Abstract
At central glutamatergic synapses, neurotransmitter often saturates postsynaptic AMPA receptors (AMPARs), thereby restricting the dynamic range of synaptic efficacy. Here, using simultaneous pre- and postsynaptic whole-cell recordings, at the calyx of Held synapse of immature rats, we have investigated the mechanism by which transmitter glutamate saturates postsynaptic AMPARs. When we loaded l-glutamate (1–100 mm) into presynaptic terminals, the quantal EPSC (qEPSC) amplitude changed in a concentration-dependent manner. At physiological temperature (36–37°C), the qEPSC amplitude increased when intraterminal l-glutamate concentration was elevated from 1 mm to 10 mm, but it reached a plateau at 10 mm. This plateau persisted after bath-application of the low affinity AMPAR antagonist kynurenate, suggesting that it was caused by saturation of vesicular filling with glutamate rather than by saturation of postsynaptic AMPARs. In contrast to qEPSCs, action potential-evoked EPSCs remained unchanged by increasing intraterminal l-glutamate from 1 mm to 100 mm, even at room temperature, indicating that multi-quantal glutamate saturated postsynaptic AMPARs. This saturation could be relieved by blocking AMPAR desensitization using cyclothiazide (100 μm). The concentration of ambient glutamate in the slice, estimated from NMDA receptor current fluctuations, was 55 nm; this was far below the concentration required for AMPAR desensitization. We conclude that rapid AMPAR desensitization, caused by glutamate released from multiple vesicles during synaptic transmission, underlies postsynaptic AMPAR saturation at this immature calyceal synapse before the onset of hearing.
The mean quantal size, together with the mean number of vesicles released by a presynaptic impulse, is a critical parameter determining the efficacy of synaptic transmission (Del Castillo & Katz, 1954). However, the mechanism, by which quantal size is determined, is not entirely clear. If a single quantal, or vesicular, transmitter transient saturates postsynaptic receptors, as previously proposed (Jack et al. 1981; Larkman et al. 1991), quantal size will be determined predominantly by the number of postsynaptic receptors. At a variety of synapses, however, it has been suggested that transmitter from a single vesicle may not saturate postsynaptic receptors (Silver et al. 1996b; Auger et al. 1998; Liu et al. 1999; Mainen et al. 1999; Sargent et al. 2005). At the calyx of Held, direct infusion of the transmitter glutamate into the presynaptic terminal enhances the qEPSC amplitude, suggesting that glutamate from a single vesicle does not saturate postsynaptic AMPARs (Ishikawa et al. 2002; Yamashita et al. 2003; Wu et al. 2007). However, as these experiments were made mostly at room temperature (RT), it remains unclear whether uni-quantal glutamate saturates postsynaptic AMPARs at physiological temperature (PT), where vesicles may be filled with glutamate to higher concentrations.
At glutamatergic synapses with a high release probability, neurally evoked synaptic currents often fail to increase their amplitude when transmitter release probability is increased, by elevation of extracellular Ca2+ concentration ([Ca2+]o) (Iwasaki & Takahashi, 2001; Foster et al. 2002), or by direct stimulation of the release machinery downstream of Ca2+ influx (Kaneko & Takahashi, 2004). Since this type of saturation in synaptic efficacy can be relieved by low affinity AMPAR antagonists (Meyer et al. 2001; Neher & Sakaba, 2001; Foster et al. 2002; Scheuss et al. 2002; Wong et al. 2003; Kaneko & Takahashi, 2004), it is thought to result from saturation of postsynaptic AMPARs by the transmitter glutamate. However, these antagonists can also reduce AMPAR desensitization by protecting the receptors during glutamate transients (Wong et al. 2003). Therefore, it remains to be investigated whether desensitization contributes to saturation of postsynaptic AMPARs.
Rodents start to hear sound at postnatal day (P) 10–12 (Jewett & Romano, 1972). At the calyx of Held synapse in the auditory brainstem, across the hearing onset period, release probability decreases, roughly by half (from 0.3–0.5 at P5–8 to 0.1–0.2 at P12–15; Taschenberger et al. 2002; Koike-Tani et al. 2008). At immature calyceal synapses, in the prehearing period, postsynaptic AMPARs are thought to be saturated by glutamate during synaptic transmission (Meyer et al. 2001; Neher & Sakaba, 2001; Sun & Wu, 2001; Iwasaki & Takahashi, 2001; Kaneko & Takahashi, 2004; Taschenberger et al. 2005). Cyclothiazide (CTZ) effectively reduces synaptic depression at calyceal synapses in the prehearing, but not in the posthearing, period (Taschenberger et al. 2002). Similarly, only in the prehearing period, CTZ increases the qEPSC amplitude (Koike-Tani et al. 2005). These results suggest that ambient or vesicular glutamate may desensitize postsynaptic AMPARs at immature calyces, thereby promoting saturation of AMPARs during synaptic transmission. By loading l-glutamate into nerve terminals, at P7–8 rat calyces of Held, we have examined the mechanism by which postsynaptic AMPARs are saturated during synaptic transmission. Our results indicate that postsynaptic AMPARs can be saturated only when glutamate from multiple vesicles overlaps in the synaptic cleft and promotes receptor desensitization.
Methods
Slice preparation and solutions
All experiments were performed in accordance with the guidelines of the Physiological Society of Japan. Wistar rats (P7–8, 81 rats in total) were decapitated under inhalation anaesthesia with halothane (1–2%). Transverse brainstem slices (200 μm thick) containing the medial nucleus of trapezoid body (MNTB) were cut as described previously (Yamashita et al. 2003). Slices were incubated for 1 h at 36–37°C in artificial cerebrospinal fluid (aCSF) containing (in mm); 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 10 glucose, 3 myo-inositol, 2 sodium pyruvate and 0.5 ascorbic acid (pH 7.4 when bubbled with 95% O2 and 5% CO2, 310–320 mosmol l−1), and maintained thereafter at room temperature (RT, 22–28°C). The aCSF contained bicuculline methiodide (10 μm) and strychnine hydrochloride (0.5 μm) to block inhibitory synaptic responses, and also d(-)2-amino-5-phosphonovaleric acid (d-APV, 50 μm) to block NMDA receptor (NMDAR)-mediated currents, unless otherwise noted. Patch pipettes for postsynaptic recordings were filled with (in mm): 110 CsF, 30 CsCl, 10 Hepes, 5 EGTA, 1 MgCl2 and 5 N-(2, 6-diethylphenylcarbamoylmethyl)-triethyl-ammonium chloride (QX-314) (pH adjusted to 7.3–7.4 with CsOH, 290–295 mosmol l−1). Tetrodotoxin (TTX, 1 μm) was added to the aCSF for recording spontaneous miniature EPSCs (mEPSCs), i.e. qEPSCs. To record NMDAR currents, MgCl2 and d-APV in the aCSF were omitted and glycine (10 μm) was supplemented. Presynaptic pipette solutions contained (in mm): 105 potassium gluconate, 30 KCl, 10 Hepes, 0.5 EGTA, 12 phosphocreatine (Na salt), 3 ATP (Mg salt), 0.5 GTP (Na salt), and 1 MgCl2 (pH 7.3–7.4 adjusted with KOH, 290–300 mosmol l−1). The l-glutamate solution for presynaptic infusion was prepared by replacing potassium gluconate in the pipette solution by l-glutamate (K salt) to maintain iso-osmolarity. Patch pipettes for outside-out recordings were filled with (in mm): 140 CsCl, 10 Hepes, 10 EGTA, 2 ATP (Mg salt), 0.5 GTP (Na salt) (pH 7.3 adjusted with CsOH).
