Abstract
A number of potassium channels including members of the KCNQ family and the Ca2+ activated IK and SK, but not BK, are strongly and reversibly regulated by small changes in cell volume. It has been argued that this general regulation is mediated through sensitivity to changes in membrane stretch. To test this hypothesis we have studied the regulation of KCNQ1 and BK channels after expression in Xenopus oocytes. Results from cell-attached patch clamp studies (∼50 μm2 macropatches) in oocytes expressing BK channels demonstrate that the macroscopic volume-insensitive BK current increases with increasing negative hydrostatic pressure (suction) applied to the pipette. Thus, at a pipette pressure of −5.0 ± 0.1 mmHg the increase amounted to 381 ± 146% (mean ±s.e.m., n= 6, P < 0.025). In contrast, in oocytes expressing the strongly volume-sensitive KCNQ1 channel, the current was not affected by membrane stretch. The results indicate that (1) activation of BK channels by local membrane stretch is not mimicked by membrane stress induced by cell swelling, and (2) activation of KCNQ1 channels by cell volume increase is not mediated by local tension in the cell membrane. We conclude that stretch and volume sensitivity can be considered two independent regulatory mechanisms.
Previous studies have investigated the regulation of several types of K+ channels by small changes in cell volume after expression in Xenopus oocytes or mammalian cells. In particular, it has been shown that members of the KCNQ family (KCNQ1, 4 and 5) are strictly regulated by small, fast changes in cell volume (Grunnet et al. 2003; Hougaard et al. 2004; Jensen et al. 2005). This is a newly identified regulatory mechanism which may be important in a number of water and salt transporting cells, e.g. in epithelia and the inner ear. BK channels are activated by membrane stretch in vascular smooth muscle cells and epithelial cells (Kirber et al. 1992; Mienville et al. 1996; Wu et al. 2003; Sheu et al. 2005). Some reports claim that BK channels are regulated by cell volume; however this conclusion appears to be based on the assumption that stretch-activated channels should be expected to be regulated by changes in cell volume (Davidson, 1993; Gasull et al. 2003). Further, Grunnet et al. (2002) found that the Ca2+- and voltage-activated BK potassium channel does not respond to volume changes when expressed in Xenopus oocytes. The mechanisms linking mechanical stimuli to the activation of mechanosensitive ion channels are still not clearly defined. Possibilities include dilution of an intracellular messenger and subsequent signal amplification by a signalling cascade, or direct or indirect mechanical activation by changes in tension of the plasma membrane and the underlying cytoskeleton. Implicitly it has been assumed that cell swelling is associated with parallel changes in membrane stretch, and that cell swelling and membrane stretch constitute a common mechanism in the regulation of ion channels. Therefore cell swelling experiments have frequently been used as surrogate model for membrane stretch. The experiments in the present study were designed to clarify whether regulation of K+ channels by small changes in cell volume and by membrane stretch are independent regulatory mechanisms. The approach is simple: two types of channels were employed in a series of experiments, one which is known to be regulated by cell volume (the KCNQ1 and KCNQ4 channels) and one, which is known to be regulated by membrane stretch (the BK channel). Experiments were conducted to determine whether the volume-sensitive channel can be regulated by membrane stretch, and if the stretch-sensitive channel can be regulated by changes in cell volume.
