Abstract
Recent studies have defined roles for STIM1 and Orai1 as calcium sensor and calcium channel, respectively, for Ca2+-release activated Ca2+ (CRAC) channels, channels underlying store-operated Ca2+ entry (SOCE). In addition, these proteins have been suggested to function in signalling and constructing other channels with biophysical properties distinct from the CRAC channels. Using the human kidney cell line, HEK293, we examined the hypothesis that STIM1 can interact with and regulate members of a family of non-selective cation channels (TRPC) which have been suggested to also function in SOCE pathways under certain conditions. Our data reveal no role for either STIM1 or Orai1 in signalling of TRPC channels. Specifically, Ca2+ entry seen after carbachol treatment in cells transiently expressing TRPC1, TRPC3, TRPC5 or TRPC6 was not enhanced by the co-expression of STIM1. Further, knockdown of STIM1 in cells expressing TRPC5 did not reduce TRPC5 activity, in contrast to one published report. We previously reported in stable TRPC7 cells a Ca2+ entry which was dependent on TRPC7 and appeared store-operated. However, we show here that this TRPC7-mediated entry was also not dependent on either STIM1 or Orai1, as determined by RNA interference (RNAi) and expression of a constitutively active mutant of STIM1. Further, we determined that this entry was not actually store-operated, but instead TRPC7 activity which appears to be regulated by SERCA. Importantly, endogenous TRPC activity was also not regulated by STIM1. In vascular smooth muscle cells, arginine-vasopressin (AVP) activated non-selective cation currents associated with TRPC6 activity were not affected by RNAi knockdown of STIM1, while SOCE was largely inhibited. Finally, disruption of lipid rafts significantly attenuated TRPC3 activity, while having no effect on STIM1 localization or the development of ICRAC. Also, STIM1 punctae were found to localize in regions distinct from lipid rafts. This suggests that TRPC signalling and STIM1/Orai1 signalling occur in distinct plasma membrane domains. Thus, TRPC channels appear to be activated by mechanisms dependent on phospholipase C which do not involve the Ca2+ sensor, STIM1.
Elevations in cytoplasmic Ca2+ concentration are used by all cells as a signalling mechanism for a variety of distinct physiological processes (Berridge, 1993; Carafoli, 2002). Therefore, it is important to understand the mechanisms by which cells regulate Ca2+ concentrations. Cytoplasmic Ca2+ concentrations are tightly regulated by pumps (such as the sarcoplasmic–endoplasmic reticulum Ca2+ ATPase (SERCA) and plasma membrane Ca2+ ATPase (PMCA)) and high-affinity Ca2+ binding proteins (Milner et al. 1992), and Ca2+ signals (rises in intracellular Ca2+ concentrations) can occur through Ca2+ release from internal Ca2+ stores or flux through plasma membrane Ca2+ channels. One ubiquitous Ca2+ signalling pathway involved in a variety of physiological processes is known as store-operated Ca2+ entry (SOCE) or capacitative Ca2+ entry (Putney, 1986; Berridge, 1995). SOCE is a process in which the depletion of Ca2+ stores located in the endoplasmic reticulum (ER) activates Ca2+ channels in the plasma membrane. Physiologically, this occurs in many cell types by the receptor-mediated activation of phospholipase C (PLC) and generation of the second messenger inositol 1,4,5-trisphosphate (IP3). IP3 binds to and activates specific ion channels located in the ER known as IP3 receptors, thus allowing Ca2+ ions to move out of the lumen of the ER and into the cytoplasm. This depletion of luminal ER Ca2+ then signals in a retrograde fashion to the plasma membrane and leads to the activation of SOCE. This process has proved critical not only in the maintenance of ER Ca2+ pools, but also in providing signals for numerous physiological functions, especially in haematopoietic cells (Gwack et al. 2007; Luik & Lewis, 2007).
While advances in the basic understandings of SOCE have been made over the past 20 years, it wasn't until recently that the molecular components of SOCE were described. We now know that STIM1 functions as the ER Ca2+ sensor (Liou et al. 2005; Roos et al. 2005), and Orai (also known as CRACM) family proteins function as the pore-forming subunits of SOCE channels known as the Ca2+ release-activated Ca2+ (CRAC) channels (Feske et al. 2006; Vig et al. 2006b; Zhang et al. 2006). Orai-formed CRAC channels exhibit low conductance, strong inward rectification and high Ca2+ selectivity. However, evidence exists for SOCE channels that have biophysical properties distinct from CRAC currents (ICRAC) (Parekh & Putney, 2005). One such class of channels which has been suggested to function as SOCE channels under certain conditions are the canonical transient receptor potential, or TRPC, channels.
Like SOCE channels, TRPC channels are activated downstream of PLC. Therefore, these channels were first suggested to be the channels underlying ICRAC (Hardie & Minke, 1992, 1993). However, electrophysiological analysis of cloned mammalian TRPCs revealed ion channels which are non-selective, conducting Na+ and K+ as well as Ca2+ (Hurst et al. 1998; Hofmann et al. 1999). Nonetheless, there are numerous reports of apparent store-operated channels which are much less selective for Ca2+ than the prototypical CRAC channels (Parekh & Putney, 2005). Further, there are a number of reports of reduced SOCE when TRPC expression is knocked down or knocked out (Parekh & Putney, 2005; Liu et al. 2007), and in some instances, TRPC channels can display apparent SOCE activity when exogenously expressed (Liu et al. 2000; Vazquez et al. 2001; Lievremont et al. 2004). However, there is also extensive literature indicating that TRPC channels do not operate as SOCE channels (Zitt et al. 1997; McKay et al. 2000; Trebak et al. 2003a; Dietrich et al. 2007; Varga-Szabo et al. 2008). In spite of this controversy, we have learned a great deal about store-independent TRPC channel activation and regulation. While TRPC3, TRPC6 and TRPC7 appear to be activated by the second messenger diacylglycerol (DAG), a product of PLC hydrolysis of phosphatidylinositol-4,5-bisphosphate, TRPC1, TRPC4 and TRPC5 activation is less defined, albeit still downstream of PLC activity (Schaefer et al. 2000; Ma et al. 2001; Hofmann et al. 2002; Venkatachalam & Montell, 2007). Interestingly, recent reports suggest that certain TRPC channels are also regulated by STIM1, and that TRPC activity (at least for TRPC1, TRPC4 and TRPC5) requires both PLC activity and STIM1 interaction (Huang et al. 2006; Lopez et al. 2006; Yuan et al. 2007). In one study, the gating of TRPC channels by STIM1 was seen to occur through electrostatic interactions between the polybasic domain of STIM1 and conserved aspartate residues in TRPCs, in this case TRPC1 and TRPC3 (Zeng et al. 2008). Further, at least in the case for TRPC1, it has also been suggested that TRPC gating by STIM1 is mediated somehow through interactions with Orai proteins (Cheng et al. 2008; Jardin et al. 2008a) and requires lipid raft clustering of signalling proteins (Alicia et al. 2008; Pani et al. 2008).
In this laboratory, we have published results showing that TRPC channels are not generally activated by store depletion (Trebak et al. 2003a), but under certain conditions it appears they may be (Vazquez et al. 2003; Lievremont et al. 2004). However, in contrast to previous studies by ourselves and others on Orai channels, we have not investigated the function of TRPC channels in the context of STIM1 signalling. Thus, in the current study, we have sought to determine whether TRPC channels are functionally regulated by the ER Ca2+ sensor, STIM1, regardless of whether or not they are activated by Ca2+ store depletion. Using both imaging and electrophysiological techniques on expressed and native proteins, we have concluded that TRPC1, TRPC3, TRPC5, TRPC6 and TRPC7 function independently of STIM1 when activated by agonist. Further, we report that while TRPC3 activity was attenuated by the disruption of lipid rafts, ICRAC and STIM1 movements in response to store depletion remained impervious to the loss of cholesterol from the lipid bilayer. Taken together, we believe these data strongly suggest that TRPC channels are activated by mechanisms dependent on phospholipase C and do not involve interactions with the Ca2+ sensor, STIM1.
Methods
Ethical information
No animals or human subjects were used in these studies.
Cell culture
Wild-type (WT) HEK293, A7r5 and A10 cells were obtained from the American Type Culture Collection (ATCC). HEK293 cells stably expressing TRPC3, TRPC6 and TRPC7 protein were described previously (Lievremont et al. 2004). Briefly, both WT and TRPC expressing HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 2 mm glutamine. Continuous selection of TRPC-expressing cells was maintained by the application of 500 μg ml−1 geneticin (Invitrogen). A7r5 and A10 cells were also cultured using DMEM (ATCC) supplemented with 10% FBS. Cells were passed by treating with trypsin–EDTA and maintained at 37˚C in a humidified incubator set at 5% CO2. All experiments were carried out on cells plated onto 30 mm round glass coverslips mounted in a Teflon chamber.
Plasmids
Full-length STIM1, STIM2 and Orai1 cDNA plasmids were purchased from Origene in the pCMV6-XL5 and pCMV-XL4 vectors, respectively. An eYFP-STIM1 plasmid was obtained from Dr Tobias Meyer, Stanford University. A multi-amino acid mutation to the putative EF-hand of eYFP-STIM1 (D76N/D78N) was made by site-directed mutagenesis with the QuikChange Site-Directed Mutagenesis Kit (Stratagene), and was sequence confirmed. M5-muscarinic receptor, TRPC1, TRPC3, and TRPC5 plasmids were obtained from Dr Lutz Birnbaumer at the NIEHS (Research Triangle Park, NC, USA), TRPC6 was obtained from Dr Thomas Gudermann (University of Marburg; Marburg, Germany), and TRPC7 was jointly supplied by Christine Murphy and Adrian Wolstenholm of University of Bath, UK, and John Westwick of Novartis, UK.
Transfections
For experiments carried out on A10 vascular smooth muscle cells or stable TRPC-expressing cells (and their WT controls), cells were transfected with the Amaxa electroporation system (Amaxa Inc., a Lonza Cologne company) following the guidelines set forth by the company for each cell line. A10 cells were electroporated using program D-030 and the nucleofector kit L buffer. HEK293 cells stably expressing TRPC channels were electroporated using the HEK293 (ATCC) cell line setting and the nucleofector kit V buffer. All other experiments utilized cells transfected with Lipofectamine 2000 (Invitrogen; 2 μl per well). For studies of muscarinic receptor activation of TRPCs, HEK293 cells were transfected with 1 μg per well of M5-muscarinic receptor cDNA together with 0.2 μg per well of eYFP cDNA, cDNA encoding one of several TRPCs (see below), with or without 0.32 μg per well of STIM1 cDNA. In order to permit comparison with previously published results, amounts of TRPC1, TRPC3, TRPC5, TRPC6 and STIM1 cDNA for experiments carried out in Figs 1 and 2 (as well as Supplemental Fig. 1) corresponded to those used by Yuan and co-workers for both high and low expression levels of the TRPC channels, adjusted only for different final transfection volumes (see Fig. 1) (Yuan et al. 2007). For all other experiments, the amounts of cDNA were as follows: eYFP cDNA (0.5 μg per well), eYFP-STIM1 cDNA (0.5 μg per well), eYFP-STIM1 D76N/D78N cDNA (0.5 μg per well), STIM2 cDNA (2.0 μg per well), and Orai1 cDNA (0.5 μg per well). After a 6–8 h incubation period, the medium bathing the cells was replaced with complete DMEM and maintained in culture. The following day, cDNA-treated cells were transferred to 30 mm glass coverslips in preparation for imaging or electrophysiological studies as described below.
