), also suppressed strongly the bradykinin-induced glutamate release (Fig. 5C). By contrast, pretreatment with BAPTA-AM failed to affect glutamate release (Fig. 5C), although this manoeuvre completely eliminated the intracellular Ca2+ rise in response to stimulation with bradykinin in mouse astrocytes (n= 26; data not shown). These results suggest that a Ca2+-independent and ROS-dependent mechanism underlies the bradykinin-induced glutamate release from mouse astrocytes. We also tested the effect of a Clostridial neurotoxin, tetanus toxin A, because bradykinin-induced glutamate release from rat astrocytes was reported to be mediated by vesicular exocytosis (Jeftinija et al. 1996, 1997) and because mouse astrocytes express the toxin target protein, synaptobrevin II (Zhang et al. 2004b). However, pretreatment of mouse astrocytes with tetanus toxin A (1 μg ml–1 for 24 h) failed to suppress significantly glutamate release (Fig. 5C).
Finally, in the co-culture system, NAC (10 mm) eliminated the bradykinin-induced Ca2+ responses in neurons without affecting those in astrocytes (Fig. 5Da). Similarly, DPI (10 μm) selectively abolished neuronal Ca2+ responses to stimulation with bradykinin, without preventing those of astrocytes (Fig. 5Db). As a control, we tested the effects of DPI (10 μm) pre-treatment on the Ca2+ response of neurons to exogenous glutamate (10 μm) and found that the NOX inhibitor was without effect (n= 31; data not shown). Thus, these data suggest that bradykinin-induced Ca2+ signal transmission from astrocytes to neighbouring neurons is mediated by ROS.
Discussion
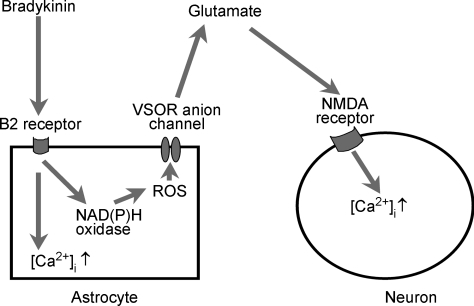
Taking all the present results together, we conclude that, in mouse astrocytes, the release of glutamate involved in bradykinin-induced astrocyte-to-neuron signalling occurs via anion channels of the VSOR-type; furthermore, these channels are activated by ROS generated upon stimulation of bradykinin B2 receptors, as summarized schematically in Fig. 6. In this regard, Mongin and collaborators have recently demonstrated that activation of rat microglia with zymosan promotes release of excitatory amino acids (monitored by aspartate efflux) via VSOR in a manner dependent on ROS (Harrigan et al. 2008).
Figure 6. Schematic illustration of the mechanisms of bradykinin-induced astrocyte-to-neuron signalling.
See text for details.
Bradykinin, a nine-amino-acid peptide, is generated in plasma and tissues from kininogens by the action of kallikrein and represents an initial mediator of inflammation which induces pain and vascular permeability (Bhoola et al. 1992). The actions of bradykinin are mediated by the G-protein-coupled receptors that are classified into constitutive B2 and inducible B1 receptor subtypes (Ding-Zhou et al. 2003; Leeb-Lundberg et al. 2005). In the central nervous system, bradykinin is known to be rapidly released after brain trauma and stroke (Francel, 1992; Gröger et al. 2005). Bradykinin receptors are expressed in both brain neurons (Murone et al. 1997; Raidoo & Bhoola, 1997; Chen et al. 2000; Mahabeer et al. 2000) and astrocytes (Cholewinski et al. 1991; Lin & Chuang, 1992; Hösli & Hösli 1993). Bradykinin induces glutamate release from rat astrocytes, thereby leading to an NMDA receptor-mediated Ca2+ increase in rat neurons (Parpura et al. 1994). Bradykinin-induced glutamate release is mediated by the B2 receptor in rat Schwann cells (Parpura et al. 1995c) and in rat astrocytes (Jeftinija et al. 1996). The present study provides evidence that the B2 receptor in astrocytes is involved in Ca2+ signal transmission from astrocytes to neurons during stimulation with bradykinin in mouse astrocyte–neuron co-cultures.