Methods
Mammalian expression system
HEK 293 cells stably expressing human KCNQ4 or human BK were kindly provided by NeuroSearch (Ballerup, Denmark). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)–high glucose Glutamax (31966, Gibco-Invitrogen) supplemented with 10% fetal calf serum and 100 U ml−1 penicillin–100 μg ml−1 streptomycin at 37°C and 5% CO2. Patch clamp whole-cell experiments were performed on a 16-channel silicon chip-based automated patch clamp system, the QPatch 16 (Sophion Bioscience, Ballerup, Denmark). For further details on the operation of the QPatch 16 system, see Willumsen (2006). After splitting, the cells were kept in culture medium in a stirred storage tank on the QPatch platform. Prior to each experiment cells were automatically washed and transferred to the disposable QPlate which comprises all 16 patch clamp sites. The intracellular Ringer solution for KCNQ4 studies contained (in mm): 2 NaCl, 8 KCl, 108 potassium gluconate, 1.2 MgCl2, 10 Hepes, 0.1 EGTA, 1.5 Na2ATP, pH 7.4. The osmolarity was adjusted to 295 mosmol l−1 using d-mannitol. The intracellular Ringer solution for BK studies contained (in mm): 5.17 CaCl2, 1.42 MgCl2, 30/10 KOH/EGTA, 110 KCl, 10 Hepes, pH 7.2 and adjusted to 295 mosmol l−1 using d-mannitol. The free Ca2+ concentration was calculated to 210 nm using the WinMaxChelator software (http://www.stanford.edu/~cpatton/maxc.html). The extracellular hypoosmotic (200 mosmol l−1) Ringer solution contained: 1 NaCl, 5 KCl, 90 sodium gluconate, 1 MgCl2, 1 CaCl2, 10 Hepes, pH 7.4. Isoosmotic (300 mosmol l−1) and hyperosmotic (400 mosmol l−1) solutions were made by addition of d-mannitol.
Expression in Xenopus laevis oocytes
Xenopus laevis oocytes were isolated and prepared as previously described (Grunnet et al. 2002). Sequences of hKCNQ1 (NM-000218) were cloned into a pXOOM vector (Jespersen et al. 2002) and hBK (NM-004137) into a pCDNA3.1 vector and linearized with XbaI and Not1, respectively (New England Biolabs, Ipswich, MA, USA). Synthetic RNA was prepared by in vitro transcription (mCAP RNA Capping Kit from Stratagene) from linearized DNA templates. RNA was extracted by phenol–chloroform, ethanol precipitated and dissolved in RNase-free TE buffer (10 mm Tris-HCl, 1 mm EDTA, pH 7.5). Fifty nanolitres of mRNA was injected into oocytes which were then kept in Kulori medium (in mm: 90 NaCl, 1 KCl, 1 MgCl2, 1 CaCl2, 5 Hepes-Tris, pH 7.4) at 19°C. Prior to patch clamp measurements, the vitelline membrane, surrounding the Xenopus oocyte, was manually removed with a pair of forceps to get access to the plasma membrane. Macroscopic currents from KCNQ1-injected oocytes were obtained in cell-attached macropatches using patch pipettes with tip diameters of 7–10 μm and resistances ranging between 350 and 800 kΩ. Pipettes with lower tip diameter and higher resistance were used (0.8–1.5 MΩ) for recording macroscopic currents from BK-injected oocytes. These pipettes were fabricated from borosilicate capillary tubes (Vitrex, cat. no. 1395; Modulohm A/S, Herlev, Denmark) on a vertical puller (Hans Ochotzki, Homburg, Germany). Pipettes were filled with extracellular solution (Kulori medium). Conventional patch clamp experiments were performed with an Axopatch 200B amplifier (Molecular devices, Sunnyvale, CA, USA) interfaced to a Digidata 1440A digitizer (Molecular Devices). The amplified and filtered signals were sampled and stored on a computer. An external low-pass 8-pole Bessel filter (LP900, Frequency Devices, Ottawa, IL, USA) was also used. KCNQ1 and KCNQ4 currents were sampled at 2.5 kHz and filtered at 1 kHz. BK currents were sampled at 40 kHz and filtered at 5 kHz. Stretching of the membrane patch was elicited by applying negative pressure with a syringe to the back end of the patch electrode holder at the cell-attached patch configuration. Pipette pressure was monitored with a pressure manometer (Eirelec, Dundalk, Ireland). Data acquisition and analysis were performed with Clampex 10 and Clampfit 10 (Molecular Devices) software programs, respectively. OriginPro 7.5 (OriginLab Corp., Northampton, MA, USA) was used for fitting and preparing graphical displays.
Statistics
Unless stated otherwise, data are presented as means ±s.e.m. (for n observations). Comparisons are made by using Student's t-test. Statistical differences were considered significant when P < 0.05.