Recordings
Whole-cell recordings were made from the calyx of Held presynaptic terminals and postsynaptic principal neurons in the MNTB, using a patch-clamp amplifier (Multiclamp 700A, Axon Instruments, USA or EPC 9/2, HEKA, Germany) as described previously (Ishikawa et al. 2002; Yamashita et al. 2003). In order to compare results with those previously reported, most experiments were carried out at RT (22–28°C). However, experiments in Figs 1 and 2 were performed at both RT and physiological temperature (PT, 36–37°C). Postsynaptic pipettes had a resistance of 1.5–3 MΩ. The access resistance of whole-cell recordings was 3–10 MΩ, which was compensated by up to 40% to keep the value constant at 3–6 MΩ throughout each experiment. Presynaptic pipettes had resistances of 5–9 MΩ and resulted in access resistances of 10–20 MΩ. The postsynaptic cells were voltage-clamped at a holding potential of −70 mV. EPSCs were evoked by presynaptic action potentials elicited by 1–3 ms depolarizing current injections in current-clamp mode. For stable EPSC recordings, stimulation frequency was kept at 0.03 or 0.05 Hz to let EPSCs fully recover from depression (Iwasaki & Takahashi, 2001) and l-glutamate was included in presynaptic patch pipettes at 1 mm to minimize run-down of quantal size (Ishikawa et al. 2002). Infusion of l-glutamate (100 mm) into the presynaptic terminal was made by the pipette perfusion technique using a plastic tube installed in the presynaptic pipette as described previously (Hori et al. 1999; Ishikawa et al. 2002; Yamashita et al. 2003).
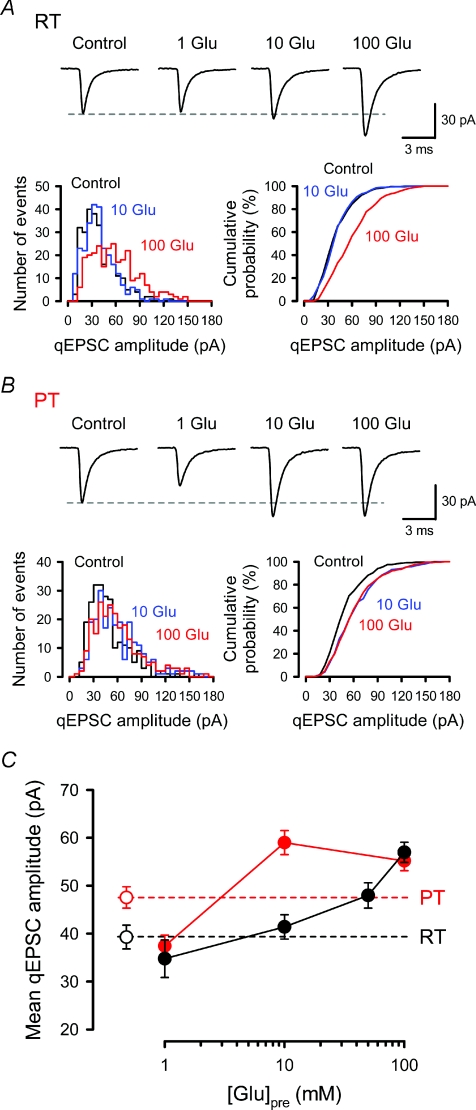
Figure 1. Dependence of mean quantal amplitude on presynaptic cytoplasmic l-glutamate concentration.
In simultaneous pre- and postsynaptic whole-cell recordings, presynaptic pipettes contained 1–100 mm l-glutamate, and qEPSCs were recorded from postsynaptic MNTB neurons in the presence of TTX (1 μm). Data were sampled more than 10 min after rupturing the presynaptic membrane. A and B, sample traces represent averaged qEPSCs recorded without presynaptic dialysis (Control) or with 1, 10 or 100 mm l-glutamate (1 Glu, 10 Glu, 100 Glu) in the presynaptic pipette at RT (A) and PT (B). Amplitude histograms (left columns) and cumulative plots (right columns) of qEPSCs in control (black) or with 10 mm (blue) or 100 mm (red) l-glutamate are shown (superimposed). The total number of events is 270 for each in A and 240 for each in B. Presynaptic loading of 100 mm (at RT and PT) and 10 mm (PT) l-glutamate significantly increased the proportion of larger qEPSCs (P < 0.001 for A and B, Kolmogorov–Smirnov test). C, the mean qEPSC amplitudes (filled circles) from 5–14 synapses (201–505 events for each) were plotted against l-glutamate concentrations in the presynaptic pipette at RT (black) and PT (red). Open circles and dashed lines represent the mean amplitude of qEPSCs recorded from MNTB neurons with a single postsynaptic pipette at RT (n= 8, black) and at PT (n= 13, red).
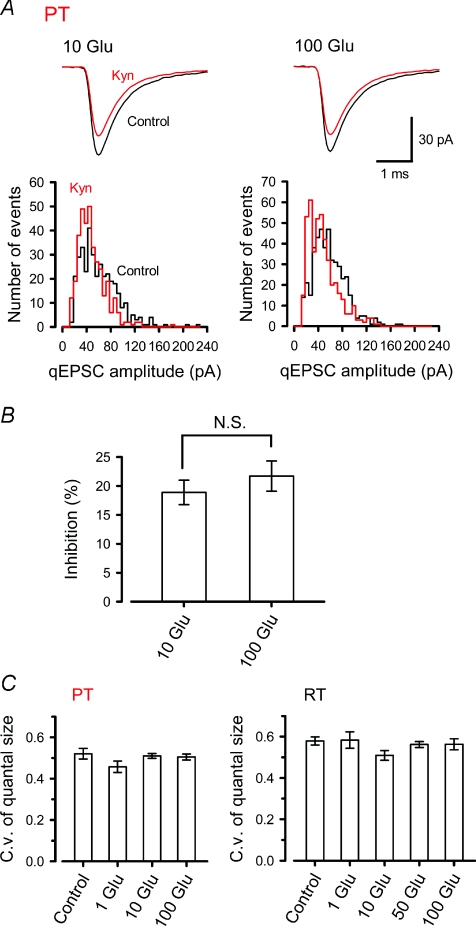
Figure 2. Saturation of vesicular glutamate filling at physiological temperature.