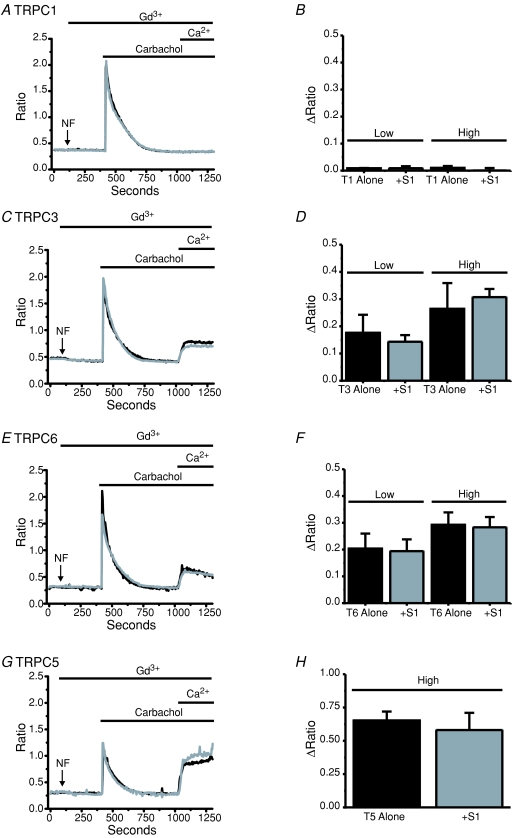
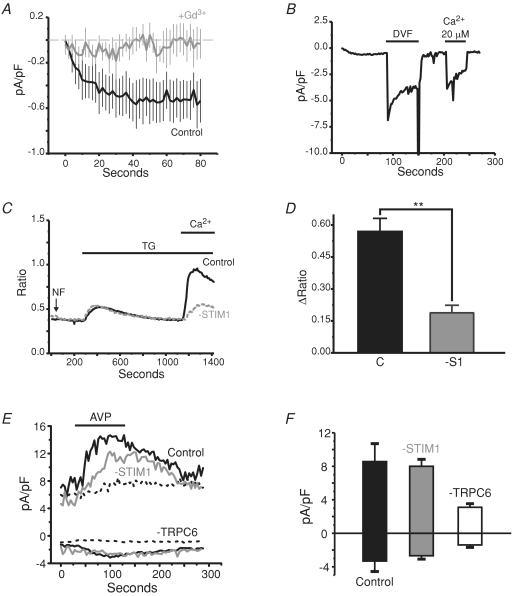
Figure 1. STIM1 does not increase transiently expressed TRPC1, TRPC3, TRPC5 or TRPC6 activity.
Ca2+ imaging experiments (fura-5f) were carried out on HEK293 cells expressing TRPC1 (A), TRPC3 (C), TRPC6 (E) and TRPC5 (G) with (grey traces) and without (black traces) the co-expression of STIM1. Carbachol (200 μm) was applied under nominally Ca2+-free (NF) external conditions and 1.8 mm Ca2+ was re-administered after 10 min in order to assess TRPC activities. Gd3+ was present as indicated in order to block endogenous SOCE. Bar graphs show no significant increase (unpaired t test) in TRPC-evoked Ca2+ entries when STIM1 is co-expressed with TRPC1 (B), TRPC3 (D), TRPC6 (F) or TRPC5 (H). Except for TRPC5, bar graphs also summarize experiments carried out following transfection with two different TRPC cDNA plasmid concentrations (Low: 0.32 μg per well or High: 1.0 μg per well using a 6-well plate) on TRPC-mediated Ca2+ influx. The data represent means ±s.e.m. from three to four coverslips with at least 25 cells for each coverslip.
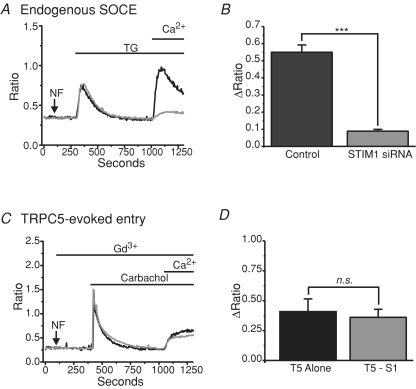
Figure 2. Knocking down STIM1 does not reduce agonist-activated TRPC5 activity.
A, representative Ca2+ imaging experiment (fura-5f) showing the effects of RNAi against STIM1 on endogenous SOCE evoked by store depletion with the SERCA pump inhibitor, thapsigargin (TG; 2 μm). B, bar graph showing STIM1 siRNA treatment significantly reduces (unpaired t test; P= 0.00047) endogenous SOCE in HEK293 cells. C, experiments carried out as in A; however, TRPC5 was transiently expressed in these cells (see Methods) and 200 μm carbachol was used instead of thapsigargin in order to activate TRPC5. Note also the presence of 5 μm Gd3+ in these experiments to inhibit endogenous SOCE. D, bar graph showing no effect (unpaired t test) of STIM1 knockdown on TRPC5-evoked Ca2+ entry after stimulation with carbachol. Three to four coverslips with at least 25 cells for each coverslip were carried out for each condition.
siRNA knockdown
The RNAi experiments in Fig. 2 were carried out as previously described (Wedel et al. 2007). Briefly, HEK293 cells were plated on 6-well plates on day 1. On day 2, cells were transfected with siRNA (100 nm) against STIM1 (Dharmacon, Lafayette, CO, USA) using Metafectene (Biontex Laboratories GmbH, Martinsried/Planegg, Germany; 7 μl per well), and including siGLO (Dharmacon) as a marker. After an 8 h incubation period, the medium bathing the cells was replaced by DMEM and maintained in culture. On day 3, siRNA-treated cells were split into additional 6-well plates. On day 4, control and STIM1 siRNA-treated cells were transfected with TRPC5 cDNA using Lipofectamine 2000 (as described above). On day 5, cells were seeded onto coverslips for imaging experiments carried out on day 6.
For the remaining RNAi experiments on stable TRPC6 and TRPC7 cells, as well as on A10 cells, the Amaxa electroporation system was used. Once again, kit V was used for the stable TRPC HEK293 cells (and their WT controls), and kit L was used for the A10 vascular smooth muscle cells. All knock-down experiments were carried out 72 h post-electroporation. Preliminary dosing experiments revealed higher concentrations of siRNA were required for consistent knockdowns compared to the metafectene experiments (data not shown), probably because of the large number of cells (around 1 million cells per reaction) suspended in a small volume of electroporation buffer (100 μl). Therefore, we used 1 μm siRNA for STIM1, Orai1 and TRPC6 when using the Amaxa system. At this concentration, all cells show significant reduction in the expected responses, compared to lower concentrations in which a few cells still respond normally. These levels of siRNA are similar to the recommended concentrations by Amaxa. The sequences of the siRNAs used were: human Orai1, cccuucggccugaucuuuaucgucu (Mercer et al. 2006); human STIM1, agaaggagcuagaaucucac (Mercer et al. 2006); rat STIM1, gugauacaguggcugauua; and rat and human TRPC6, ugauaugggcugaauguaa. All knockdowns using Amaxa were compared to cells electroporated with siControl (1 μm) 72 h prior to the experiments being carried out.
Calcium measurements
Fluorescence measurements of intracellular Ca2+ were made in all experiments by loading the cells with either fura-2 AM or fura-5f AM (indicated in the figure legends), as previously described (Lievremont et al. 2004; Mercer et al. 2006). Fura-2 was used rather than fura-5f in experiments in which Ba2+ was used as a surrogate for Ca2+ (stable TRPC7 studies) or in the A10 vascular smooth muscle cell studies because of the different affinities of the indicators for the divalent cations and in order for comparison with previously published results, respectively. The fluorescent indicator dye was loaded in FBS-supplemented DMEM at 37°C in the dark for 25 min. For [Ca2+]i measurements, cells were bathed in room temperature Hepes-buffered salt solution (HBSS) containing (in mm): 140 NaCl, 3 KCl, 2 MgCl2, 2.0 CaCl2, 10 glucose and 10 Hepes (pH to 7.4 with NaOH). Nominally Ca2+-free solutions were HBSS with no added CaCl2. Fluorescence images of the cells were recorded and analysed with a digital fluorescence imaging system (InCyt2, Intracellular Imaging Inc.). Cells were alternately excited at 340 nm and 380 nm, and the 530 nm emission was captured using a CCD camera. Typically, greater than 50 cells for HEK293 and greater than 25 cells for A10 were monitored per experiment. In all cases, ratio of fluorescence with excitation at 340 nm over that at 380 nm is reported, after correction for contributions by autofluorescence, measured after treating cells with 10 μm ionomycin and 20 mm MnCl2.
Electrophysiology
Whole-cell currents were investigated at room temperature using the patch-clamp technique in the whole-cell configuration, as previously described (DeHaven et al. 2007). The standard Hepes-buffered saline solution was the same as that used in the Ca2+ imaging experiments, except the Ca2+ concentration varied depending on the experiment (see figure legends). In A10 vascular smooth muscle cells, 5 μm nimodipine was present throughout all experiments to block voltage-gated Ca2+ channels, and 10 mm CsCl2 was also added to block inwardly rectifying K+ channels. The standard divalent-free solution (DVF) was prepared by removing CaCl2 and MgCl2 from HBSS and adding 0.1 mm EGTA. Fire-polished pipettes fabricated from borosilicate glass capillaries (WPI, Sarasota, FL, USA) with 3–5 MΩ resistance were filled with (in mm): 145 caesium methanesulfonate, 20 BAPTA, 10 Hepes and 8 MgCl2 (pH to 7.2 with CsOH) for Ca2+- and Na+-ICRAC measurements, while experiments using clamped Ca2+ (TRPC currents) contained (in mm): 145 caesium methanesulfonate, 10 BAPTA, 10 Hepes, 1 MgCl2 and 3.2 CaCl2 (pH to 7.2 with CsOH) (free Ca2+≈ 100 nm). Voltage ramps were recorded immediately after gaining access to the cell, and the currents were normalized to cell capacitance. In all Na+-ICRAC measurements, ramps were applied from positive to negative potentials (to avoid activation of voltage-gated Na+ channels) every 2 s, while all TRPC recordings were made from negative to positive potentials. For ICRAC measurements, leak currents were subtracted by taking an initial ramp current before Icrac developed and subtracting this from all subsequent ramp currents. Leak currents were not subtracted in the TRPC studies. Access resistance was typically between 5–10 MΩ. Cell-attached patch-clamp experiments were carried out using a bath and pipette solution containing (In mm): 140 KCl, 1 MgCl2, 10 glucose, 2 CaCl2 and 10 Hepes (pH 7.3 with KOH), as previously described by our laboratory (Vazquez et al. 2006; Lemonnier et al. 2008). For these cell-attached experiments, data were low-pass filtered at 2 kHz and digitized at 20 kHz. For all electrophysiological experiments, the currents were acquired with pCLAMP-10 (Axon Instruments) and analysed with Clampfit (Axon Instruments) and Origin 6 (Microcal) software. All solutions were applied by means of a gravity-based multi-barrel local perfusion system with an extremely low dead volume common delivery port (Perfusion pencil, Automate Scientific, Inc.) with flow rates set at around 0.25 ml min−1.