Results
Effect of changes in cell volume on KCNQ4 and BK channels
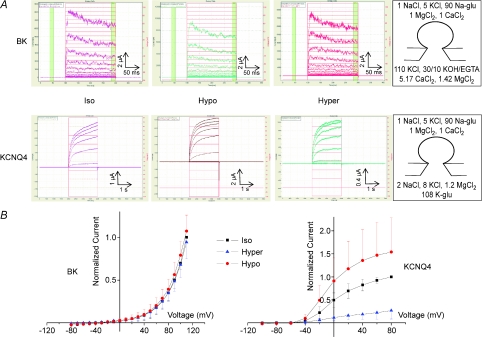
Previous studies have demonstrated that KCNQ1 and KCNQ4 channels co-expressed with AQP1 in Xenopus oocytes are regulated, in a similar way, by small changes in cell volume (5–10%), whereas BK channels are not (Grunnet et al. 2002, 2003). We tested whether the volume sensitivity of KCNQ channels and the volume insensitivity of BK channels are inherent properties, or if they are dependent on the employed expression system. For this purpose HEK 293 cells stably expressing KCNQ4 and BK were available to us. Figure 1 demonstrates that when expressed in HEK 293 cells, KCNQ4 and BK respond to osmotically induced cell swelling and shrinkage in the same way as when expressed in Xenopus oocytes: (i) hypoosmotic cell swelling activated KCNQ4 channels inducing current increases at all potentials whereas hyperosmotic cell shrinkage inhibited the KCNQ4 current; and (ii) BK currents were not affected by osmotic challenges.
Figure 1. KCNQ4 channels, but not BK channels, are sensitive to osmotically induced challenges.
A, original QPatch I–t traces showing the effects of changes in extracellular osmolarity on BK and KCNQ4 currents recorded from QPatch experiments on HEK 293 cells (whole-cell configuration). KCNQ4 and BK channels were activated by a step-protocol under iso-, hypo- and hyperosmotic conditions. For BK, from a holding potential of −80 mV, steps of 10 mV (200 ms duration) were applied from −80 mV to 110 mV. For KCNQ4, from a holding potential of −80 mV, steps of 20 mV (2 s duration) were applied from −100 mV to 80 mV. Zero current is indicated by the horizontal line. Ion composition on the cis and trans sides is given in mm (rightmost panels). B, effects of changes in extracellular osmolarity for all experiments on KCNQ4 (n= 12) and BK (n= 10) current–voltage relationship recorded in QPatch experiments on HEK 293 cells. Currents were normalized relative to maximal steady-state currents under isosmotic conditions. The data are means (±s.d.) currents.
Effect of membrane stretch on KCNQ1 and BK channels
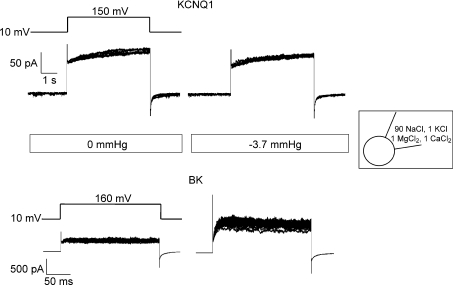
To examine whether the volume-sensitive KCNQ1 channel and the volume-insensitive BK channels are sensitive to membrane stretch, Xenopus oocytes were injected with mRNA encoding the respective ion channel proteins. Channel currents were recorded before and after application of negative pressure during repetitive depolarizing pulses at cell-attached patch configuration. Figure 2 shows superimposed current traces from KCNQ1 and BK channels under control conditions (0 mmHg pipette pressure) and under application of local membrane stretch (−3.7 mmHg pipette pressure), respectively. BK currents increased to 280 ± 9% (25 sweeps) at −3.7 mmHg relative to control (100%, at 0 mmHg) as reported previously (see Introduction). In contrast, KCNQ1 currents were not significantly affected, 92 ± 4% (5 sweeps) relative to control.
Figure 2. Example of current traces for KCNQ1 (top) and BK (bottom) channel in cell-attached macropatches from Xenopus oocytes exposed to 0 and to −3.7 mmHg pipette pressure.
KCNQ1 and BK currents were recorded at repeated depolarization pulses (5 and 25 sweeps, respectively). Currents were elicited by the voltage protocol shown above the traces. KCNQ1 were depolarized for 5 s and BK for 200 ms. Inset indicates pipette solution composition.