A, in simultaneous pre- and postsynaptic whole-cell recordings, qEPSCs were recorded in the presence of TTX (1 μm), with the presynaptic pipette solution containing 10 mm or 100 mm l-glutamate at PT. After obtaining control qEPSCs, kynurenate (100 μm) was bath-applied. Sample average traces (top) and amplitude histograms (bottom) of qEPSCs in the presence (Kyn, red) or absence (Control, black) of kynurenate (superimposed), with 10 mm (left) or 100 mm (right) l-glutamate in the presynaptic pipette solution. The total number of events is 362 for each with 10 mm l-glutamate and 455 for each with 100 mm l-glutamate. B, the percentage of qEPSC inhibition by kynurenate with 10 mm (10 Glu, n= 5) and 100 mm (100 Glu, n= 7) l-glutamate in the nerve terminals. No significant difference was observed (N.S., P > 0.4). C, similar c.v.s of the qEPSC amplitudes in the presence of l-glutamate loaded at 1–100 mm, or without presynaptic whole-cell recording (Control), at PT (left) and RT (right).
Fast l-glutamate application
Double-barrelled pipettes for l-glutamate application to outside-out patches were fabricated from theta glass tubes (outer diameter, 2.0 mm, TGC200-10, Harvard Apparatus, USA) pulled for a tip diameter of 200–500 μm (Koike et al. 2000; Koike-Tani et al. 2005). Conditioning and test solutions were continuously passed through each barrel of the application pipette using static pressure. The application pipette was rapidly moved every 4–5 s by a square voltage pulse (10–30 V, 100 ms) applied to a piezoelectric device (Burleigh Instruments, USA). The 10–90% rise time and 10–90% decay time of solution change in this system was 0.15 ms and 0.12 ms, respectively (Koike-Tani et al. 2005). d-APV (50 μm) was routinely included in the aCSF to block NMDAR activation.
Data analysis and statistics
Records were low-pass filtered at 3 kHz and stored on DAT tapes (sampling rate 48 kHz). Data were subsequently re-digitized at 20 kHz (Digidata 1320A, Axon Instruments) and analysed off-line using Axograph (Axon Instruments). Spontaneous qEPSCs were detected using a sliding template method implemented in Axograph, and only those having amplitudes more than 3 times larger than the baseline noise s.d. (2.2–3.4 pA) were included. The templates were made by initially averaging 50–150 qEPSCs selected by eye. Overlapped events were excluded from analyses by visual inspection. Presynaptic l-glutamate infusion did not affect the baseline noise level. The decay of averaged eEPSCs or qEPSCs was fitted by double exponential functions, and its weighted mean time constant (τm) was calculated from individual time constants (τ1, τ2) and their relative amplitude (a1, a2) as follows: τm=a1τ1+a2τ2. To examine the tonic activation of NMDARs, the mean and s.d. of 10 ms baseline currents were measured every 2 s. Sections contaminated by spontaneous EPSCs were discarded from the analysis. Ambient glutamate concentration ([glu]) was estimated from whole-cell NMDAR current fluctuations as follows. Assuming the binomial law for NMDAR channel openings, the σ2–I relationship follows a parabolic function described by the equation; σ2=iI− (I2/N) +σ2B, where I represents the mean d-APV-sensitive current amplitude, σ2 the current variance, N the maximal number of channels in the open state, i the single-channel current amplitude and σ2B the background noise variance. When ambient [glu] is low, the equation can be approximated by:
| (1) |
The relationships between I and [glu] can be described by the Hill equation:
where Imax represents the response at a saturating concentration of glutamate, EC50 the concentration of glutamate producing a half-maximal response, and n the Hill coefficient. When [glu] is low, the Hill equation can be approximated by:
| (2) |
From (1) and (2) the σ2–[glu] relationship can be described as:
| (3) |
We measured σ2 values in two cases, with and without 0.1 μm l-glutamate, in the presence of dl-threo-β-benzyloxyaspartate (TBOA), and measured σ2B values in the presence of d-APV. From these values and by adopting 1.5 for n and 2.3 μm for EC50 (Patneau & Mayer, 1990), we simultaneously obtained ambient [glu] in TBOA, and iImax/(EC50)n values. Using σ2 values measured in the absence of TBOA, and assuming that the iImax/(EC50)n is unchanged by TBOA, we estimated ambient [glu] in the absence of TBOA.
All values are given as means ±s.e.m. and significance of differences was evaluated by Student's t-test, unless otherwise noted. ANOVA followed by Dunnett's post hoc test was also used for multiple comparisons against control. P < 0.05 was taken as the level of significance.
Results
Non-saturation of AMPARs by glutamate from single vesicles at physiological temperature
To assess whether a single vesicular packet of transmitter saturates postsynaptic AMPARs at the calyx of Held of P7–8 rats, we loaded l-glutamate into calyceal terminals at various concentrations via presynaptic whole-cell patch pipettes, and recorded spontaneous qEPSCs at both RT (Fig. 1A) and PT (Fig. 1B). At RT, quantal size measured without presynaptic dialysis (control) showed skewed distribution, with its skewness (1.28 ± 0.12, mean ±s.e.m., n= 8) being apparently greater than that reported for P14–15 rat calyces (skewness, 1.06; Sahara & Takahashi, 2001). The mean quantal size increased as intraterminal l-glutamate concentration was raised, but showed no plateau even at 50 mm (Fig. 1C). The skewness of qEPSC distribution tended to decrease as intraterminal l-glutamate concentration was raised to 100 mm (1.02 ± 0.10, n= 6, Fig. 1A). The mean quantal size measured without a presynaptic pipette (control) fell between those recorded with 1 mm and 10 mm presynaptic l-glutamate (Fig. 1C), suggesting the range of endogenous glutamate concentration. Thus, the glutamate concentration dependent nature of mean quantal size at P7–8 calyces was similar to those observed at P14–15 (Ishikawa et al. 2002). With 100 mm presynaptic l-glutamate, mean quantal size (57.0 ± 2.1 pA, n= 6) was 1.46 times larger than control (P < 0.01, Dunnett's post hoc test), suggesting that glutamate released from a single vesicle does not saturate postsynaptic AMPARs, even at P7–8 (Wu et al. 2007). Might it then saturate AMPARs at physiological temperature? To test this issue, we recorded qEPSCs at PT (36–37°C). Mean quantal size measured without presynaptic dialysis (control) was 47.5 ± 2.2 pA (n= 13) at PT, which was larger than that at RT (39.3 ± 2.5 pA, n= 8, P < 0.03, Fig. 1C), as previously reported (Kushmerick et al. 2006; Postlethwaite et al. 2007). As at RT, mean quantal size fell between those recorded with 1 mm and 10 mm (Fig. 1C). Unlike at RT, however, at PT, mean quantal size reached a plateau at 10 mm l-glutamate, with its size (59.0 ± 2.5 pA, n= 12) being greater than control (P < 0.01, Dunnett's post hoc test). These results suggest that postsynaptic AMPARs are not saturated by glutamate released from single vesicle even at PT. The qEPSC size distribution was clearly skewed in control (skewness, 1.53 ± 0.13, n= 13), but its skewness was significantly reduced (P < 0.05, Dunnett's post hoc test) after loading l-glutamate at 10 mm (1.17 ± 0.08, n= 12) or 100 mm (1.15 ± 0.09, n= 14) (Fig. 1B). These results suggest that incomplete filling of synaptic vesicles with glutamate contributes to the skewed distribution of qEPSCs.