Live cell confocal and TIRFM imaging
Live cell confocal and total internal reflection fluorescence (TIRF) microscopy were carried out as previously described (Smyth et al. 2005; DeHaven et al. 2008). Briefly, cells were maintained in HBSS at room temperature. Confocal imaging was carried out with a Zeiss LSM 510 laser scanning system with a 63× oil-immersion (NA 1.4) objective. All confocal images were collected with the pinhole set at 1 Airy Unit. For eYFP-STIM1, 514 nm excitation was provided by an argon laser and emission was selected with a 530–600 nm bandpass filter. For fluorescently labelled GM1, cells were excited by 543 nm wavelength and emission was selected with a 560 nm long-pass filter. Appropriate controls were established to verify separation between the signals carried out in the experiments shown in Fig. 10 (data not shown). GM1 labelling, with and without cholera toxin subunit B-specific antibody crosslinking, was carried out following the guidelines provided with the Vybrant AlexaFluor Lipid raft labelling kit (Invitrogen). TIRFM measurements of eYFP-STIM1 was carried out essentially as previously described (Smyth et al. 2005; DeHaven et al. 2008). For fluorescence intensity profiles, data are represented as the fluorescence intensity at each time point divided by the fluorescence intensity at the start of the experiment (F/F0). Fluorescence intensities were collected from regions of interest encompassing the visible footprints of single cells and were background subtracted.
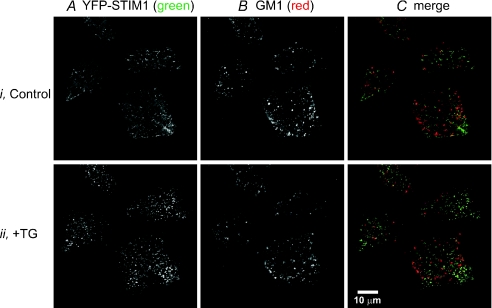
Figure 10. eYFP-STIM1 does not co-localize with the lipid raft marker, GM1.
Representative confocal images of live HEK293 cells expressing eYFP-STIM1 (A) and labelled for the lipid raft marker, GM1 (B) before (Control) and after (+TG) 15 min treatment with the SERCA pump inhibitor, thapsigargin. C, merge of the eYFP-STIM1 (green) and GM1 (red) images before and after TG treatment showing the lack of co-localization between STIM1 and GM1. Results are shown from an experiment utilizing a cross-linking antibody directed against cholera toxin; similar results were obtained in the absence of the cross-linking antibody (not shown).
Western blotting
Western blot analysis was performed as previously described (Smyth et al. 2008) Briefly, normalized cell lysates were electrophoresed into 10% polyacrylamide gels. Proteins were then transferred to PVDF membranes, followed by blocking with 3% bovine serum albumin (BSA) in TBS-T (24.7 mm Tris base, 137 mm NaCl, 2.7 mm KCl, 0.1% Tween-20, pH 7.4). Next, the membranes were incubated in primary antibody directed against STIM1 (rabbit polyclonal; ProSci) in TBS-T with BSA overnight at 4°C. For TRPC1, the primary antibody was a rabbit monoclonal from AbCam applied at 1 : 10000 dilution, and incubated overnight at 4°C. The blocking agent was 5% milk. Following consecutive washes, the secondary antibody was applied (horseradish peroxidase-conjugated anti-rabbit IgG; Pierce) in TBS-T with BSA for 1 h at room temperature. After three 10 min washes in TBS-T, the membranes were developed with ECL plus reagent (GE Life Sciences). Lastly, the membranes were stripped with ReStore (Invitrogen) and re-probed with an actin primary antibody (AbCam). Band intensities were analysed using ImageJ software and normalized to actin.
FLIPR-based membrane potential dye assays
Experiments shown in Supplemental Fig. 6 were carried out on cells plated onto poly-lysine-coated 96-well plates, and relative membrane potential effects were measured using a membrane potential assay kit (Molecular Devices) and a fluorometric imaging plate reader (FLIPR384; Molecular Devices), following a similar protocol suggested by the vendor.
Results
STIM1 does not increase activity of transiently expressed TRPC1, TRPC3, TRPC5 or TRPC6
It is well established that STIM1 interacts with Orai proteins, either directly or indirectly, to form store-operated CRAC channels (Frischauf et al. 2008). A key and general finding is the striking synergism between transiently expressed STIM1 and Orai producing extremely large currents and Ca2+ entry signals (Mercer et al. 2006; Peinelt et al. 2006). To assess the potential for STIM1 to similarly regulate TRPC channels, we co-transfected HEK293 cells with STIM1 and various TRPC channel subunits. Of the six TRPCs functioning in human cells, we examined effects of STIM1 on TRPC1, 3, 5 and 6. TRPC4 was not investigated as in our hands, in HEK293 cells it appears to act only constitutively (McKay et al. 2000), while others have reported that it acts similarly to TRPC5 (Schaefer et al. 2000). TRPC7 is examined in depth in another context as a subsequent part of this study.
We utilized a standard method for examining TRPC function independently of endogenous store-operated channel activity. We activated the transfected HEK293 cells through PLC-linked muscarinic receptors with carbachol in the presence of Gd3+, which blocks the endogenous store-operated channels (Broad et al. 1999b). We monitored agonist-activated Ca2+ entry with a protocol of agonist addition in the absence of Ca2+ followed by re-addition of extracellular Ca2+. In cells that had not been transfected with TRPCs, we confirmed that no Gd3+-insensitive carbachol-activated Ca2+ entry was observed (not shown). Figure 1 shows the results from TRPC1 (panels A and B), TRPC3 (panels C and D), TRPC6 (panels E and F) and TRPC5 (panels G and H) expressing HEK293 cells with (black traces) and without (grey traces) the co-expression of STIM1. In TRPC3-, TRPC5- and TRPC6-expressing cells, but not in TRPC1 cells, the re-addition of Ca2+ revealed significant Gd3+-insensitive, and therefore TRPC-mediated, Ca2+ entry. However, the expression of STIM1 did not increase TRPC-mediated Ca2+ influx under these conditions. Calcium entry under these conditions is well below saturation as shown by the substantial increase in signal observed in cells transfected with STIM1 and Orai1 (Mercer et al. 2006).
We considered the possibility that expression level of the TRPC channels may affect the outcome of these results, as previously reported by this laboratory in experiments with DT40 B-lymphocytes (Vazquez et al. 2003), and recently reported for TRPCs in HEK293 cells (Yuan et al. 2007). Therefore, we carried out the experiments under both high and low plasmid concentrations for TRPC1 (Fig. 1, panel B), TRPC3 (panel D) and TRPC6 (panel F), concentrations similar to those previously described (Yuan et al. 2007). With the lower plasmid concentrations, for TRPC3 and 6 there was diminished Ca2+ entry, and a greater number of cells showing no entry at all, confirming that protein expression was decreased to a limiting level. However, there still was no difference between the Gd3+-insensitive entry in cells co-expressing STIM1 versus controls (Fig. 1). TRPC5 (panel H) studies were carried out using only the higher plasmid concentration, because TRPC5 was previously reported to interact with STIM1 independently of expression level (Yuan et al. 2007). We also carried out the above experiments in the absence of extracellular Gd3+, and the results are summarized in Supplemental Fig. 1. Note that TRPC1 did not produce any entry in the presence of Gd3+, in agreement with Hofmann et al. (2002) who concluded that TRPC1 does not traffic to the plasma membrane correctly in the absence of TRPC4 or TRPC5. Co-expression of STIM1 did not remedy the failure of TRPC1 to give an agonist-activated Gd3+-insensitive Ca2+ entry, nor did low levels of TRPC1, in the presence or absence of STIM1, influence thapsigargin-activated Ca2+ entry in the absence of Gd3+ (data not shown). We confirmed by Western analysis that our plasmid and transfection protocol successfully increased TRPC1 protein expression several-fold (not shown). For TRPC5 we also examined the effects of STIM1 and TRPC5 transfections on carbachol-activated TRPC5 currents and on CRAC currents. There was no increase in TRPC5 current associated with STIM1 transfection, nor was there any increase in Icrac in cells trasfected with STIM1 plus TRPC5 compared to STIM1 alone (Supplemental Fig. 2).
It has previously been suggested that STIM1 is obligatory for the activation of TRPC5 by agonist (Yuan et al. 2007). Thus, we carried out similar experiments and examined the effects of STIM1 knockdown on TRPC5 activation by agonist. Our laboratory has previously published similar approaches using siRNA directed against STIM1 (Mercer et al. 2006; DeHaven et al. 2007; Wedel et al. 2007). Figure 2A and B shows that endogenous SOCE in cells treated with STIM1 siRNA was significantly reduced by 80% compared to control cells when thapsigargin was used to deplete stores, confirming that STIM1 knockdown was successful. However, in the presence of Gd3+, carbachol-evoked activation of TRPC5-mediated Ca2+ entry was unaffected by knockdown of STIM1 (Fig. 2C and D).
TRPC7, previously reported to function in a store-dependent manner, is not regulated by STIM1 or Orai1
To this point, we have examined a potential role for STIM1 in agonist-induced Ca2+ entry through TRPC channels with the view that this protocol would reveal effects on either store-operated or non-store-operated entry. In the past, we have consistently failed to see activation of these channels by store depletion alone when transiently expressed in HEK293 cells (Trebak et al. 2003a; Lievremont et al. 2004; and Supplemental Fig. 1). However, our laboratory previously described increased TRPC7-mediated Ba2+ influx after store depletion with thapsigargin in HEK293 cells stably (but not transiently) expressing TRPC7 (Lievremont et al. 2004). Ba2+ was used as a surrogate for Ca2+ to avoid complications that might arise from alterations in Ca2+ transport and Ca2+ regulation of the channels themselves. The use of Ba2+ avoids these problems since it is a poor substrate for Ca2+-sensitive regulatory sites and transport mechanisms (Vanderkooi & Martonosi, 1971; Uvelius et al. 1974). We hypothesized that this might be an example of STIM1/Orai1 interaction with a TRPC channel, conferring store dependency to the TRPC7 channels (for example, Liao et al. 2007). To test this, RNAi experiments were carried out to determine whether the proteins Orai1 and STIM1 play a role in this apparent TRPC7 store-dependent entry. Figure 3A shows single cell fura-2 imaging results in HEK293 cells stably expressing TRPC7. ER Ca2+ stores were depleted using thapsigargin under nominally Ca2+-free conditions, and Ba2+ was added after the cytoplasmic Ca2+ levels returned to baseline (15 min). In all experiments, 5 μm Gd3+ was present to block endogenous store-operated channels. In these cells, thapsigargin treatment caused a significant increase in the rate of Ba2+ flux compared to the leak controls (Fig. 3A and B), as previously reported (Lievremont et al. 2004). However, the knockdown of neither STIM1 nor Orai1 reduced TRPC7-mediated Ba2+ entry (Fig. 3B).