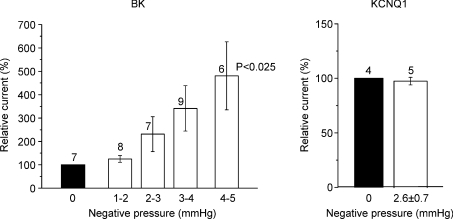
Figure 3 collects the effect of increasing negative pipette pressures on KCNQ1 and BK channel currents from all experiments. Negative pressures of up to 5 mm Hg could be applied in the BK experiments whereas only up to ∼3.7 mmHg could be applied in the KCNQ1 experiments. This is due to the fact that the large macropatches used in the KCNQ1 experiments were very fragile compared to the conventional patches employed for the BK experiments. Furthermore, the negative pressure needed to be maintained for much longer times in the KCNQ1 experiments due to the long depolarization pulses used in the KCNQ1 experiments (5 s) as compared to the BK experiments (200 ms). This made pressure pulse application to KCNQ1 channels a challenging task. It is seen from Fig. 3 that KCNQ1 currents were not affected by 2.6 ± 0.7 mmHg negative pressure whereas BK currents increased up to 381 ± 146% (mean ±s.e.m., n= 6, P < 0.025) corresponding to an ∼5-fold increase at 4–5 mmHg negative pressures.
Figure 3. Effect of increasing negative pipette pressure on cell-attached macropatches containing BK or KCNQ1 channels for all experiments.
BK and KCNQ1 data are mean (±s.e.m.) currents measured at different pipette pressures compared to control (100%, 0 mmHg). For KCNQ1, a pressure of 2.6 ± 0.7 mmHg represents an average pressure of five experiments exposed to a pressure interval between 1.8 and 3.7 mmHg.
Discussion
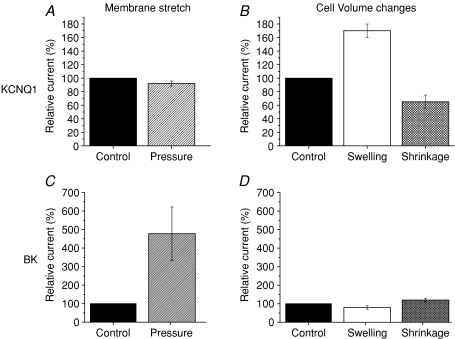
In the present study, the effect of membrane stretch on KCNQ1 and BK macroscopic currents was examined. Local membrane stretch was induced by application of suction, i.e. negative hydrostatic pressure to the pipette. Depolarization-activated BK currents increased at negative pipette pressures whereas depolarization-activated KCNQ1 currents were not affected. These results combined with results from Grunnet et al. (2002, 2003) summarized in Fig. 4 show that the volume regulated KCNQ1 channel is not affected by membrane stretch, when expressed in Xenopus oocytes (Fig. 4A and B). In contrast, BK channels are stretch activated, but insensitive to cell volume changes (Fig. 4C and D). The volume sensitivity of KCNQ channels and the volume insensitivity of BK channels were tested in a human expression system (HEK 293) and it was found that the regulatory properties of the two channels were similar to those observed in Xenopus oocytes (Fig. 1). These observations point out that regulation by cell volume changes is not a phenomenon restricted to expression systems, but most likely should be considered as a general regulatory mechanism. The results of the present study demonstrate that (i) activation of KCNQ channels in response to cell swelling is not dependent on membrane stretch, and (ii) the stretch-activated BK channel does not react on cell swelling. Based on this finding, it is concluded that cell volume changes and membrane stretch basically constitute independent regulatory signals for the activity of ion channels.
Figure 4. Membrane stretch vs. cell volume changes.
Previous studies (B and D, see Grunnet et al. 2002, 2003) on the effect of cell volume changes performed in Xenopus oocytes expressing either KCNQ1 or BK channels showed that KCNQ1 channels are sensitive to small changes in cell volume: the relative current increased by cell swelling (70%) and decreased after cell shrinkage (40%). In contrast, cell volume changes had no significant effect on BK currents. The present study (A and C), demonstrated that the volume-sensitive KCNQ1 channels are not affected by membrane stretch, whereas the volume insensitive BK channels are highly stretch-activated; the BK current increased by 480% in response to pressure (−5.0 ± 0.3 mmHg).