It has been shown that upon stimulation with bradykinin, astrocytes exhibit a variety of responses including increased cytosolic Ca2+ (Gimpl et al. 1992; Stephens et al. 1993; Parpura et al. 1994; Ikeda et al. 2000; Wang et al. 2007), release of glutamate (Parpura et al. 1994; Jeftinija et al. 1996, 1997), release of PGE2 and NGF (Noda et al. 2007), depolarization (Hösli et al. 1992), and increased membrane currents (Gimpl et al. 1992). Here, we demonstrated, for the first time, that bradykinin activates anion channel currents in mouse astrocytes. Although mouse astrocytes stimulated with bradykinin did not undergo detectable cell swelling, for the following reasons, we conclude that the bradykinin-activated anion channel is of the VSOR-type. First, bradykinin-activated anion currents and VSOR currents of mouse astrocytes share biophysical properties (e.g. outward rectification, inactivation kinetics at large positive voltages and glutamate permeability (bradykinin-activated anion currents, Pglutamate/PCl= 0.17; swelling-activated VSOR currents, Pglutamate/PCl= 0.15; Liu et al. 2006)). Second, both bradykinin-activated anion currents and swelling-activated VSOR currents in mouse astrocytes exhibited the same physiological properties such as dependence on intracellular ATP and sensitivity to osmotic shrinkage. Third, the pharmacological profiles of both currents were essentially identical.
VSOR is principally activated by osmotic cell swelling (Strange et al. 1996; Okada, 1997; Nilius et al. 1997). However, recently, ROS has been demonstrated to activate VSOR without eliciting cell swelling (Browe & Baumgarten, 2004; Shimizu et al. 2004; Varela et al. 2004), although ROS-activated VSOR currents are sensitive to osmotic shrinkage (Shimizu et al. 2004). Here, we demonstrated that B2 receptor-mediated activation of VSOR is induced by ROS generated by NAD(P)H oxidases in mouse astrocytes in the absence of detectable cell swelling and that the bradykinin-activated VSOR current is sensitive to osmotic cell shrinkage. Stimulation of B2 receptors that activate downstream Gαq/PLCβ is known to increase the intracellular Ca2+ concentration in rat astrocytes (Stephens et al. 1993; Ikeda et al. 2000). In agreement with these data, in the present study, bradykinin was found to increase intracellular Ca2+ in mouse astrocytes in a manner sensitive to a B2 receptor antagonist. However, it was found that the Ca2+ signalling pathway downstream of astrocytic B2 receptor activation was independent of bradykinin-induced ROS production and VSOR activation (see Fig. 6), because the bradykinin-induced ROS increase was not inhibited by pretreatment with BAPTA-AM, and vice versa, the astrocytic Ca2+ responses to bradykinin were not prevented by a ROS scavenger.
The pathway for bradykinin-induced glutamate release from astrocytes has been classified into two types: Ca2+-dependent release via vesicular exocytosis and Ca2+-independent release via transporters/channels. The glutamate-releasing channels may include gap junction hemi-channels, P2X7 receptor cation channels, and anion channels. Since ATP release was not induced by stimulation with bradykinin in the present study, P2X7 receptors are not likely to be involved in the glutamate release. An involvement of gap junction hemi-channels, which are known to be sensitive to Gd3+ but not to DIDS (Eskandari et al. 2002), may also be ruled out, because bradykinin-induced glutamate release was insensitive to Gd3+ but sensitive to DIDS. The present study provides the following compelling evidence for the involvement of VSOR in the pathway for bradykinin-induced glutamate release. First, the bradykinin-activated VSOR exhibited sizable permeability to glutamate. Second, both bradykinin-induced glutamate release and VSOR currents share sensitivity to anion channel blockers. Third, bradykinin-induced glutamate release was inhibited by hypertonicity, which could also inhibit the bradykinin-induced VSOR activity. Fourth, both bradykinin-induced glutamate release and VSOR currents were inhibited by pretreatment with a ROS scavenger (NAC) or a NOX inhibitor (DPI) but were not essentially affected by pretreatment with BAPTA-AM in mouse astrocytes.
Bradykinin is known to induce glutamate release from rat astrocytes and thereby cause an intracellular Ca2+ rise in adjacent neurons (Parpura et al. 1994). In the present study, we demonstrated that this is also the case in mouse astrocyte–neuron co-cultures. In contrast to rat astrocytes, however, we conclude that when stimulated with bradykinin, mouse astrocytes release glutamate via VSOR, not by exocytosis. We speculate that a species difference is likely to account for the different mechanisms of glutamate release by mouse and rat astrocytes.
Both bradykinin-induced activation of VSOR currents and glutamate release started within 1 min and gradually increased over several minutes after bradykinin application. However, the onset of the neuronal Ca2+ response occurred earlier (within 30–40 s). Thus, it is possible that the concentration of released glutamate in the vicinity of the neuronal membrane was much higher than the concentration measured in the bulk solution and rapidly reached the level sufficient for activation of neuronal NMDA receptors. It is also possible that the actual time course of bradykinin-induced VSOR activation in intact mouse astrocytes is different from that in the cells subjected to washout of cytosolic constituents under the whole-cell configuration.