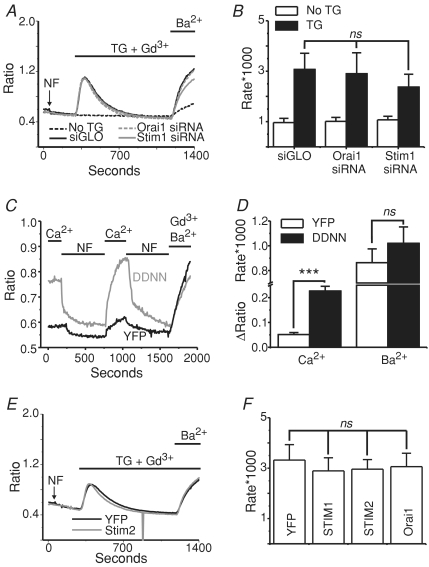
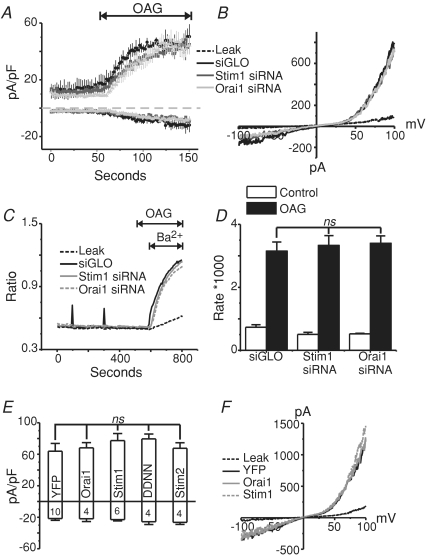
Figure 3. TRPC7 previously reported to function in a store-dependent manner is not regulated by STIM or Orai.
A, Gd3+ (5 μm) was present throughout the TRPC7 experiments to block endogenous store-operated Ca2+ entry. Shown are mean traces (fura-2 AM) from stable TRPC7 HEK293 cells confirming increased TRPC7-dependent (Gd3+-insensitive) Ba2+ entry in response to 2 μm thapsigargin (leak control: dashed black trace vs. siGLO control: continuous black trace), and showing no significant affect of knocking down STIM1 (STIM1 siRNA: continuous grey trace) or Orai1 (Orai1 siRNA: dashed grey trace). Ba2+ was used as a surrogate for Ca2+ to avoid Ca2+ buffering problems, as previously described for TRPC7 cells (Lievremont et al. 2004). B, bar graph depicting no significant difference (ANOVA) between the rates of Ba2+ entry (under the presence of Gd3+) in TRPC7 cells transfected with Orai1 (n= 5 coverslips) or STIM1 (n= 7 coverslips) siRNA when compared to control cells (n= 7 coverslips) (siGLO). C, imaging experiments demonstrating that stable TRPC7 cells transfected with the constitutively active mutant of STIM1 (D76N/D78N; grey trace) show a significant increase in basal Ca2+ levels when compared to eYFP control cells (black trace), but do not show any increase in the basal activity of TRPC7 as seen by the influx of Ba2+ in the presence of 5 μm Gd3+. Nominally Ca2+-free (NF) and 2 mm external Ca2+ conditions, as well as the addition of Ba2+ and Gd3+ are indicated by the lines above the graph. D, bar graph depicting a significant increase in basal Ca2+ concentrations (unpaired t test, P= 0.00013) in TRPC7 cells transiently expressing STIM1 D76N/D78N; however, there was no difference between the rates of Ba2+ entry (under the presence of Gd3+; unpaired t test, P= 0.42387) in stable TRPC7 cells expressing either eYFP alone (n= 4 coverslips) or in conjunction with STIM1 D76N/D78N (n= 6 coverslips). E, ratiometric measurements showing stable TRPC7 cells transfected with STIM2 (grey trace) have no change in the rate of Ba2+ entry compared to eYFP control cells (black trace). F, bar graph depicting no significant difference (ANOVA) between the rates of Ba2+ entry in TRPC7 cells expressing either eYFP (n= 5 coverslips), or in conjunction with STIM1 (n= 5 coverslips), STIM2 (n= 5 coverslips) or Orai1 (n= 5 coverslips). Leak entry rates between conditions were also not significantly different (not shown). Each coverslip (each n) was taken as the mean of at least 25 individual cells.
The RNAi findings were supported by experiments carried out using a constitutively active construct of STIM1 in which aspartic acids 76 and 78 are mutated to asparagines (D76N/D78N). STIM1 D76N/D78N presumably no longer binds Ca2+ in its EF-hand domain, and therefore oligomerizes with other STIM1 proteins independently of store depletion, and constitutively activates store-dependent plasma membrane Orai channels (Liou et al. 2005; Mercer et al. 2006). We hypothesized that if STIM1 interacts functionally with TRPC7, then the constitutively active mutant of STIM1 should increase TRPC7 activity. However, while the expression of STIM1 D76N/D78N did significantly increase basal Ca2+ levels in these cells, presumably due to constitutive activation of endogenous Orai1 channels, it did not increase the rate of Gd3+-insensitive Ba2+ entry which occurs through TRPC7 (Fig. 3C and D).
We also examined the effects of over-expressing STIM1, STIM2 and Orai1 on the thapsigargin-evoked Gd3+-insensitive Ba3+ entry seen in these TRPC7 expressing cells. We and others have previously reported that the expression of STIM2 or Orai1 alone causes a suppression of endogenous SOCE (Mercer et al. 2006; Soboloff et al. 2006). While the cause of this suppression is not completely understood, we hypothesized that the TRPC7-dependent entry might also be suppressed by the expression of STIM2 and Orai1, or augmented by STIM1 if they functionally interact. However, the expression of neither STIM1, STIM2 nor Orai1 had any effect on the TRPC7-mediated Ba2+ entry seen after thapsigargin treatment, compared to eYFP controls (Fig. 3E and F). Thus, the increase in TRPC7 activity after thapsigargin treatment in these cells is completely independent of STIM1, STIM2 and Orai1. Unlike Orail, transient expression of TRPC7 did not suppress SOCE in HEK293 cells (Supp. Fig. 4). It is important to note that all of the experiments carried out in Fig. 3 were carried out in parallel with wild-type HEK293 cells, verifying that STIM1 and Orai1 were indeed knocked down as previously described (panels A and B), that STIM1 D76N/D78N caused constitutive activity that was sensitive to Gd3+ (panels C and D), and that STIM2 and Orai1 caused a suppression of endogenous SOCE (panels E and F) (Supplemental Fig. 3).
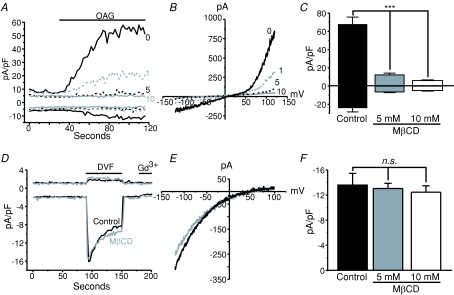
We next examined the effects of knocking down STIM1 and Orai1 on the second messenger-activated currents in these TRPC7 stable cells. In order to by-pass signalling and the production of other second messengers that might play a role in regulation of these channels, the synthetic diacylglycerol, OAG, was used as an agonist. Figure 4A shows whole-cell voltage-clamp experiments in TRPC7 cells treated with either siGLO (control; black trace), STIM1 (dark grey trace), or Orai1 (light grey trace) siRNA, 72 h post-transfection. One minute after break-in with an intracellular pipette solution containing 100 nm clamped Ca2+, 50 μm OAG was focally applied to the cells to activate the TRPC7 channels. Neither the knockdown of STIM1 nor Orai1 altered the peak TRPC7 currents after the application of OAG. The kinetics of activation also were unaffected by STIM1 and Orai1 RNAi. Figure 4B shows representative current–voltage relationships for the conditions shown in panel A. Similar results were seen in fura-2 imaging assays, in which knockdown of STIM1 or Orai1 did not affect the TRPC7-mediated Ba2+ entries in response to OAG application (Fig. 4C and D). Similar to the RNAi experiments in Fig. 4A and B, the transient expression of STIM and Orai1 proteins also did not influence TRPC7 channel activity when activated by OAG (Fig. 4E and F).
Figure 4. TRPC7 currents activated by OAG are not altered by the knockdown of STIM1 or Orai1, or by the over-expression of STIM1, STIM1 D76N/D78N, STIM2 or Orai1.
A, whole-cell OAG-activated currents (means ±s.e.m.) taken at −100 and +100 mV from stable TRPC7 cells transfected with siGLO (n= 3), STIM1 siRNA (n= 4) or Orai1 siRNA (n= 3) 72 h prior to the experiments. The external HBSS contained 2 mm Ca2+, and OAG (50 μm) was externally applied as indicated by the line above the graph. B, current–voltage (I–V) relationships taken from stable TRPC7 cells before (leak; broken black trace) and after (siGLO, STIM1 siRNA, Orai1 siRNA) focal OAG application. In all conditions, the outwardly rectifying I–V relationships were nearly identical. C, mean Ba2+ entries (fura-2 AM) in response to OAG (50 μm) application in stable TRPC7 expressing HEK293 cells 72 h after transfection with siGLO (Control), STIM1 or Orai1 siRNA. D, summary of data in C, showing no significant difference (ANOVA) of OAG-mediated Ba2+ influx in stable TRPC7 expressing cells expressing siGLO (n= 7 coverslips), STIM1 siRNA (n= 8 coverslips) or Orai1 siRNA (n= 4 coverslips). E, summary of OAG-mediated whole-cell currents (−100 mV and +100 mV) in stable TRPC7 cells transfected with eYFP (control) (n= 10), Orai1 (n= 4), STIM1 (n= 6), STIM1 D76N/D78N (n= 4) or STIM2 (n= 4). Nominally Ca2+-free HBSS was used as the external bathing solution in these studies. F, I–V relationship showing the currents recorded under the same conditions as E.
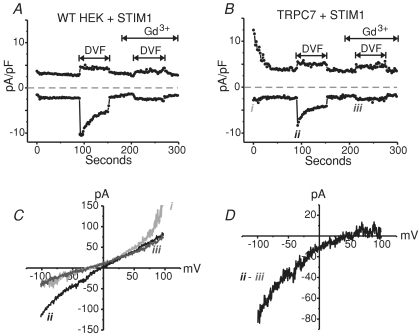
We were puzzled by the finding that an apparent store-operated activity of TRPC7 was so clearly independent of the Ca2+ store sensor, STIM1. In the Ca2+ imaging studies, Ca2+ store depletion was always accomplished by treatment with the Ca2+ pump inhibitor, thapsigargin. However, store-operated channels should become active no matter how stores are depleted. Therefore, we decided to deplete the stores using IP3 in the patch pipette in a similar approach described by Hoth and Penner in the original description of ICRAC (Hoth & Penner, 1992). If in these cells TRPC7 is indeed store operated, then IP3-mediated store depletion should also activate the TRPC7 channels in a store-dependent manner. As the experiments were carried out in parallel with wild-type HEK293 cells, and because endogenous ICRAC is difficult at best to record in these cells, STIM1 was expressed in order to amplify ICRAC through endogenous Orai1 channels in the control and TRPC7 conditions. We previously reported a 2- to 3-fold increase in Na+-ICRAC in HEK293 cells expressing STIM1 compared to their wild-type counterparts (DeHaven et al. 2007). Figure 5A and B shows representative recordings (−100 mV and +100 mV taken every 2 s) taken from WT HEK293 and stable TRPC7 cells, respectively, both transiently expressing eYFP-STIM1, in which IP3 was dialysed into the cell to deplete ER Ca2+ stores. Importantly, these results indicate that no Gd3+-insensitive non-selective TRPC7 currents were ever activated by ER Ca2+ store depletion with BAPTA and IP3 in the patch pipette in the TRPC7 cells (Fig. 5B). Further, the only currents that were detected had the same properties as the endogenous store-operated CRAC currents seen in the wild-type cells expressing STIM1 (panel A). Switching to a divalent cation-free solution (DVF) only amplified an inwardly rectifying current that was strongly suppressed by the lanthanide, Gd3+ (panels C and D).