Membrane stretch insensitivity and volume sensitivity of KCNQ1 channels
Volume sensitivity of homomeric expressed KCNQ1 channels has not only been demonstrated in Xenopus oocytes but also in COS cells (Kubota et al. 2002), guinea pig cells (Rees et al. 1995; Sasaki et al. 1994) and canine ventricular myocytes (Zhou et al. 1997). Moreover KCNQ1 has also been shown to contribute to the regulatory volume decrease response in cardiomyocytes (Vandenberg et al. 1996; Calloe et al. 2007) and airway epithelial cells (Lock & Valverde, 2000). In the present study, we demonstrated that the KCNQ1 volume responsiveness is not mediated through membrane stretch as direct application of negative pipette pressure had no effect on KCNQ1 currents (Fig. 2). Moreover, the precise sensing of minute changes in cell volume (∼10% corresponding to a hardly visually detectable ∼3% increase in cell diameter) demonstrated by Grunnet et al. (2003) are far less than what is needed for eliciting physical changes of the membrane such as unfolding of villi and membrane folds. This further demonstrates that the volume responsiveness is not membrane mediated per se, but rather induced by a cell volume related effect on an intracellular messenger cascade that leads to altered activity of the channels.
Membrane stretch sensitivity and cell volume insensitivity of BK channels
In the present study BK channels expressed in Xenopus oocytes were activated by depolarization at intracellular levels of Ca2+. The mechano-sensitivity of BK channels is independent of Ca2+, and in agreement with previous studies, the BK channels responded directly to membrane stretch (Barret et al. 1982; Kirber et al. 1992; Mienville et al. 1996; Wu et al. 2003; Sheu et al. 2005). In these studies, activation of BK channels by membrane stretch appeared to be a direct effect on the channel itself or some closely associated component, and not secondary to e.g. an elevation of internal Ca2+ caused by influx of Ca2+ through stretch-activated channels in the plasma membrane or by depletion of intracellular stores. Unlike KCNQ1 channels, BK channels are not activated by small increases in oocyte volume (∼8%, Grunnet et al. 2002). If BK channels act as a ‘biosensor of cell membrane stretch’ and if swelling stretches the membrane, then a BK response should have been elicited. However, even substantial volume changes, caused by 33% hyper- or hypoosmolarity, had no significant effect on BK channel activity in HEK293 cells (Fig. 1), indicating that the volume change did not cause increased local membrane tension or stretch comparable to that induced by pipette suction. These observations suggest that membrane stretch and cell volume increase activate the channels by separate mechanisms, and that ion channel activation by cell swelling is not a result of membrane stretch or tension, but dependent on alternative mechanisms.
Membrane stretch versus cell volume changes
Implicitly, it has been assumed that cell volume changes affect the tension within the membrane and its associated cytoskeleton, and that this mechanism somehow is involved in the activation and inactivation of mechanosensitive ion channels (Pedersen et al. 2001). However, when exposed to hypoosmotic stress, cells are able to avoid build-up of substantial internal pressures by different strategies, either by reducing the pressure gradient by diluting the internal solutes when swelling, or by unfolding membrane invaginations and adding new membranes from stores. For example the oocyte is not a smooth sphere. Instead the surface contains folds and microvilli that approximately double the total membrane area (Zampighi et al. 1995). Consequently, smoothening out all membrane invaginations would increase the diameter of the oocyte by approximately 40%. However, in our experiments the volume of the oocytes increases by about 10%, corresponding to an increase in diameter of approximately 3%. For a reduction of extracellular osmolarity of e.g. 100 mm, Van't Hoff's law predict a pressure increase of close to 2000 mmHg if the oocyte membrane were stiff. However, such a pressure is obviously not sensed by the oocyte, but instead, the volume is increased. To eliminate any pressure build-up the oocyte volume should increase by 50% assuming that no solutes are leaving the cell. Therefore, the tension must be partly dissipated within the cytoskeleton so that any measurable tension increase in the plasma membrane is avoided preventing lysis (Spagnoli et al. 2008). Thus, most likely, hypotonicity of the medium causes an increased tension in cytoskeletal elements protecting the membrane bilayer from being markedly stretched and consequently protects the oocyte from activation of stretch-activated ion channels. This is supposedly a general property of all types of cells.