In summary, the present study has clarified the mechanisms of bradykinin-induced astrocyte-to-neuron signalling in mice as follows (see Fig. 6): bradykinin stimulates B2 receptors and brings about a ROS-independent Ca2+ rise and the Ca2+-independent generation of ROS in astrocytes. ROS then activates glutamate-permeable volume-sensitive outwardly rectifying anion channels (VSORs) without inducing swelling of astrocytes, thereby causing the release of glutamate. Finally, the glutamate released from astrocytes activates NMDA receptors, leading to an increase in the cytosolic Ca2+ concentration of neurons. The pathophysiological roles of bradykinin-induced astrocyte-to-neuron signalling in vivo are not known. However, the signals transmitted from astrocytes by the pathway described above are likely to be involved in the pro-epileptic (Mazzuferi et al. 2005), injurious (Ding-Zhou et al. 2003) and anti-apoptotic (Chao & Chao, 2006) roles of bradykinin in neurons.
Acknowledgments
We thank E. L. Lee for reading the manuscript, J. Nabekura, H. Wake and Y. Takatsuru for discussion as well as T. Okayasu for secretarial assistance. This work was supported by Grants-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology-Japan (MEXT) and Japan Society for the Promotion of Science (JSPS).
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.165084/DC1
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The Origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40:312–323. doi: 10.1002/glia.10124. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- Browe DM, Baumgarten CM. Angiotensin II (AT1) Receptors and NADPH oxidase regulate Cl− current elicited by β1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol. 2004;124:273–287. doi: 10.1085/jgp.200409040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Chao L. Experimental therapy with tissue kallikrein against cerebral ischemia. Front Biosci. 2006;11:1323–1327. doi: 10.2741/1886. [DOI] [PubMed] [Google Scholar]
- Chen EY, Emerich DF, Bartus RT, Kordower JH. B2 bradykinin receptor immunoreactivity in rat brain. J Comp Neurol. 2000;427:1–18. [PubMed] [Google Scholar]
- Chen X, Wang L, Zhou Y, Zheng L-H, Zhou Z. “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewinski AJ, Stevens G, McDermott AM, Wilkin GP. Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem. 1991;57:1456–1458. doi: 10.1111/j.1471-4159.1991.tb08314.x. [DOI] [PubMed] [Google Scholar]
- Decher N, Lang HJ, Nilius B, Brüggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of ICl,swell and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding-Zhou L, Margaill I, Palmier B, Pruneau D, Plotkine M, Marchand-Verrecchina C. LF 16-0687 Ms, a bradykinin B2 receptor antagonist, reduces ischemic brain injury in a murine model of transient focal cerebral ischemia. Br J Pharmacol. 2003;139:1539–1547. doi: 10.1038/sj.bjp.0705385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559:799–812. doi: 10.1113/jphysiol.2004.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- Fan H-T, Morishima S, Kida H, Okada Y. Phloretin differentially inhibits volume-sensitive and cyclic AMP-activated, but not Ca-activated, Cl− channels. Br J Pharmacol. 2001;133:1096–1106. doi: 10.1038/sj.bjp.0704159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem. 2006;281:4274–4284. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- Francel PC. Bradykinin and neuronal injury. J Neurotrauma. 1992;9(Suppl 1):S27–S45. [PubMed] [Google Scholar]
- Gimpl G, Walz W, Ohlemeyer C, Kettenmann H. Bradykinin receptors in cultured astrocytes from neonatal rat brain are linked to physiological responses. Neurosci Lett. 1992;144:139–142. doi: 10.1016/0304-3940(92)90735-p. [DOI] [PubMed] [Google Scholar]
- Gröger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N. Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:978–989. doi: 10.1038/sj.jcbfm.9600096. [DOI] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourďheuil D, Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hazama A, Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol. 1988;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje M, Hofmann F, Lux R, Veh RW, Just I, Ahnert-Hilger G. Glutamate uptake and release by astrocytes are enhanced by Clostridium botulinum C3 protein. J Biol Chem. 2008;283:9289–9299. doi: 10.1074/jbc.M706499200. [DOI] [PubMed] [Google Scholar]
- Hösli E, Hösli L. Autoradiographic localization of binding sites for neuropeptide Y and bradykinin on astrocytes. Neuroreport. 1993;4:159–162. doi: 10.1097/00001756-199302000-00011. [DOI] [PubMed] [Google Scholar]
- Hösli L, Hösli E, Kaeser H, Lefkovits M. Colocalization of receptors for vasoactive peptides on astrocytes of cultured rat spinal cord and brain stem: electrophysiological effects of atrial and brain natriuretic peptide, neuropeptide Y and bradykinin. Neurosci Lett. 1992;148:114–116. doi: 10.1016/0304-3940(92)90817-q. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ueno A, Naraba H, Matsuki N, Oh-Ishi S. Intracellular Ca2+ increase in neuro-2A cells and rat astrocytes following stimulation of bradykinin B2 receptor. Jpn J Pharmacol. 2000;84:140–145. doi: 10.1254/jjp.84.140. [DOI] [PubMed] [Google Scholar]