Figure 5. Whole-cell patch clamp experiments using high BAPTA and IP3 to deplete internal Ca2+ stores activates only ICRAC in TRPC7 cells.
A, representative whole-cell CRAC current in a HEK293 cell expressing STIM1. A divalent-free (DVF) solution exchange protocol was used, as previously described (DeHaven et al. 2007), in order to amplify the extremely small endogenous CRAC current in these cells. STIM1 was expressed to further amplify the small CRAC currents in HEK293 cells. Current was recorded at −100 and +100 mV and stores were depleted with 20 μm IP3 and 20 mm BAPTA in the pipette. Lines above trace indicate where different extracellular solutions were focally applied. Gd3+ (10 μm) was used to block the Na+ICRAC, revealing a linear increase in leak currents under these external conditions in which all Ca2+ and Mg2+ is removed and 10 μm Gd3+ is the only cation present. B, same as A, except in a HEK293 cell stably expressing TRPC7. Note that there is constitutive TRPC7 activity at break in, but this activity diminishes over time. In stable TRPC7-expressing HEK293 cells, normally functioning inwardly rectifying Na+-CRAC currents are detectable with similar current densities to control cells (A). C, current–voltage (I–V) relationships taken at specific time points indicated as i, ii and iii for current trace shown in B. D, I–V for the Gd3+-sensitive current shown in B and C.
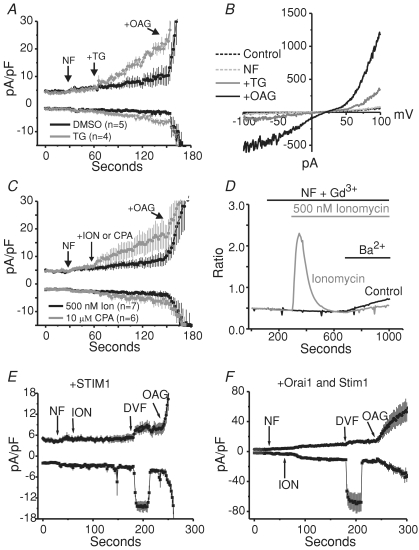
TRPC channels may be regulated by cytoplasmic Ca2+ in a concentration-dependent manner, in which too little or too much cytoplasmic Ca2+ suppresses channel activity (Shi et al. 2004). Experiments shown in Fig. 5 were carried out with millimolar concentrations of BAPTA in the patch pipette; therefore, it is feasible that the cytoplasmic Ca2+ concentrations were below that which is required for TRPC7 activation. Evidence for this can be seen in Fig. 5B and C, in which the basal TRPC7 activity seen just after break-in is reduced over time, presumably due to BAPTA diffusing into the cell and chelating cytoplasmic Ca2+. Therefore, under these conditions, we could not definitively conclude whether we did not see store-operated TRPC7 currents because TRPC7 is actually not store operated, or because our internal conditions were too stringent to allow for activation of the channels in a store depletion-dependent manner. To address this uncertainty, we carried out experiments on stably expressing TRPC7 cells in which the internal Ca2+ concentration was clamped to physiological concentrations (∼100 nm). Further, we decided to deplete the stores with other store-depleting agents (thapsigargin, cyclopiazonic acid (CPA) and ionomycin) in order to avoid any complications which might occur with the IP3 receptors which are also sensitive to cytoplasmic Ca2+ concentrations. Figure 6A shows the mean ±s.e.m. of independent voltage-clamp experiments in which thapsigargin (grey trace) was focally applied to stably expressing TRPC7 cells under nominally Ca2+-free (NF) external conditions, followed by the application of 50 μm OAG. As a control, experiments were carried out with DMSO, the vehicle for thapsigargin (black trace). Consistent with the results from the Ba2+ entry imaging experiments, in all cells tested, the focal application caused a small, but significant increase in a whole-cell current, which had a current–voltage relationship as expected for TRPC7 (Fig. 6B). This increase in current was not seen in the DMSO-treated cells. When thapsigargin was replaced with CPA, a competitive SERCA pump inhibitor (Fig. 6C, grey trace), small increases in current densities were also seen similar to those when thapsigargin was applied; however, depletion of Ca2+ stores with the ionophore ionomycin (black trace) was ineffective at activating TRPC7 (Fig. 6C and D), even though it did activate ICRAC in these TRPC7 cells (Fig. 6E and F). We conclude that the activation of TRPC7 by thapsigargin results in some manner from inhibition of SERCA per se, rather than from depletion of Ca2+ stores. Therefore, TRPC7 would not be considered a store-operated channel, a conclusion consistent with the lack of involvement of STIM1 or Orai1.
Figure 6. Thapsigargin-evoked increases in TRPC7 current.
A, whole-cell currents in HEK293 cells stably expressing TRPC7. Focally applied thapsigargin (n= 4, grey trace) increases outwardly rectifying currents in these cells, while DMSO does not (n= 5, black trace). Internal Ca2+ was clamped to around 100 nm free Ca2+, and external solutions were added as indicated by the arrows. Voltage ramps were applied from −100 mV to +100 mV (250 ms), every 2 s to record the currents which developed over time. B, representative current–voltage relationships showing the peak currents recorded after break-in (in the presence of 2 mm Ca2+, Control, dashed black trace), after switching to nominally Ca2+-free external solution (NF, grey dashed trace), after 1.5 min of focally applied thapsigargin (+TG, grey continuous trace), and after the application of 50 μm OAG (+OAG, black trace). C, experiments similar to A; however, using the ionophore ionomycin (n= 7, black trace) or the SERCA pump antagonist CPA (n= 6, grey trace), instead of thapsigargin. D, fura-2 AM imaging experiment showing the effects of ionomycin application (grey trace) compared to leak control (black trace). Ionomycin did not increase the rate of Ba2+ entry compared to the control. Shown are the means of single coverslips which are representative of three similar experiments for each condition. E, experiments (n= 7) similar to those inC (100 nm clamped Ca2+), in which ionomycin was used to deplete internal stores. However, in these cells, eYFP-STIM1 was transiently expressed and divalent cation-free (DVF) external solution was focally applied in order to demonstrate that ionomycin did indeed deplete the stores critical for ICRAC development. The inward currents seen after the application of DVF solution represent ICRAC. Note there is no depotentiation of these currents because the starting solution was devoid of Ca2+ (NF). F, same as in E; however, in conjunction with STIM1, Orai1 was also transiently expressed to further amplify the Na+-ICRAC currents recorded (n= 4). All whole-cell currents shown in this figure are represented as means ±s.e.m.
Knockdown of STIM1 inhibits SOCE but not endogenous TRPC6 currents in smooth muscle cells
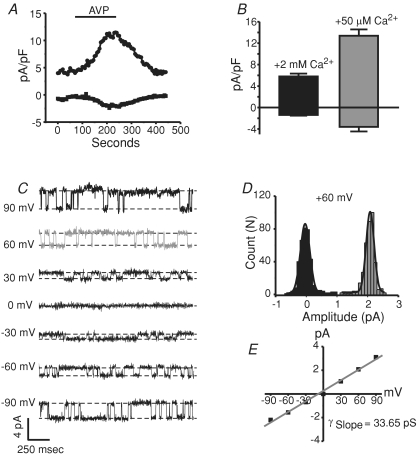
All of the findings to this point suggested no interaction between STIM1 and exogenously expressed TRPC channels. However, it is possible that regulation might be lost or less obvious with overexpressed channels. Thus, we next investigated the role of STIM1 on the activation of arginine–vasopressin (AVP)-evoked non-selective cation currents (NSCC) in A10 vascular smooth muscle cells. In a related cell line (A7r5), a NSCC has been molecularly identified using RNAi as TRPC6 (Soboloff et al. 2005; Takahashi et al. 2008). We therefore first determined whether the AVP-activated NSCC in A10 cells is similarly mediated by TRPC6. Figure 7A shows a representative whole-cell patch-clamp experiment in which the focal application of 500 nm AVP activated non-selective cation channels in A10 vascular smooth muscle cells. Similar results were seen in the A7r5 cells (data not shown). Figure 7B shows that the mean current densities of the AVP-activated currents recorded at −100 and +100 mV were larger when the extracellular Ca2+ was reduced from 2 mm (black bar) to around 50 μm Ca2+ (grey bar). This increase in current density when extracellular Ca2+ is reduced is presumably from electrostatic interaction of Ca2+ in the pore of the channels reducing monovalent permeation under the presence of millimolar external Ca2+. These effects mirror what is commonly seen in many TRPC channels (Kamouchi et al. 1999; Okada et al. 1999; McKay et al. 2000; Lemonnier et al. 2006). Cell-attached experiments revealed in some patches the presence of single channel events as shown in Fig. 7C, which were dependent on the presence of extracellular AVP. At +60 mV, the single channel current amplitude was 2.1 pA (Fig. 7D), and the slope conductance for the recording shown in panel C was calculated as 33.65 pS, similar to that published for TRPC6 (panel E) (cfTrebak et al. 2007). Further, RT-PCR analysis of TRPC transcripts in A10 cells revealed the predominant expression of TRPC1 and TRPC6 mRNA, with very low copies of TRPC4 and TRPC7 detected, and virtually no TRPC3 and TRPC5 (data not shown). Finally, RNAi directed towards TRPC6 significantly reduced the AVP-activated current densities in these cells compared to control cells (Fig. 8E and F). RNAi knockdown of TRPC6 was verified by quantitative RT-PCR, which showed greater than 70% reduction of TRPC6 message (Supplemental Fig. 5F). Further, because the siRNA sequence used was compatible with both rat and human message, cells stably expressing human TRPC6 were tested for functional knockdown of TRPC6. Supplemental Fig. 5C–E shows the effects of TRPC6 siRNA treatment on these TRPC6 stable cells, in which the whole-cell currents were reduced by 80% compared to siControl cells. Taken together, these results suggest the presence of AVP-activated TRPC6 currents in A10 vascular smooth muscle cells.
Figure 7. AVP-activated non-selective cation currents in A10 vascular smooth muscle cells.
A, a representative whole-cell current (−100 mV and +100 mV) recorded from an A10 cell before, during and after the focal application of 500 nm arginine–vasopressin (AVP). External solution contained 2 mm Ca2+ and the patch pipette contained 100 nm clamped Ca2+. AVP was applied as indicated by the line above the graph. Nimodipine (5 μm) was present throughout. B, bar graph depicting the effects of extracellular Ca2+ (HBSS + 2 mm Ca2+ or HBSS + 50 μm Ca2+) on the non-selective cation currents recorded in A10 cells after the application of AVP. C, single channel events recorded in the cell-attached mode from A10 cells bathed in 500 nm AVP application at the indicated holding potentials. The single channel events were not detected in experiments in which AVP was not applied (not shown). D, all points histogram showing the amplitude (pA) of the single channel events recorded after AVP treatment at a holding potential of +60 mV. E, current–voltage relationship of the single channel events and calculated slope conductance of the AVP-activated current.