Saturation of vesicular glutamate filling at physiological temperature
At PT, mean quantal size reached a plateau with 10 mm l-glutamate dialysis (Fig. 1C). Might this plateau be produced by saturation of postsynaptic AMPARs, or saturation in the vesicular glutamate uptake mechanism? To test the former possibility, we used the low affinity AMPAR antagonist kynurenate, which enables one to assess relative glutamate concentrations in the synaptic cleft, as it competes with glutamate released from synaptic vesicles during synaptic transmission (Diamond & Jahr, 1997). The validity of this method has been demonstrated at P14 calyces of Held (at RT), where an increase in vesicular glutamate content, following presynaptic l-glutamate loading (50 mm), causes a significant reduction in the magnitude of inhibition of qEPSCs by kynurenate (Yamashita et al. 2003). At P7–8 calyces at PT, the magnitude of reduction in the mean quantal size by kynurenate (100 μm) was similar (P= 0.42) between calyces loaded with l-glutamate at 10 mm (18.9 ± 2.2%, n= 5) and 100 mm (21.7 ± 2.6%, n= 7, Fig. 2A and B), suggesting that saturation of the glutamate uptake mechanism resulted in a similar vesicular glutamate content in the presence of intraterminal l-glutamate at 10–100 mm. The coefficient of variation (c.v.; s.d./mean) of qEPSC amplitude was similar (P > 0.2 and P > 0.4 respectively, one-way ANOVA) for a wide range of presynaptic l-glutamate concentrations (1–100 mm), at both RT and PT (Fig. 2C), despite apparent difference in the extent of vesicle filling. These results argue against the idea that variation in vesicular glutamate concentration is the main cause of variation in quantal size (Wu et al. 2007). Multiple factors, including variation in postsynaptic AMPAR density (Sätzler et al. 2002), as well as vesicular glutamate content, may contribute to quantal size variation.
Saturation of postsynaptic AMPARs by glutamate released from multiple vesicles
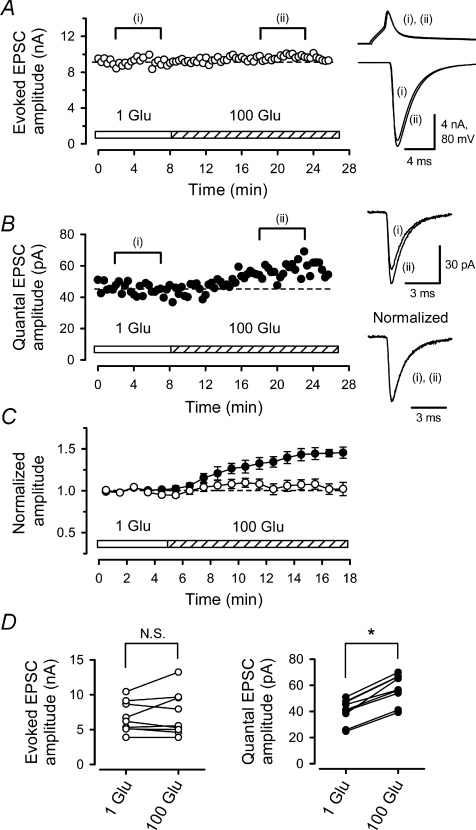
Our present results indicate that glutamate released from a single vesicle does not saturate postsynaptic AMPARs at immature calyces, even at PT. In contrast, postsynaptic AMPARs might be saturated by glutamate released in response to a presynaptic action potential (AP), even at RT (Iwasaki & Takahashi, 2001; Kaneko & Takahashi, 2004; Taschenberger et al. 2005). To obtain direct evidence, we infused 100 mm l-glutamate into presynaptic terminals using pipette perfusion, while recording AP-evoked EPSCs (eEPSCs) and spontaneous EPSCs (Ishikawa et al. 2002; Yamashita et al. 2003) in simultaneous pre- and postsynaptic recordings at RT. If postsynaptic AMPARs are saturated by the transmitter glutamate released during eEPSCs, overloading of l-glutamate should not increase the EPSC amplitude, as previously shown for EPSCs evoked by large and sustained presynaptic Ca2+ currents (Ishikawa et al. 2002). Indeed, presynaptic infusion of 100 mm l-glutamate did not increase the amplitude of eEPSCs (Fig. 3A, C and D, P= 0.36, n= 9 synapses). However, as expected, this presynaptic loading enhanced the mean amplitude of spontaneous EPSCs (Fig. 3B–D), on average by 44 ± 5% (n= 9, P < 0.01). At P7–8, as at P14–29 (Ishikawa et al. 2002; Yamashita et al. 2003), spontaneous EPSCs recorded from calyceal synapses without TTX (1 μm) were indistinguishable from mEPSCs recorded in the presence of TTX, with respect to both amplitude (102 ± 4%, n= 4, P > 0.5) and frequency (99 ± 6%, n= 4, P > 0.6), and therefore can be regarded as qEPSCs. Thus, at this immature synapse at RT, multiple quanta of glutamate saturate postsynaptic AMPARs during eEPSCs, whereas a single quantum of glutamate does not saturate them.
Figure 3. Postsynaptic AMPARs are saturated by multi-quantal glutamate released in response to presynaptic action potential.
In simultaneous pre- and postsynaptic whole-cell recording, l-glutamate in the presynaptic pipette was switched from 1 to 100 mm using pipette perfusion. A, presynaptic l-glutamate loading had no significant effect on the amplitude of EPSCs (right lower sample traces) evoked by presynaptic APs (upper traces). Averaged sample records before (i) and after (ii) 100 mm l-glutamate loading were superimposed for EPSCs and APs. Dashed lines indicate mean values before switch (A and B). B, presynaptic l-glutamate loading potentiated qEPSCs. Each data point represents the mean amplitude of qEPSCs sampled every 20 s. Sample records are averaged qEPSCs before (i) and after (ii) the switch, with (bottom) or without (top) normalization at the peak (superimposed). C, summary data from 9 synapses. The mean amplitude of eEPSCs (open circles) before the switch was 6.7 ± 0.7 nA (normalized in ordinate). Mean amplitude of qEPSCs (filled circles) before the switch was 40.2 ± 3.1 pA. Means and s.e.m. are shown by symbols and error bars. D, left, mean amplitudes of eEPSCs before and after presynaptic l-glutamate loading. No significant difference (N.S., P > 0.3) was observed. Right, mean amplitudes of qEPSCs before and after presynaptic l-glutamate loading. The asterisk indicates significant difference (P < 0.01).