- Inoue H, Mori S, Morishima S, Okada Y. Volume-sensitive chloride channels in mouse cortical neurons: characterization and role in volume regulation. Eur J Neurosci. 2005;21:1648–1658. doi: 10.1111/j.1460-9568.2005.04006.x. [DOI] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G. Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res. 1997;750:41–47. doi: 10.1016/s0006-8993(96)00610-5. [DOI] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G, Liu F. Neuroligand-evoked calcium-dependent release of excitatory amino acids from cultured astrocytes. J Neurochem. 1996;66:676–684. doi: 10.1046/j.1471-4159.1996.66020676.x. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Lin WW, Chuang DM. Regulation of bradykinin-induced phosphoinositide turnover in cultured cerebellar astrocytes: possible role of protein kinase C. Neurochem Int. 1992;21:573–579. doi: 10.1016/0197-0186(92)90090-e. [DOI] [PubMed] [Google Scholar]
- Liu H-T. Bradykinin-induced communication between astrocytes and neurons is mediated by glutamate released via volume-sensitive outwardly rectifying anion channels. J Physiol Sci. 2007;57(Suppl.):S126. [Google Scholar]
- Liu H-T, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–357. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- Mahabeer R, Naidoo S, Raidoo DM. Detection of tissue kallikrein and kinin B1 and B2 receptor mRNAs in human brain by in situ RT-PCR. Metab Brain Dis. 2000;15:325–335. doi: 10.1023/a:1011131510491. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuferi M, Binaschi A, Rodi D, Mantovani S, Simonato M. Induction of B1 bradykinin receptors in the kindled hippocampus increases extracellular glutamate levels: A microdialysis study. Neuroscience. 2005;135:979–986. doi: 10.1016/j.neuroscience.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murone C, Paxinos G, McKinley MJ, Oldfield BJ, Muller-Esterl W, Mendelsohn FA, Chai SY. Distribution of bradykinin B2 receptors in sheep brain and spinal cord visualized by in vitro autoradiography. J Comp Neurol. 1997;381:203–218. doi: 10.1002/(sici)1096-9861(19970505)381:2<203::aid-cne7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA, Desai S, Pulsinelli WA. Acid-induced death in neurons and glia. J Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Noda M, Sasaki K, Ifuku M, Wada K. Multifunctional effects of bradykinin on glial cells in relation to potential anti-inflammatory effects. Neurochem Int. 2007;51:185–191. doi: 10.1016/j.neuint.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995a;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG. α-Latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett. 1995b;360:266–270. doi: 10.1016/0014-5793(95)00121-o. [DOI] [PubMed] [Google Scholar]
- Parpura V, Liu F, Jeftinija KV, Haydon PG, Jeftinija SD. Neuroligand-evoked calcium-dependent release of excitatory amino acids from Schwann cells. J Neurosci. 1995c;15:5831–5839. doi: 10.1523/JNEUROSCI.15-08-05831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raidoo DM, Bhoola KD. Kinin receptors on human neurones. J Neuroimmunol. 1997;77:39–44. doi: 10.1016/s0165-5728(97)00048-9. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. Hydroxyl radical mediates the endothelium-dependent relaxation produced by bradykinin in mouse cerebral arterioles. Cir Res. 1987;61:601–603. doi: 10.1161/01.res.61.4.601. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci U S A. 2004;101:6770–6773. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Heistad DD, Faraci FM. Mechanisms of bradykinin-induced cerebral vasodilation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke. 1997;28:2290–2295. doi: 10.1161/01.str.28.11.2290. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Cholewinski AJ, Wilkin GP, Djamgoz MB. Calcium-mobilizing and electrophysiological effects of bradykinin on cortical astrocyte subtypes in culture. Glia. 1993;9:269–279. doi: 10.1002/glia.440090405. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A. NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem. 2004;279:13301–13304. doi: 10.1074/jbc.C400020200. [DOI] [PubMed] [Google Scholar]
- Wang Y-B, Peng C, Liu Y-H. Low dose of bradykinin selectively increases intracellular calcium in glioma cells. J Neurol Sci. 2007;258:44–51. doi: 10.1016/j.jns.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Ye Z-C, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci U S A. 2004a;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangršič T, Kreft M, Kržan M, Li N, Sul J-Y, Halassa M, van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004b;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.