Figure 8. Knockdown of STIM1 does not alter endogenous TRPC6 currents, while significantly suppressing SOCE in smooth muscle cells.
A, whole-cell ICRAC measurements taken at −120 mV from A10 smooth muscle cells with (n= 6; grey trace) or without (n= 7; black trace) the presence of 3 μm Gd3+. Stores were actively depleted with IP3 and BAPTA in the patch pipette, and 5 μm nimodipine was present in the external HBSS containing 10 mm Ca2+. B, ICRAC experiment showing in smooth muscle cells the Na+ currents are half-maximally blocked by 20 μm external Ca2+ at −120 mV. Ramps were applied from positive to negative potentials (see Methods) to avoid voltage-gated channel contamination of the CRAC currents. C, Ca2+ imaging experiment (fura-2 AM) showing the effects of RNAi targeted against STIM1 on thapsigargin-evoked SOCE in A10 cells. External solutions were exchanged as indicated by the lines above the graph. D, bar graph showing STIM1 siRNA-treated cells (-S1, n= 4 coverslips, grey bar) have significantly less (unpaired t test; P= 0.00152) SOCE compared to siControl-treated control (C, n= 5 coverslips, black bar) cells. E, representative whole-cell currents obtained as in Fig. 7, in which AVP-activated currents were reduced in cells treated with siRNA against TRPC6 (n= 9; broken black trace), but not in cells treated with STIM1 siRNA (n= 5; continuous grey trace), compared to siControl-treated cells (n= 7; continuous black trace). F, bar graph showing the summary of the data collected in A10 cells for the AVP-activated non-selective cation currents in which STIM1 siRNA had little effect, but TRPC6 siRNA reduced the overall current densities.
We next verified the presence of store-operated Ca2+ entry and ICRAC in these smooth muscle cells, and determined that STIM1 was a critical mediator of this pathway. Figure 8A shows voltage-clamp experiments in which BAPTA and IP3 were included in the patch pipette to deplete internal Ca2+ stores and 10 mm Ca2+ was in the bathing solution. Just after breaking in, a small but detectable ICRAC developed in these smooth muscle cells. Similar currents have been measured from A7R5 cells (Brueggemann et al. 2006). Importantly, this Ca2+ current is not detected in cells treated with low micromolar concentrations of the lanthanide, Gd3+. The identity of these currents was further assessed by examining Na+ currents under divalent-free conditions and the half-maximal blocking concentration of external Ca2+. Figure 8B shows an experiment in which 20 μm external Ca2+ was required to half-maximally inhibit Na+ permeation through these store-dependent channels, a concentration similar to that seen for both endogenous ICRAC in non-excitable cells and over-expressed Orai (Prakriya & Lewis, 2003; Prakriya et al. 2006; DeHaven et al. 2007). The current–voltage relationships for the Ca2+ and Na+ CRAC currents in these cells were taken from voltage ramps run from positive to negative direction in order to avoid contamination with depolarization activated currents (see Methods) and were strongly inwardly rectifying, indicative of ICRAC.
Next, we evaluated the dependence of SOCE on STIM1 by using RNAi directed against STIM1. Figure 8C shows representative Ca2+ imaging experiments (means of single coverslips) using thapsigargin to deplete internal Ca2+ stores and activate SOCE. Seventy-two hours post-Amaxa electroporation with siRNAs, the STIM1 siRNA-treated cells (grey dashed trace) showed a much smaller SOCE phase compared to siControl-treated cells (black continuous trace). The bar graph in Fig. 8D shows that knocking down STIM1 reduces SOCE in these A10 smooth muscle cells to around 30% of the control SOCE. The knockdown of STIM1 was verified by Western blot analysis (Supplemental Fig. 5A–B) in which the majority of the protein was knocked down in three independent experiments. Further, similar, but less effective results were obtained in these cells when an shRNA directed against a different site on STIM1 was used (data not shown). We take these data to strongly suggest that ICRAC underlies SOCE in A10 smooth muscle cells, and that STIM1 is required for activation of SOCE in these cells.
In order to determine whether STIM1 regulates endogenous TRPC6 in these A10 smooth muscle cells, whole-cell patch-clamp experiments were carried out similar to those shown in Fig. 7. AVP was focally applied to cells under voltage clamp, and voltage ramps (−100 to +100 mV) were applied every few seconds to measure the AVP-activated currents that developed over time. Figure 8E shows representative experiments for a siControl cell (black trace) and a STIM1 siRNA-treated cell (grey trace), in which the focal application of AVP activated TRPC6 currents which were unaffected by the loss of STIM1 protein. Figure 8F shows the summary of the data collected, in which the current densities activated by AVP were not altered by the knockdown of STIM1. Thus, we believe endogenous TRPC6 is not regulated by STIM1.
TRPC and STIM1/Orai1 signalling occur in distinct plasma membrane domains
A functional interaction between STIM1 and TRPC channels would suggest that STIM1 and TRPC localize to similar domains within the cell. Several studies have reported that TRPC channels are localized to lipid raft domains of the plasma membrane, and that this localization is necessary for proper channel function. However, whether STIM1 similarly localizes to lipid rafts has not been determined. We therefore tested whether TRPC and/or STIM1 and CRAC signalling is associated with lipid rafts by performing experiments using the cholesterol sequestering agent methyl β cyclodextrin (MβCD) (Christian et al. 1997; Graziani et al. 2006) in HEK293 cells stably expressing TRPC3. TRPC3 cells were pre-treated with 1, 5 or 10 mm MβCD for 1 h, and then the drug was washed out three times with normal HBSS. Whole-cell patch-clamp experiments were then carried out to determine the effects of lipid raft disruption on TRPC3 channel activity. OAG was used to activate the TRPC3 channels in order to bypass any effects MβCD might have on signalling upstream of channel activity. Figure 9A–C shows that disruption of lipid rafts by MβCD completely suppresses TRPC3 activity, compared to control cells. One millimolar MβCD significantly reduced the OAG-activated TRPC3 current, and 10 mm MβCD completely abolished it. Further, 10 mm MβCD also completely suppressed constitutive TRPC3 activity, as revealed by the reduction in the initial current density just after break-in seen in panel A.
Figure 9. TRPC and STIM1/Orai1 signalling occur in distinct plasma membrane domains.
A, whole-cell voltage clamp experiments (−120 to +120 mV voltage ramp applied every 2 s) taken from HEK293 cells stably expressing TRPC3 and pre-treated with vehicle (0, black continuous trace), 1 mm (1, grey dashed trace), 5 mm (5, black dashed trace) or 10 mm (10, grey continuous trace) MβCD for 30 min. Whole-cell TRPC3 currents were activated by focal application of 30 μm OAG as indicated by the line above the graph. B, current–voltage relationships taken from the recordings shown in A after OAG application. Currents were not leak subtracted. C, bar graph showing significant inhibition of TRPC3 currents in cells treated with millimolar concentrations of MβCD (Control: n= 7; 5 mm MβCD: n= 8; 10 mm MβCD: n= 6). D, Na+-ICRAC measurements taken from HEK293 cells expressing eYFP-STIM1 (+100 to −120 mV every 2 s) with (grey trace) or without (black trace) 10 mm MβCD treatment prior to break-in. Stores were depleted using 25 μm IP3 in the patch pipette and all external divalent cations were removed in order to amplify CRAC currents. External solution exchanges were focally applied as indicated by the lines above the graph. E, leak subtracted I–V plots taken from the peak Na+ CRAC currents shown in D. F, bar graph showing in eYFP-STIM1-expressing cells that sequestration of cholesterol from the lipid bilayer by pre-treating the cells with MβCD has no effect on ICRAC (Control: n= 7; 5 mm MβCD: n= 10; 10 mm MβCD: n= 5). Plotted are peak Na+ CRAC currents. Error bars represent means ±s.e.m. and data analyses were carried out using ANOVA followed by Tukey's test for pairwise comparisons.
In stark contrast to the effects of MβCD on TRPC3 activity, ICRAC measurements in HEK293 cells expressing eYFP-STIM1 were completely insensitive to lipid raft disruption by MβCD pre-treatment. Figure 9D shows representative ICRAC measurements with (grey trace) and without (black trace) the pre-treatment of 5 mm MβCD. Experiments were carried out using IP3 to actively deplete the stores, and divalent-free external solutions were focally applied in order to amplify the small ICRAC currents in these cells, as previously described (DeHaven et al. 2007) (similar to Fig. 5). Figure 9E and F show the current–voltage relationships for the recordings shown in panel D, and the means ±s.e.m. for all cells tested, respectively.
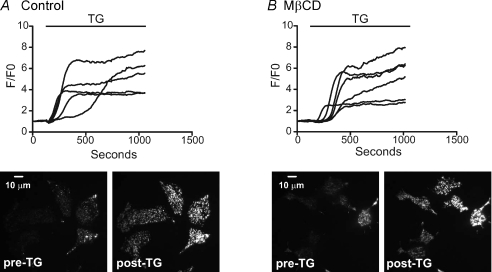
MβCD effects on TRPC3 and CRAC channel activity and SOCE were also investigated using fura-5f imaging. In these assays, TRPC3 activity was also reduced after MβCD pre-treatment. In contrast to the results with current measurement, Ca2+ imaging experiments on WT HEK293 cells with thapsigargin treatment and re-addition of Ca2+ revealed SOCE was partially reduced in the MβCD pre-treated cells (data not shown). However, in experiments utilizing membrane potential-sensitive dyes, MβCD caused apparent depolarization when added acutely, and pre-treatment with MβCD almost completely inhibited the depolarizing effects of high KCl. This may indicate that MβCD treatment causes substantial plasma membrane depolarization (α-cyclodextrin did not depolarize these cells, Supplemental Fig. 6). Since measurement of Icrac under voltage clamp did not detect any effects of MβCD, we conclude that the inhibitory effect on SOCE in imaging experiments most probably reflect a decrease in driving force. Therefore, caution should be used when working with MβCD and Ca2+ indicator dyes.