High release probability contributes to saturation of postsynaptic AMPARs by multiple glutamate quanta
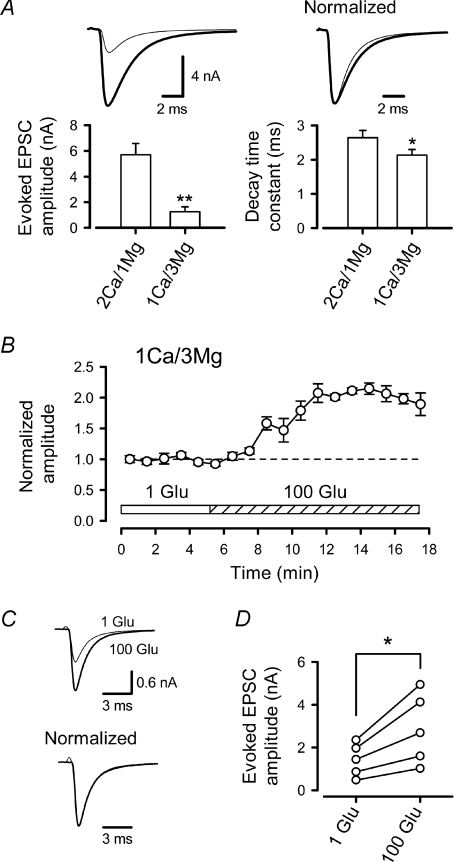
At a high release probability, the transmitter, which is released from multiple vesicles from a single release site, or from neighbouring sites, can overlap in the synaptic cleft, thereby increasing occupancy of postsynaptic AMPARs (Silver et al. 1996b; Wadiche & Jahr, 2001; Foster et al. 2002; Harrison & Jahr, 2003). When the glutamate concentration in the synaptic cleft becomes higher, its clearance tends to become slower, resulting in slow EPSC decay (Silver et al. 1996b; Foster et al. 2005). When the release probability was lowered by reducing the Ca2+/Mg2+ concentration ratio ([Ca2+]o/[Mg2+]o) in aCSF from 2 mm/1 mm to 1 mm/3 mm, EPSCs became smaller and decayed more rapidly (Fig. 4A), with the mean time constant (τm) being reduced by 27 ± 4% (n= 5, P < 0.01), suggesting that, under normal conditions, glutamate released from multiple vesicles overlaps. When we loaded 100 mm l-glutamate into the calyceal terminal, in the low [Ca2+]o/[Mg2+]o solution, the eEPSC amplitude markedly increased (Fig. 4B–D). These results suggest that an overlap of glutamate released from multiple vesicles underlies saturation of postsynaptic AMPARs.
Figure 4. Non-saturation of postsynaptic AMPARs in low Ca2+ solution.
A, reduction in the [Ca2+]o/[Mg2+]o ratio from 2 mm/1 mm (thick line) to 1 mm/3 mm (thin line), reduced the eEPSC amplitude by 79 ± 3% (n= 5, **P < 0.001) and shortened the decay time (right panel, *P < 0.01, n= 5). Sample records are averaged eEPSCs in normal and low [Ca2+]o/[Mg2+]o solutions (superimposed, peak amplitudes are normalized in the right sample traces). B, presynaptic loading of 100 mm l-glutamate increased the amplitudes of eEPSCs in low [Ca2+]o/[Mg2+]o (1 mm/3 mm) solution (n= 5). C, sample records are averaged eEPSCs before (1 Glu, thin line) and after (100 Glu, thick line) l-glutamate loading (superimposed) with (lower traces) or without (upper traces) normalizing amplitude. D, significant increase in the mean amplitude of eEPSCs (*P < 0.01) after loading 100 mm l-glutamate in low [Ca2+]o/[Mg2+]o solution.
Involvement of desensitization in saturation of postsynaptic AMPARs by multiple glutamate quanta
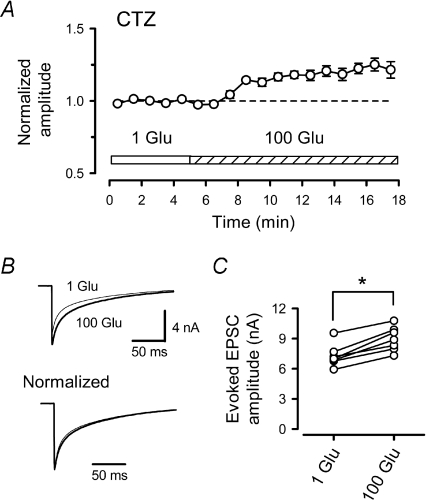
At the calyx of Held, before the onset of hearing, desensitization of postsynaptic AMPARs contributes significantly to shaping the EPSC decay and to short-term synaptic depression (Taschenberger et al. 2002, 2005; Koike-Tani et al. 2005, 2008). Might AMPAR desensitization also contribute to saturation of AMPARs? We addressed this issue by using CTZ (100 μm), which blocks AMPAR desensitization (Trussell et al. 1993; Yamada & Tang, 1993; Koike-Tani et al. 2005, 2008). CTZ also increases transmitter release probability (Diamond & Jahr, 1995), via inhibition of presynaptic voltage-gated potassium channels (Ishikawa & Takahashi, 2001), and enhances the affinity of AMPARs for glutamate (Dzubay & Jahr, 1999; Fucile et al. 2006). Both of these ‘side effects’ can augment AMPAR saturation. Nevertheless, when we infused 100 mm l-glutamate into the calyceal terminals in the presence of CTZ, the eEPSC amplitude became larger (Fig. 5), on average by 24 ± 3% (n= 7, P < 0.01), even in the normal [Ca2+]o/[Mg2+]o solution. These results suggest that CTZ, via block of AMPAR desensitization, relieved AMPARs from saturation during synaptic transmission.
Figure 5. Non-saturation of postsynaptic AMPARs in the presence of CTZ.
A, in the presence of CTZ (100 μm, n= 7) presynaptic loading of 100 mm l-glutamate potentiated eEPSCs. B, sample records are averaged eEPSCs before (1 Glu, thin line) and after 100 mm l-glutamate (100 Glu, thick line) loading (superimposed) with (lower traces) or without (upper traces) normalizing amplitude. C, mean amplitudes of eEPSCs before and after presynaptic loading of 100 mm l-glutamate in the presence of CTZ. The asterisk indicates a significant difference (P < 0.01).
Does ambient glutamate desensitize AMPARs?