Possible molecular sensors of membrane stretch
The membrane or membrane-associated component that transmits forces to the channel and the part of the channel that senses forces remain unknown. However, recent studies have shown that deletion of the cytoplasmic c-terminus, the so called stress-axis regulated exon (STREX), of BK channels cloned from chick hearts, abolishes stretch-sensitivity of the channel (Qi et al. 2005). This finding suggests that STREX constitutes a part of the mechano-sensing apparatus of the channel. STREX may not directly sense the stress in the membrane as it is facing the cytoplasm and is not interacting directly with the plasma membrane. Whether there is a specific mechano-sensing motif for the BK variant (Zero) which was used in our study needs to be further investigated.
Perspectives
The present study specifically relates to KCNQ and BK potassium channels. The key finding is that membrane stretch and cell volume changes constitute independent ion channel regulators. This attribute may be a general property relating to all mechanosensitive ion channels. Whether this is so can only be addressed in detailed studies of the mechanosensitive properties of a number of stretch and/or volume regulated ion channels. Future investigations are also required to determine whether ion channels are regulated by membrane stretch and tension per se, i.e. without any involvement of other cellular components, or whether cytoplasmic or cytoskeletal components are involved. Testing this could involve studies in which reconstituted purified channel proteins are introduced in artificial lipid bilayers or pure liposomes leaving the mechanosensitive ion channels devoid of any cellular or structural component. Application of membrane stretch to naked lipid bilayer could indicate whether the imposed tension alone gates mechanosensitive ion channels (Sukharev et al. 1993).
Acknowledgments
The authors thank Sophion Bioscience A/S for use of the QPatch16 system. Ms Z. Rasmussen and T. Soland are thanked for expert technical assistance. This work has been supported by grants from the Danish Natural Science Foundation (FNU), the Novo Nordisk Foundation, the Carlsberg Foundation, Fonden til Lægevidenskabens Fremme, the Lundbeck Foundation Center for Neurovascular Signaling (LUCENS), and the Fouger Hartmann Foundation.
References
- Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloe K, Nielsen MS, Grunnet M, Schmitt N, Jorgensen NK. KCNQ channels are involved in the regulatory volume decrease response in primary neonatal rat cardiomyocytes. Biochim Biophys Acta. 2007;1773:764–773. doi: 10.1016/j.bbamcr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Davidson RM. Membrane stretch activates a high-conductance K+ channel in G292 osteoblastic-like cells. J Membr Biol. 1993;131:81–92. doi: 10.1007/BF02258536. [DOI] [PubMed] [Google Scholar]
- Gasull X, Ferrer E, Llobet A, Castellano A, Nicolas JM, Pales J, Gual A. Cell membrane stretch modulates the high-conductance Ca2+-activated K+ channel in bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:706–714. doi: 10.1167/iovs.02-0384. [DOI] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, MacAulay N, Jorgensen NK, Schmitt N, Pongs O, Olesen SP, Klaerke DA. KCNQ1 channels sense small changes in cell volume. J Physiol. 2003;549:419–427. doi: 10.1113/jphysiol.2003.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, MacAulay N, Jorgensen NK, Jensen S, Olesen SP, Klaerke DA. Regulation of cloned, Ca2+-activated K+ channels by cell volume changes. Pflugers Arch. 2002;444:167–177. doi: 10.1007/s00424-002-0782-4. [DOI] [PubMed] [Google Scholar]