The functional experiments showing that ICRAC is not dependent upon lipid rafts was supported by confocal experiments looking at the localization of eYFP-STIM1 and the raft constituent ganglioside, GM1, which is used extensively for imaging lipid rafts (Galbiati et al. 2001). Figure 10 shows images of live HEK293 cells expressing eYFP-STIM1 (panel A) and labelled with a fluorophore conjugated to the cholera toxin β-subunit, which binds to GM1 (panel B), before (I, Control) and after (ii.+TG) 15 min treatment with the SERCA pump inhibitor, thapsigargin. Images were taken at the cell–coverslip interface in order to see near-plasma membrane events. After store depletion with thapsigargin, eYFP-STIM1 rearranged into punctate structures (Fig. 10A). However, store depletion had little or no effect on the arrangement of the GM1 in the plasmalemma (Fig. 10B). More importantly, there was essentially no co-localization between STIM1 (green) and GM1 (red) (lipid rafts) after store depletion (Fig. 10C). Accordingly, there was no effect of MβCD pre-treatment on STIM1 movements to near-plasma membrane areas, as assessed by TIRF microscopy. Similar to the experiments carried out on TRPC3-expressing cells, eYFP-STIM1-expressing HEK293 cells were pre-treated with MβCD for 1 h, followed by three washes in HBSS. Then, TIRF microscopy experiments were carried out before, during and after store depletion with thapsigargin. Figure 11A and B shows by TIRF microscopy that lipid raft disruption by 10 mm MβCD had no effect on the ability of STIM1 to re-arrange into punctate-like structures after store depletion. In control cells (panel A), the application of thapsigargin caused significant rearrangement of STIM1, causing the molecule to become punctate and approach the plasma membrane, as previously described (Liou et al. 2005). Notably, identical results were seen in the STIM1-expressing cells pre-treated with 10 mm MβCD. Thus, our data suggest TRPC3 channel function depends on the lipid make-up of the plasma membrane; however, STIM1 signalling and activation of Orai channels apparently does not require or involve lipid raft microdomains.
Figure 11. Disruption of lipid rafts by MβCD does not prevent eYFP-STIM1 from rearranging into puncta in response to store depletion by thapsigargin.
A, time-controlled experiments showing the changes in near-plasma membrane fluorescence intensities measured by TIRF microscopy in HEK293 cells expressing eYFP-STIM1 and treated with 2 μm thapsigargin to deplete internal Ca2+ stores. Each trace represents an individual cell taken from a single coverslip. B, same as in A, however, in cells pre-treated with 10 mm MβCD. Lines above graphs indicate where thapsigargin was applied.
Discussion
Since the first description of store-operated Ca2+ entry (SOCE) over 20 years ago, investigators have focused on determining the molecular make-up of the signal within the ER and the ion channels in the plasma membrane which form SOCE (Putney, 1997; Parekh & Putney, 2005). Within those 20 years, many hypotheses have developed for the signal; however, the recent discovery of STIM1 has clarified this issue. We now know that STIM1 functions as an ER Ca2+ sensor that can signal to the plasma membrane when the stores have been depleted of their Ca2+ and activate, either directly or indirectly, SOC channels (Luik & Lewis, 2007; Csutora et al. 2008). The inception of whole genome RNAi screens led to the discovery of the SOC channels, Orai1, (also known as CRACM1), which is now believed to be the pore-forming subunits of the SOCE channels known as the Ca2+ release-activated Ca2+ (CRAC) channels (Prakriya et al. 2006; Vig et al. 2006a). Two homologues of Orai1, Orai2 and 3, have been shown to be similarly activated by store depletion in transfection experiments but their function in native SOC channels is yet to be established. While CRAC channels are the conventional highly Ca2+ selective SOC channels, evidence exists for SOC channels which are much less Ca2+ selective (Parekh & Putney, 2005). The canonical transient receptor potential class of channels (TRPC), which are non-selective cation channels, are the leading candidates for alternative SOC channels functioning in a store dependent manner under certain conditions (Albert et al. 2007). Like CRAC channels, TRPC channels are activated downstream of PLC activity, and it is has been suggested that SOC currents, which are less Ca2+ selective, may involve channels composed of TRPC subunits.
However, to date STIM1 (and to some extent the closely related STIM2) is the only known ER Ca2+-sensing molecule. Thus, it could function as a regulatory protein capable of universally regulating specific ion channels, including the archetypical Orai, but also possibly TRPC channels, in a store-dependent manner. It has been proposed that store-operated channels should be redefined as any channels regulated by STIM1 (Yuan et al. 2007). It is also possible that STIM1 could be a structural protein required for channel activity (in this case, TRPC), but which may not necessarily confer store dependence to the channels. Indeed, there is evidence for STIM1 regulation of one non-store-operated channel, apparently gated by arachidonic acid (Mignen et al. 2007). While the hypothesis that STIM1 functionally regulates TRPC channels has been investigated by many groups (Huang et al. 2006; Liao et al. 2007; Ong et al. 2007; Alicia et al. 2008; Cheng et al. 2008; Jardin et al. 2008a,b; Li et al. 2008; Liao et al. 2008; Ma et al. 2008), the experiments were in many cases carried out with agonists which activate both the second-messenger and store-operated pathways. Thus, there are two key questions: one, are TRPC channels activated by depletion of Ca2+ stores; and two, is STIM1 involved in the activation mechanism of TRPC channels, whether by store depletion or by products of phospholipase C. The interaction of STIM1 with Orai channel subunits is readily demonstrated by co-expression of STIM1 with Orai, resulting in profound synergistic increases in agonist- or thapsigargin-activated Ca2+ entry (Mercer et al. 2006; Peinelt et al. 2006; Zhang et al. 2006). Thus, in the current study, we examined the activation of Ca2+ entry through the PLC-linked muscarinic receptor in HEK293 cells co-transfected with selected TRPCs and STIM1. We carried out experiments in the presence and absence of the Icrac inhibitor, Gd3+. Thus any positive interaction of STIM1 with either store-operated or PLC-dependent TRPC channels should have been revealed in these experiments. However, no significant augmentation by STIM1 of agonist-induced entry was observed with any of the TRPCs, regardless of the presence or absence of Gd3+, and regardless of expression level. Thus, in our hands, STIM1 appears incapable of interacting with these TRPC channels in any functional way.
In our past experiences, we have generally found that store depletion protocols do not activate TRPC channels (McKay et al. 2000; Trebak et al. 2003a; Lievremont et al. 2004, 2005; Lemonnier et al. 2006). However, we published two instances in which store depletion apparently activated TRPCs. In one instance, TRPC3 could be activated by thapsigargin but only when expressed at low levels in DT40 B-cells (Vazquez et al. 2003). In a second case, TRPC7 could be activated by thapsigargin, but only in HEK293 cells stably transfected with TRPC7 (Lievremont et al. 2004). In both cases, no currents were reported, and the only means of activating the cells was by application of the SERCA inhibitor, thapsigargin. In the current study, we examined in detail the activation of TRPC7 in stably transfected HEK293 cells by thapsigargin. Surprisingly, we found that only thapsigargin, and no other means of store depletion, was capable of activating TRPC7 in these cells. In addition, the activation of TRPC7 current began almost immediately upon addition of thapsigargin or another SERCA inhibitor, CPA. This is not expected for a store-operated channel which generally requires much longer times before passive depletion protocols begin to activate (Broad et al. 1999a). We conclude that TRPC7 is activated as a result of SERCA inhibition per se rather than by depletion of Ca2+ stores. Interestingly, in a previous study, we showed that in HEK293 cells transiently transfected with TRPC7, SERCA inhibitors inhibited channel activation by diacylglycerols (Lemonnier et al. 2006). The mechanisms underlying these interesting but complex interactions between SERCA pumps and TRPC7 channels merits further investigation. Nonetheless, since it is clear that TRPC7 is not being activated by Ca2+ store depletion, it is perhaps not surprising, and consistent with the findings with transiently transfected TRPCs, that TRPC7 activity is not affected by STIM1 expression, regardless of the means of its activation.
The results on stably expressing TRPC7 HEK293 cells also underscore the importance of utilizing multiple approaches to depleting internal Ca2+ stores in determining whether an apparent store-operated entry pathway is truly store operated. In this instance, we misinterpreted our previous work using thapsigargin-activated entry as sufficient evidence for SOCE, when clearly that is not the case.
All of the findings discussed to this point involved studies of TRPC channels transfected into cells. It is for this reason that we also investigated STIM1 function in a vascular smooth muscle cell line, A10, which appears to express a native TRPC6 channel. A similar and widely investigated vascular smooth muscle cell line, A7r5, has previously been shown to express a non-selective cation current which is activated by arginine–vasopressin (AVP) signalling through a G-protein receptor, and has many of the biophysical properties of TRPC6 (cfTrebak et al. 2007). Recently, RNAi studies have molecularly identified that TRPC6 is indeed essential in making up these channels (Soboloff et al. 2005; Takahashi et al. 2008). We detected a similar current in A7r5 cells (data not shown), as well as a related smooth muscle cell line (A10 cells). Both cells are very similar morphologically, and respond identically to AVP (Van et al. 1988; Korbmacher et al. 1989). TRPC6 siRNA attenuated the whole-cell currents in these A10 vascular smooth muscle cells, as well as TRPC6-expressing HEK293 control cells. Importantly, we also detected small but reproducible ICRAC, and we found that thapsigargin-evoked SOCE in these cells was dependent on the proteins STIM1 (Fig. 8) and Orai1 (data not shown). Thus, these cells proved useful in examining possible regulation by STIM1 of an endogenous TRPC. RNAi directed against STIM1 had little to no inhibitory effect on the AVP-activated non-selective currents, while it significantly reduced SOCE. Therefore, studies on endogenous proteins recapitulate what was observed for expressed proteins: no detectable functional interaction between STIM1 and TRPC channels after the activation of PLC.
Along with the receptors that activate them, TRPC channels have long been suggested to be associated with and regulated by lipids in the plasma membrane (PM), especially with respect to the role of cholesterol-rich lipid rafts (Bergdahl et al. 2003; Brownlow et al. 2004; Groschner et al. 2004; Graziani et al. 2006; Alicia et al. 2008; Jardin et al. 2008b; Pani et al. 2008). Less has been published on SOCE and membrane lipids, and nothing has been published suggesting STIM1/Orai1 signalling requires lipid raft domains in order to function properly. However, recent reports have described clustering of STIM1 and TRPC1 complexes (Alicia et al. 2008; Pani et al. 2008) or STIM1–Orai1–TRPC1 complexes (Jardin et al. 2008b). In order to examine the possible involvement of cholesterol-rich lipid rafts, experiments were carried out using the agent MβCD, which disrupts lipid rafts by sequestering cholesterol from the plasma membrane (Christian et al. 1997). Using stable TRPC3 HEK293 cells, we showed that MβCD pre-treatment completely blocked OAG-activated TRPC3 activity, as assessed by whole-cell patch-clamp experiments. However, MβCD pre-treatment had no effect on ICRAC measured from HEK293 cells expressing STIM1. Accordingly, there was no effect of MβCD pre-treatment on STIM1 movements in response to store depletion. However, our co-localization experiments using GM1 as a marker for lipid rafts suggested that STIM1 puncta do not associate with lipid raft domains in the plasma membrane. Therefore, we feel it is unlikely that STIM1 could be specifically regulating an ion channel associated with lipid rafts.