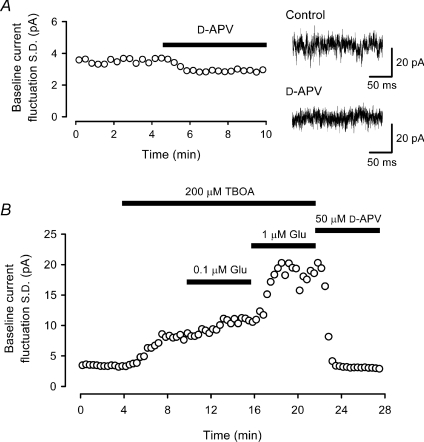
Our results with CTZ (Fig. 5) suggest that AMPAR desensitization by glutamate is involved in promoting saturation of synaptic transmission at immature calyces of Held. Desensitization of postsynaptic AMPARs can be produced by ambient glutamate, or by glutamate released from synaptic vesicles during transmission. In excised patches from P7–8 MNTB neurons, a steady-state glutamate concentration higher than 1 μm is required to attenuate AMPAR currents (Koike-Tani et al. 2008). We estimated ambient glutamate concentration from baseline fluctuations of NMDAR currents recorded from postsynaptic MNTB neurons. In Mg2+-free aCSF, baseline fluctuations of NMDAR currents could be revealed by bath-application of d-APV (50 μm), which reduced the magnitude of the current fluctuations (Fig. 6A). To estimate ambient glutamate concentration, we applied l-glutamate at given concentrations, and measured the magnitude of any increase in the NMDAR current fluctuation. To minimize glutamate uptake by surrounding glial cells or neurons, we included a blocker of glutamate transport (TBOA, 200 μm) (Shimamoto et al. 1998; Cavelier & Attwell, 2005) in the bath solution. Without TBOA, l-glutamate concentrations higher than 10 μm were required to appreciably increase NMDAR current fluctuations (data not shown), suggesting the presence of a strong glutamate uptake system (Renden et al. 2005). Although TBOA has no effect on NMDAR activation (Jabaudon et al. 1999), it increased NMDAR current fluctuations (from 3.71 ± 0.26 pA to 9.27 ± 0.66 pA in s.d., n= 5, P < 0.01), suggesting an elevation of ambient glutamate. In the presence of TBOA, l-glutamate at 0.1 μm increased the s.d. of NMDAR current fluctuations to 11.2 ± 0.6 pA (n= 5, Fig. 6B). From these values, using the variance–mean relationship and Hill equation (see Methods), we estimated ambient glutamate concentrations to be 320 nm in the presence of TBOA, and 55 nm in its absence. These results are comparable to those estimated in hippocampal slices; 200 nm and 25–30 nm in the presence and absence of TBOA, respectively (Cavelier & Attwell, 2005; Herman & Jahr, 2007). Thus, the ambient glutamate concentration in the slice is too low to desensitize AMPARs.
Figure 6. Estimation of ambient glutamate concentration from whole-cell NMDAR current fluctuations.
A, reduction of baseline current fluctuation in an MNTB neuron by d-APV (50 μm) in Mg2+-free solution. Before d-APV application slices were washed with Mg2+-free solution (containing 10 μm glycine) for at least 20 min. Sample records are baseline currents before and after application of d-APV. The AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX, 10 μm) had no effect on the current fluctuations (101 ± 2% of control, n= 5). B, increased baseline NMDAR current fluctuations caused by l-glutamate (0.1 μm and 1 μm) after blocking glial glutamate uptake by TBOA (200 μm) in Mg2+-free solution containing NBQX (10 μm).
Concomitant activation and desensitization of AMPARs
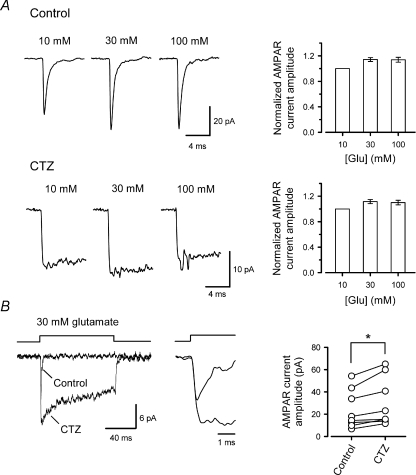
Given that the ambient glutamate concentration was far below the concentration required to promote AMPAR desensitization, the alternative possibility is that glutamate released from vesicles, during the rise time of eEPSCs, desensitizes postsynaptic AMPARs. AMPARs undergo rapid desensitization at avian and rodent auditory synapses (Raman & Trussell, 1992; Koike-Tani et al. 2005). Using CTZ, in rapid glutamate application experiments to excised patches from MNTB neurons, we examined whether desensitization was sufficiently rapid to take place during the rise time of eEPSCs. To evaluate the effect of AMPAR desensitization block by CTZ, in isolation from its effect on AMPAR affinity (Dzubay & Jahr, 1999; Fucile et al. 2006), we tested the effect of CTZ on maximal AMPAR currents. As we increased the applied glutamate concentration, the amplitude of the AMPAR currents reached maximal value at 30 mm (Fig. 7A). CTZ increased the amplitude of AMPAR patch currents induced by 30 mm l-glutamate by 26 ± 7% (n= 7, P < 0.02, Fig. 7B). The AMPAR patch currents had a 10–90% rise time of 207 ± 11 μs (n= 7), which was comparable to that of synaptic currents (qEPSCs, 258 ± 7 μs, n= 9). These results support the possibility that at immature calyces glutamate released from vesicles can desensitize a fraction of postsynaptic AMPARs before EPSCs reach their peak.
Figure 7. Desensitization of AMPAR patch currents at their rising phase.
A, AMPAR patch currents induced by rapid application of 10, 30 and 100 mm l-glutamate in the presence (lower panels) or absence (upper panels) of CTZ (100 μm). Sample records in control and CTZ derived from different patches. Bar graphs show AMPAR current amplitudes, in the presence or absence of CTZ, normalized to that at 10 mm l-glutamate (n= 5–6). B, AMPAR currents induced by 30 mm l-glutamate in a patch, before and after CTZ application (superimposed at different time scales). CTZ significantly increased the peak amplitude of AMPAR patch currents (n= 7, *P < 0.02).
Discussion
Mechanisms by which quantal size is determined
Using intraterminal glutamate loading, we have demonstrated that a single packet of glutamate does not saturate postsynaptic AMPARs at immature calyx of Held synapses, even at physiological temperature (Fig. 1). Thus, at immature calyceal synapses in the prehearing period, vesicular transmitter content is an important determinant of quantal size and synaptic efficacy, just as it is at more mature calyceal synapses (Ishikawa et al. 2002; Yamashita et al. 2003). At PT, unlike at RT, the quantal size reached a plateau when the intraterminal glutamate concentration was raised to 10 mm. The experiments using kynurenate indicated that this plateau was not caused by AMPAR saturation, but by saturation in vesicular glutamate filling, presumably because of more efficient vesicular filling at PT (Fig. 2). These results further suggest that the larger mean quantal size observed at PT compared with RT (Fig. 1C), as previously reported (Kushmerick et al. 2006: Postlethwaite et al. 2007), might result from higher glutamate concentration in vesicles at PT, in addition to postsynaptic factors such as an increase in AMPAR channel conductance (Postlethwaite et al. 2007).
Wu et al. (2007) reported that quantal size is unrelated to the membrane capacitance change associated with qEPSCs, suggesting that vesicle volume is not a dominant source of qEPSC variation. Furthermore, as larger qEPSCs are enhanced to a lesser extent by intraterminal glutamate loading, and reduced to a lesser extent by a low affinity AMPAR agonist, they concluded that larger qEPSCs result from higher intravesicular glutamate concentration. It was postulated that variation in vesicular glutamate concentration is a main source of quantal size variation. However, even after vesicles were filled maximally with glutamate at PT (Figs 1 and 2), the c.v. of quantal size remained unchanged (Fig. 2C), suggesting that the variation of quantal size does not solely result from that of vesicular glutamate concentration, but may also result from variations of postsynaptic AMPAR density among release sites.