- Hougaard C, Klaerke DA, Hoffmann EK, Olesen SP, Jorgensen NK. Modulation of KCNQ4 channel activity by changes in cell volume. Biochim Biophys Acta. 2004;1660:1–6. doi: 10.1016/j.bbamem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Jensen HS, Callo K, Jespersen T, Jensen BS, Olesen SP. The KCNQ5 potassium channel from mouse: a broadly expressed M-current like potassium channel modulated by zinc, pH, and volume changes. Brain Res Mol Brain Res. 2005;139:52–62. doi: 10.1016/j.molbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jespersen T, Grunnet M, Angelo K, Klaerke DA, Olesen SP. Dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytes. Biotechniques. 2002;32:536–8. 540. doi: 10.2144/02323st05. [DOI] [PubMed] [Google Scholar]
- Kirber MT, Ordway RW, Clapp LH, Walsh JV, Jr, Singer JJ. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- Kubota T, Horie M, Takano M, Yoshida H, Otani H, Sasayama S. Role of KCNQ1 in the cell swellinginduced enhancement of the slowly activating delayed rectifier K+ current. Jpn J Physiol. 2002;52:31–39. doi: 10.2170/jjphysiol.52.31. [DOI] [PubMed] [Google Scholar]
- Lock H, Valverde MA. Contribution of the IsK (MinK) potassium channel subunit to regulatory volume decrease in murine tracheal epithelial cells. J Biol Chem. 2000;275:34849–34852. doi: 10.1074/jbc.C000633200. [DOI] [PubMed] [Google Scholar]
- Mienville J, Barker JL, Lange GD. Mechanosensitive properties of BK channels from embryonic rat neuroepithelium. J Membr Biol. 1996;153:211–216. doi: 10.1007/s002329900124. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Hoffmann EK, Mills JW. The cytoskeleton and cell volume regulation. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:385–399. doi: 10.1016/s1095-6433(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Qi Z, Chi S, Su X, Naruse K, Sokabe M. Activation of a mechanosensitive BK channel by membrane stress created with amphipaths. Mol Membr Biol. 2005;22:519–527. doi: 10.1080/09687860500370703. [DOI] [PubMed] [Google Scholar]
- Rees SA, Vandenberg JI, Wright AR, Yoshida A, Powell T. Cell swelling has differential effects on the rapid and slow components of delayed rectifier potassium current in guinea pig cardiac myocytes. J Gen Physiol. 1995;106:1151–1170. doi: 10.1085/jgp.106.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Mitsuiye T, Wang Z, Noma A. Increase of the delayed rectifier K+ and Na+-K+ pump currents by hypotonic solutions in guinea pig cardiac myocytes. Circ Res. 1994;75:887–895. doi: 10.1161/01.res.75.5.887. [DOI] [PubMed] [Google Scholar]
- Sheu SJ, Wu SN, Hu DN. Stretch-stimulated activity of large conductance calcium-activated potassium channels in human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2005;21:429–435. doi: 10.1089/jop.2005.21.429. [DOI] [PubMed] [Google Scholar]
- Spagnoli C, Beyder A, Besch S, Sachs Frederick. Atomic force microscopy analysis of cell volume regulation. Physical Review E. 2008;78:031916. doi: 10.1103/PhysRevE.78.031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Martinac B, Arshavsky VY, Kung C. Two types of mechanosensitive channels in the Eschexrichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993;65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Rees SA, Wright AR, Powell T. Cell swelling and ion transport pathways in cardiac myocytes. Cardiovasc Res. 1996;32:85–97. [PubMed] [Google Scholar]
- Willumsen NJ. Increased throughput in ion channel drug development and exploration by automation of electrophysiology. American Biotechnology Laboratory, March issue. 2006 [Google Scholar]
- Wu SN, Lin PH, Hsieh KS, Liu YC, Chiang HT. Behavior of nonselective cation channels and large-conductance Ca2+-activated K+ channels induced by dynamic changes in membrane stretch in cultured smooth muscle cells of human coronary artery. J Cardiovasc Electrophysiol. 2003;14:44–51. doi: 10.1046/j.1540-8167.2003.02040.x. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Kreman M, Boorer KJ, Loo DDF, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- Zhou YY, Yao JA, Tseng GN. Role of tyrosine kinase activity in cardiac slow delayed rectifier channel modulation by cell swelling. Pflugers Arch. 1997;433:750–757. doi: 10.1007/s004240050341. [DOI] [PubMed] [Google Scholar]