All of the findings in this multi-faceted study lead one to the same conclusion: store-activated channels, composed specifically of Orai subunits, require STIM1 as an endoplasmic reticulum Ca2+ sensor, while TRPC channels, regardless of their mechanism of activation or expression level, apparently do not depend upon or interact with STIM1 in any detectable functional manner. We are aware that there are numerous studies that have come to a different conclusion, generally based on somewhat alternative strategies and often different cell backgrounds (Zhu et al. 1996; Kiselyov et al. 1998; Liu et al. 2000; Rosado & Sage, 2000; Beech et al. 2003; Liao et al. 2007; and others; reviewed in Parekh & Putney, 2005). Some studies have utilized more complex combinations of STIM1, Orai1 and TRPCs (Cheng et al. 2008; Jardin et al. 2008a; Liao et al. 2008) which are not addressed in this study. It is, of course, not possible to attempt to reproduce every experimental protocol that has utilized either overexpressed TRPCs or TRPC knockdown to evaluate their function. Rather, in the current study, we have focused on what we believe to be a simple approach to studying TRPC function, and specifically its potential interaction with STIM1. Following the logic of Popper's black swan problem (Taleb, 2007) we have tested the idea that TRPC channels do not functionally interact with STIM1 by attempting falsification, and in every instance we have failed. We feel that if such an interaction were to occur, it would readily have been detected in our experiments. We cannot speculate on the many variables that may affect the experimental findings from other laboratories. We have illustrated one example of a misleading result that led us in the past to wrongly attribute store-dependent activation to a TRPC channel. It has also been recently shown that TRPC1 can appear to contribute to a store-operated current when, in fact, it is being activated by IP3, independently of intracellular stores (Zarayskiy et al. 2007).
TRPC channels support a number of important physiological functions, including smooth muscle activity (Dietrich et al. 2005), exocrine gland secretion (Liu et al. 2007), neuronal migration (Greka et al. 2003; Hui et al. 2006) to name a few. At the cellular level, they have been shown to be regulated by a myriad of signals, including IP3 receptors (Kiselyov et al. 1998; Rosado & Sage, 2000; Vazquez et al. 2006), diacylglycerols (Hofmann et al. 1999), inositol lipids (Kwon et al. 2007; Lemonnier et al. 2008), src (Vazquez et al. 2004; Kawasaki et al. 2006), myosin light chain kinase (Shimizu et al. 2006), and by Ca2+ in complex ways (Trebak et al. 2003b; Lemonnier et al. 2006). Continued research is needed to establish the physiological pathways that regulate and activate this important ion channel family so that useful pharmacological interventions can be developed to aid in the treatment of associated diseases.
Acknowledgments
This work was supported in part by the Intramural Research Program, National Institute of Environmental Health Sciences, National Institutes of Health, project number Z01 ES090087.
Glossary
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- ER
endoplasmic reticulum
- eYFP
enhanced yellow fluorescent protein
- Icrac
calcium release activated Ca2+ current
- IP3
inositol trisphosphate
- OAG
oleyl acetyl glycerol
- PLC
phospholipase C
- SERCA
sarcoplasmic endoplasmic reticulum Ca2+ ATPase
- siRNA
small inhibitory RNA
- SOCE
store-operated Ca2+ entry
- STIM1
stromal interacting molecule 1
- TRPC
cannonical transient receptor potential channel
Author contributions
W.I.D., conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published; B.F.J., conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published; J.G.P., conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published; J.T.S., conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published; T.T., conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published; G.S.B., conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published; J.W.P. Jr, conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2009.170431/DC1
References
- Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583:25–36. doi: 10.1113/jphysiol.2007.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a storeoperated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad LM, Armstrong DL, Putney JW. Role of the IP3 receptor in Ca2+ feedback inhibition of calcium release-activated calcium current (Icrac. J Biol Chem. 1999a;274:32881–32888. doi: 10.1074/jbc.274.46.32881. [DOI] [PubMed] [Google Scholar]
- Broad LM, Cannon TR, Taylor CW. A noncapacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J Physiol. 1999b;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow SL, Harper AG, Harper MT, Sage SO. A role for hTRPC1 and lipid raft domains in store-mediated calcium entry in human platelets. Cell Calcium. 2004;35:107–113. doi: 10.1016/j.ceca.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium signaling: a tale for all seasons. Proc Nat Acad Sci U S A. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- Csutora P, Peter K, Kilic H, Park KM, Zarayskiy V, Gwozdz T, Bolotina VM. Novel role for STIM1 as a trigger for calcium influx factor production. J Biol Chem. 2008;283:14524–14531. doi: 10.1074/jbc.M709575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW., Jr Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;283:19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven WI, Smyth JT, Boyles RR, Putney JW. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos YS, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos YS, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Frischauf I, Schindl R, Derler I, Bergsmann J, Fahrner M, Romanin C. The STIM/Orai coupling machinery. Channels. 2008;2:261–268. doi: 10.4161/chan.2.4.6705. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Graziani A, Rosker C, Kohlwein SD, Zhu MX, Romanin C, Sattler W, Groschner K, Poteser M. Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy. Biochem J. 2006;396:147–155. doi: 10.1042/BJ20051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Groschner K, Rosker C, Lukas M. Role of TRP channels in oxidative stress. Novartis Found Symp. 2004;258:222–230. [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993;16:371–376. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–262. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Nat Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, Levenson R, Beech DJ, Weiss JL. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol. 2006;572:165–172. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Zhu X, Boulay G, Birnbaumer L, Stefani E. Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Letters. 1998;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem. 2008a;283:25296–25304. doi: 10.1074/jbc.M802904200. [DOI] [PubMed] [Google Scholar]
- Jardin I, Salido GM, Rosado JA. Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels. 2008b;2:401–403. doi: 10.4161/chan.2.6.7055. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol. 1999;518:345–358. doi: 10.1111/j.1469-7793.1999.0345p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci U S A. 2006;103:335–340. doi: 10.1073/pnas.0508030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Korbmacher C, Helbig H, Stahl F, Coroneo M, Haller H, Lindschau C, Quass P, Wiederholt M. Continuous membrane voltage recordings in A10 vascular smooth muscle cells: effect of AVP. Am J Physiol Cell Physiol. 1989;257:C323–C332. doi: 10.1152/ajpcell.1989.257.2.C323. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier L, Trebak M, Lievremont JP, Bird GS, Putney JW. Protection of TRPC7 cation channels from calcium inhibition by closely associated SERCA pumps. FASEB J. 2006;20:503–505. doi: 10.1096/fj.05-4714fje. [DOI] [PubMed] [Google Scholar]
- Lemonnier L, Trebak M, Putney JW., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506–514. doi: 10.1016/j.ceca.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103:e97–e104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievremont JP, Bird GS, Putney JW. Canonical transient receptor potential TRPC7 can function as both a receptor- and store-operated channel in HEK-293 cells. Am J Physiol Cell Physiol. 2004;287:C1709–C1716. doi: 10.1152/ajpcell.00350.2004. [DOI] [PubMed] [Google Scholar]
- Lievremont JP, Numaga T, Vazquez G, Lemonnier L, Hara Y, Mori E, Trebak M, Moss SE, Bird GS, Mori Y, Putney JW. The role of canonical transient receptor potential 7 in B-cell receptor-activated channels. J Biol Chem. 2005;280:35346–35351. doi: 10.1074/jbc.M507606200. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(–/–) mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- Lopez J, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol Med. 2007;13:103–107. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H-T, Venkatachalam K, Li H-S, Montell C, Kurosaki T, Patterson RL, Gill DL. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- McKay RR, Szmeczek-Seay CL, Lièvremont J-P, Bird GStJ, Zitt C, Jüngling E, Lückhoff A, Putney JW. Cloning and expression of the human transient receptor potential 4 (TRP4) gene: localization and functional expression of human TRP4 and TRP3. Biochem J. 2000;351:735–746. [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. Large store-operated calciumselected currents due to co-expression of orai1 or orai2 with the intracellular calcium sensor, stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+ selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner RE, Famulski KS, Michalak M. Calcium binding proteins in the sarcoplasmic/endoplasmic reticulum of muscle and nonmuscle cells. Mol Cell Biochem. 1992;112:1–13. doi: 10.1007/BF00229637. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, Koblan Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative Calcium Entry. Austin, TX: Landes Biomedical Publishing; 1997. pp. 1–210. [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Coupling between inositol 1,4,5-trisphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem J. 2000;350:631–635. [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Yoshida T, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, Sakurada K, Kiuchi Y, Mori Y. Ca2–calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol. 2006;570:219–235. doi: 10.1113/jphysiol.2005.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Lemonnier L, Vazquez G, Bird GS, Putney JW. Dissociation of regulated trafficking of TRPC3 channels to the plasma membrane from their activation by phospholipase C. J Biol Chem. 2005;281:11712–11720. doi: 10.1074/jbc.M510541200. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem. 2005;280:39786–39794. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, Mori MX, Honda A, Inoue R. Nitric oxide–cGMP–protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol. 2008;586:4209–4223. doi: 10.1113/jphysiol.2008.156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb NN. The Black Swan: The Impact of the Highly Improbable. London: Penguin Books; 2007. pp. 1–400. [Google Scholar]
- Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney JW., Jr Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol. 2007:593–614. doi: 10.1007/978-3-540-34891-7_35. [DOI] [PubMed] [Google Scholar]
- Trebak M, St J Bird J, McKay RR, Birnbaumer L, Putney JW. Signaling mechanism for receptor-activated TRPC3 channels. J Biol Chem. 2003a;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- Trebak M, Vazquez G, St J Bird J, Putney JW. The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003b;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- Uvelius B, Sigurdson SB, Johansson B. Strontium and barium as substitutes for calcium on electrical and mechanical activity in rat portal vein. Blood Vessels. 1974;11:245–259. doi: 10.1159/000158019. [DOI] [PubMed] [Google Scholar]
- Van RC, Romey G, Lazdunski M. Vasopressin modulates the spontaneous electrical activity in aortic cells (line A7r5) by acting on three different types of ionic channels. Proc Natl Acad Sci U S A. 1988;85:9365–9369. doi: 10.1073/pnas.85.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi JM, Martonosi A. Sarcoplasmic reticulum. XII. The interaction of 8-anilino-1-naphthalene sulfonate with skeletal muscle microsomes. Arch Biochem Biophys. 1971;144:87–98. doi: 10.1016/0003-9861(71)90457-7. [DOI] [PubMed] [Google Scholar]
- Varga-Szabo D, Authi KS, Braun A, Bender M, Ambily A, Hassock SR, Gudermann T, Dietrich A, Nieswandt B. Store-operated Ca2+ entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflugers Arch. 2008;457:377–387. doi: 10.1007/s00424-008-0531-4. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Bird GS, Mori Y, Putney JW. Native TRPC7 channel activation by an inositol trisphosphate receptor-dependent mechanism. J Biol Chem. 2006;281:25250–25258. doi: 10.1074/jbc.M604994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez G, Lièvremont J-P, St J Bird J, Putney JW. Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc Nat Acad Sci USA. 2001;98:11777–11782. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez G, Wedel BJ, Kawasaki BT, Bird GS, Putney JW. Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J Biol Chem. 2004;279:40521–40528. doi: 10.1074/jbc.M405280200. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Wedel BJ, Trebak M, St J Bird J, Putney JW. Expression level of TRPC3 channel determines its mechanism of activation. J Biol Chem. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel B, Boyles RR, Putney JW, Bird GS. Role of the store-operated calcium entry proteins, Stim1 and Orai1, in muscarinic-cholinergic receptor stimulated calcium oscillations in human embryonic kidney cells. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarayskiy V, Monje F, Peter K, Csutora P, Khodorov BI, Bolotina VM. Store-operated Orai1 and IP3 receptor-operated TRPC1 channel. Channels. 2007;1:246–252. doi: 10.4161/chan.4835. [DOI] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- Zitt C, Obukhov AG, Strübing C, Zobel A, Kalkbrenner F, Lückhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.