Mechanisms by which postsynaptic AMPARs saturate during synaptic transmission
Increasing vesicular glutamate content by direct loading of l-glutamate into P7–8 calyceal terminals did not increase the amplitude of eEPSCs, indicating that postsynaptic AMPARs are saturated by transmitter glutamate. Since glutamate released from a single vesicle does not saturate postsynaptic AMPARs (Figs 1 and 3), their saturation during eEPSCs must be caused by an overlap of glutamate released from multiple vesicles in the synaptic cleft (Silver et al. 1996b; Meyer et al. 2001). Consistent with this idea, after reducing release probability by lowering extracellular Ca2+/Mg2+ concentration ratio, the eEPSC decay became faster (Fig. 4A) and intraterminal loading of l-glutamate increased the eEPSC amplitude (Fig. 4B–D). At calyces in the prehearing period (P5–8), the average number of docked vesicles at each active zone is 6 (Taschenberger et al. 2002) and release probability is estimated to be 0.3–0.5 (Taschenberger et al. 2002; Koike-Tani et al. 2008). These features favour multivesicular release at each active zone. At calyceal terminals in the posthearing period (P13–14), the average number of docked vesicles at each active zone is reduced to 3 (Taschenberger et al. 2002), and release probability is also decreased to 0.1–0.2 (Taschenberger et al. 2002; Koike-Tani et al. 2008). Both of these developmental changes will reduce the chance of overlap of glutamate from different vesicles, thereby reducing AMPAR occupancy during synaptic transmission.
The magnitude of increase in the eEPSC amplitude after intraterminal l-glutamate loading in low [Ca2+]o/[Mg2+]o aCSF (Fig. 4B) was greater than that in the qEPSC amplitude (Fig. 3). These results are analogous to those in normal aCSF at P14–15 calyceal synapses (Ishikawa et al. 2002), suggesting that glutamate released from multiple vesicles still overlaps under conditions of lowered release probability at calyceal synapse in the prehearing period. At more mature calyceal synapses, at P29, intraterminal l-glutamate loading increases the amplitudes of qEPSCs and eEPSCs to a similar extent (Yamashita et al. 2003), suggesting that the release sites become well-segregated as animals mature.
To determine whether AMPAR desensitization is involved in promoting saturation of synaptic transmission, we loaded l-glutamate into the presynaptic terminal after blocking AMPAR desensitization by CTZ. In the presence of CTZ, l-glutamate loading enhanced the eEPSC amplitude. These results cannot be explained by the effect of CTZ on release probability (Diamond & Jahr, 1995; Ishikawa & Takahashi, 2001), because high release probability increases AMPAR occupancy toward saturation (Fig. 4). Besides blocking desensitization, CTZ increases ligand affinity of AMPARs (Dzubay & Jahr, 1999; Fucile et al. 2006). This effect of CTZ might potentially contribute to increases in the amplitudes of qEPSCs and eEPSCs, observed at P6–7 calyx synapses, after CTZ application (Koike-Tani et al. 2005). However, this effect cannot explain the apparent relief by CTZ of AMPARs from saturation, which was revealed as the enhancement of eEPSCs by l-glutamate loading in the presence of CTZ. These results altogether suggest that desensitization contributes to saturation of postsynaptic AMPARs at immature calyx of Held synapses.
The ambient glutamate concentration was not high enough to promote AMPAR desensitization, but rapid glutamate application showed that AMPAR desensitization during the rising phase of eEPSCs would be sufficient to attenuate the peak amplitude of eEPSCs. Upon binding with glutamate, AMPARs can proceed to desensitized states without passing through open states (Wadiche & Jahr, 2001; Robert & Howe, 2003). At immature calyceal synapses, eEPSCs have relatively slow rise times, because of slower qEPSC kinetics (Yamashita et al. 2003; Koike-Tani et al. 2005) and less synchronous quantal release (Taschenberger et al. 2005). The slow rise time of eEPSCs may facilitate the ability of AMPAR desensitization to truncate their peak amplitude. The higher sensitivity of immature AMPARs to l-glutamate-induced desensitization (Koike-Tani et al. 2008) may also contribute to the rapid desensitization by vesicular glutamate.
Physiological implications for the developmental decrease in the occupancy of postsynaptic AMPARs
Similar to immature calyceal synapses, AMPARs are thought to be saturated during evoked EPSCs at a subset of mossy fibre–granule cell synapses and climbing fibre–Purkinje cell synapses in the cerebellum (Silver et al. 1996b; Foster et al. 2002; Harrison & Jahr, 2003). Given that the kinetics of the EPSC rise time and AMPAR desensitization at these cerebellar synapses are comparable to those at immature calyces (Llano et al. 1991; Silver et al. 1996a; Häusser & Roth, 1997; Wall et al. 2002; Koike-Tani et al. 2005; DiGregorio et al. 2007), desensitization may also underlie saturation of AMPARs at these central synapses. At the climbing fibre–Purkinje cell synapse having high release probability (Silver et al. 1998), saturation of AMPARs reduces the amount of short-term depression and accelerates recovery from depression (Foster et al. 2002; Harrison & Jahr, 2003). By contrast, at the immature calyx of Held, the high occupancy of AMPARs restricts synaptic facilitation, contributing to strong synaptic depression (Taschenberger et al. 2002, 2005; Wong et al. 2003). A developmental decrease in release probability (Iwasaki & Takahashi, 2001; Taschenberger et al. 2002; Koike-Tani et al. 2008) reduces occupancy and desensitization of AMPARs, and saves releasable synaptic vesicles from depletion, thereby reducing synaptic depression.
In the cerebellum, climbing fibre–Purkinje cell transmission plays a modulatory role in adjusting motor co-ordination by Purkinje cells (Ito, 2000) at a frequency of up to 15 Hz (Schwarz & Welsh, 2001). Saturation of AMPARs by transmitter is thought to enhance stability of this type of synaptic transmission (Foster et al. 2002; Harrison & Jahr, 2003). In contrast, at fast relay synapses like the mature calyx of Held, synaptic transmission can occur at a high frequency, up to 400 Hz, at room temperature (Taschenberger & von Gersdorff, 2000). At such fast synapses, high AMPAR occupancy and the ensuing strong synaptic depression would not favour high-fidelity transmission, although, at other synapses, synaptic depression may be useful for detecting low frequency transient events (Abbott et al. 1997). At the calyx of Held, developmental reduction in AMPAR desensitization and occupancy reduces synaptic depression during high frequency transmission, thereby contributing to the establishment of highly reliable fast relay synapse.
Acknowledgments
This study was supported by Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology to T.T. and Research Fellowship from the Japanese Society for the Promotion of Science to T.Y. We thank Maki Koike-Tani for technical advice, and George Augustine, Mark Farrant and Ko Matsui for helpful comments.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AMPAR
AMPA receptor
- AP
action potential
- d-APV
d(-)2-amino-5-phosphonovaleric acid
- CTZ
cyclothiazide
- c.v.
coefficient of variation
- eEPSC
action potential-evoked EPSC
- mEPSC
miniature EPSC
- MNTB
medial nucleus of trapezoid body
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline
- NMDAR
NMDA receptor
- P
postnatal day
- PT
physiological temperature
- qEPSC
quantal EPSC
- RT
room temperature
- TBOA
dl-threo-β-benzyloxyaspartate
Author contributions
T.Y. and T.T. designed the experiments. T.Y., T.K. and K.E. performed the experiments and analysed the data. All authors participated in data interpretations. T.Y. and T.T. wrote the manuscript. All authors revised the manuscript and approved the final manuscript.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Auger C, Kondo S, Marty A. Multivesicular release at single functional synaptic sites in cerebellar stellate and basket cells. J Neurosci. 1998;18:4532–4547. doi: 10.1523/JNEUROSCI.18-12-04532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio DA, Rothman JS, Nielsen TA, Silver RA. Desensitization properties of AMPA receptors at the cerebellar mossy fiber-granule cell synapse. J Neurosci. 2007;27:8344–8357. doi: 10.1523/JNEUROSCI.2399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubay JA, Jahr CE. The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci. 1999;19:5265–5274. doi: 10.1523/JNEUROSCI.19-13-05265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Crowley JJ, Regehr WG. The influence of multivesicular release and postsynaptic receptor saturation on transmission at granule cell to Purkinje cell synapses. J Neurosci. 2005;25:11655–11665. doi: 10.1523/JNEUROSCI.4029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Kreitzer AC, Regehr WG. Interaction of postsynaptic receptor saturation with presynaptic mechanisms produces a reliable synapse. Neuron. 2002;36:1115–1126. doi: 10.1016/s0896-6273(02)01106-6. [DOI] [PubMed] [Google Scholar]
- Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc Natl Acad Sci U S A. 2006;103:2943–2947. doi: 10.1073/pnas.0511063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J, Jahr CE. Receptor occupancy limits synaptic depression at climbing fiber synapses. J Neurosci. 2003;23:377–383. doi: 10.1523/JNEUROSCI.23-02-00377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Roth A. Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. J Physiol. 1997;501:77–95. doi: 10.1111/j.1469-7793.1997.077bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Takai Y, Takahashi T. Presynaptic mechanism for phorbol ester-induced synaptic potentiation. J Neurosci. 1999;19:7262–7267. doi: 10.1523/JNEUROSCI.19-17-07262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Sahara Y, Takahashi T. A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron. 2002;34:613–621. doi: 10.1016/s0896-6273(02)00692-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol. 2001;533:423–431. doi: 10.1111/j.1469-7793.2001.0423a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol. 2001;534:861–871. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB, Redman SJ, Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DL, Romano MN. Neonatal development of auditory system potentials averaged from the scalp of rat and cat. Brain Res. 1972;36:101–115. doi: 10.1016/0006-8993(72)90769-x. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Tsukada S, Tsuzuki K, Kijima H, Ozawa S. Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J Neurosci. 2000;20:2166–2174. doi: 10.1523/JNEUROSCI.20-06-02166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Tani M, Saitoh N, Takahashi T. Mechanisms underlying developmental speeding in AMPA-EPSC decay time at the calyx of Held. J Neurosci. 2005;25:199–207. doi: 10.1523/JNEUROSCI.3861-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Tani M, Kanda T, Saitoh N, Yamashita T, Takahashi T. Involvement of AMPA receptor desensitization in short-term synaptic depression at the calyx of Held in developing rats. J Physiol. 2008;586:2263–2275. doi: 10.1113/jphysiol.2007.142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Gersdorff H. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci. 2006;26:1366–1377. doi: 10.1523/JNEUROSCI.3889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A, Stratford K, Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991;350:344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Neher E, Schneggenburger R. Estimation of quantal size and number of functional active zones at the calyx of Held synapse by nonstationary EPSC variance analysis. J Neurosci. 2001;21:7889–7900. doi: 10.1523/JNEUROSCI.21-20-07889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of Held. J Neurosci. 2001;21:444–461. doi: 10.1523/JNEUROSCI.21-02-00444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol. 2007;579:69–84. doi: 10.1113/jphysiol.2006.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Trussell LO. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- Renden R, Taschenberger H, Puente N, Rusakov DA, Duvoisin R, Wang LY, Lehre KP, von Gersdorff H. Glutamate transporter studies reveal the pruning of metabotropic glutamate receptors and absence of AMPA receptor desensitization at mature calyx of Held synapses. J Neurosci. 2005;25:8482–8497. doi: 10.1523/JNEUROSCI.1848-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara Y, Takahashi T. Quantal components of the excitatory postsynaptic currents at a rat central auditory synapse. J Physiol. 2001;536:189–197. doi: 10.1111/j.1469-7793.2001.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PB, Saviane C, Nielsen TA, DiGregorio DA, Silver RA. Rapid vesicular release, quantal variability, and spillover contribute to the precision and reliability of transmission at a glomerular synapse. J Neurosci. 2005;25:8173–8187. doi: 10.1523/JNEUROSCI.2051-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sätzler K, Söhl LF, Bollmann JH, Borst JGG, Frotscher M, Sakmann B, Lübke JHR. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuss V, Schneggenburger R, Neher E. Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse. J Neurosci. 2002;22:728–739. doi: 10.1523/JNEUROSCI.22-03-00728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Welsh JP. Dynamic modulation of mossy fiber system throughput by inferior olive synchrony: a multielectrode study of cerebellar cortex activated by motor cortex. J Neurophysiol. 2001;86:2489–2504. doi: 10.1152/jn.2001.86.5.2489. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. dl-Threo-β-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Silver RA, Colquhoun D, Cull-Candy SG, Edmonds B. Deactivation and desensitization of non-NMDA receptors in patches and the time course of EPSCs in rat cerebellar granule cells. J Physiol. 1996a;493:167–173. doi: 10.1113/jphysiol.1996.sp021372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA, Cull-Candy SG, Takahashi T. Non-NMDA glutamate receptor occupancy and open probability at a rat cerebellar synapse with single and multiple release sites. J Physiol. 1996b;494:231–250. doi: 10.1113/jphysiol.1996.sp021487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA, Momiyama A, Cull-Candy SG. Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses. J Physiol. 1998;510:881–902. doi: 10.1111/j.1469-7793.1998.881bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J-Y, Wu L-G. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Leão RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Scheuss V, Neher E. Release kinetics, quantal parameters and their modulation during short-term depression at a developing synapse in the rat CNS. J Physiol. 2005;568:513–537. doi: 10.1113/jphysiol.2005.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Robert A, Howe JR, Usowicz MM. The speeding of EPSC kinetics during maturation of a central synapse. Eur J Neurosci. 2002;15:785–797. doi: 10.1046/j.1460-9568.2002.01910.x. [DOI] [PubMed] [Google Scholar]
- Wong AYC, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci. 2003;23:4868–4877. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-S, Xue L, Mohan R, Paradiso K, Gillis KD, Wu L-G. The origin of quantal size variation: vesicular glutamate concentration plays a significant role. J Neurosci. 2007;27:3046–3056. doi: 10.1523/JNEUROSCI.4415-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA, Tang C-M. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ishikawa T, Takahashi T. Developmental increase in vesicular glutamate content does not cause saturation of AMPA receptors at the calyx of Held synapse. J Neurosci. 2003;23:3633–3638. doi: 10.1523/JNEUROSCI.23-09-03633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]