Abstract
We tested whether horizontal cells (HCs) provide feedback that regulates the Ca2+ current (ICa) of rods in salamander and mouse retinas. In both species, hyperpolarizing HCs by puffing a glutamate antagonist, 6,7-dinitro-quinoxaline-2,3-dione (DNQX), onto HC processes caused a negative shift in the voltage dependence of rod ICa and increased its peak amplitude. Conversely, depolarizing HCs by puffing kainic acid (KA) into the outer plexiform layer (OPL) caused a positive voltage shift and decreased rod ICa. Experiments on salamander retina showed that these effects were blocked by addition of the pH buffer, Hepes. Intracellular calcium concentration ([Ca2+]i) was examined in rods by confocal microscopy after loading salamander and mouse retinal slices with Fluo-4. Rods were depolarized to near the dark resting potential by bath application of high K+ solutions. Hyperpolarizing HCs with 2,3-dihydroxy-6-nitro-7-sulphamoylbenzo[f]quinoxaline (NBQX) enhanced high K+-evoked Ca2+ increases whereas depolarizing HCs with KA inhibited Ca2+ increases. In both species these effects of NBQX and KA were blocked by addition of Hepes. Thus, like HC feedback in cones, changes in HC membrane potential modulate rod ICa thereby regulating rod [Ca2+]i at physiological voltages, in both mouse and salamander retinas. By countering the reduced synaptic output that accompanies hyperpolarization of rods to light, HC feedback will subtract spatially averaged luminance levels from the responses of individual rods to local changes. The finding that HC to rod feedback is present in both amphibian and mammalian species shows that this mechanism is highly conserved across vertebrate retinas.
There is evidence that horizontal cells (HCs) provide negative feedback to rods in the amphibian retina (Thoreson et al. 2008; Normann & Pochobradsky, 1976) similar to the feedback from HCs to cones in many species (Baylor et al. 1971; O’Bryan, 1973; Lasansky & Vallerga, 1975; Burkhardt, 1977; Verweij et al. 2003). Like HC to cone feedback (Gerschenfeld et al. 1980; Verweij et al. 1996), hyperpolarizing HCs by current injection or light stimulation causes a leftward shift and an increase in the amplitude of L-type calcium currents (ICa) in synaptically connected rods (Thoreson et al. 2008). Also like HC to cone feedback, these effects on rod ICa are blocked by the pH buffer, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes) (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Cadetti & Thoreson, 2006; Davenport et al. 2008; Thoreson et al. 2008).
The present study provides further evidence for the hypothesis that feedback from HCs regulates ICa in amphibian rods and shows that changes in ICa regulate [Ca2+]i at membrane potentials within the normal physiological voltage range of rods. In addition, we find that feedback from HCs regulates ICa in rods from mammalian (mouse) retina. This suggests that HC to rod feedback is a feature common to all vertebrate retinas. The effects of negative feedback from HCs to rods can subtract spatially averaged light levels measured by horizontal cells from local luminance changes measured by individual rods, thereby enhancing the detection of local changes in illumination. The similarities between feedback in rods and cones and the finding that HC to rod feedback is present in mouse retina opens up the possibility of using mouse genetic models to study the mechanisms and functions of HC feedback.
Methods
Salamander (Ambystoma tigrinum) retina
Animals were handled humanely according to protocols approved by the UNMC Animal Care and Use Committee. Salamanders (Kons Scientific, Germantown, WI, USA) were decapitated with heavy shears and then the brain and spinal cord rapidly pithed. After enucleation, the anterior segment of the eye, including the lens, was removed. The resulting eyecup was cut into quarters and a section of eyecup was placed vitreal side down on a piece of filter paper (2 × 5 mm, Type AAWP, 0.8 μm pores, Millipore, Bedford, MA, USA). After adhering to the filter paper, the retina was isolated under cold amphibian superfusate. Retinal slices (125 μm) were cut with a razor blade tissue chopper (Stoelting, Wood Dale, IL, USA) and positioned in the recording chamber for viewing of the retinal layers with an upright fixed stage microscope (Olympus BHWI, Tokyo, Japan or Nikon E600 FN, Japan). For flatmount retina experiments, whole retinas were isolated – vitreal surface down – on a piece of nitrocellulose filter (Type AAWP, 0.8 μm pores, Millipore, Bedford, MA, USA) with a 4 mm diameter hole cut into its centre. Slices were prepared under dim room lights; isolated retinas were prepared under infrared illumination using night vision goggles (Nitemate NAV3, Litton Industries, Tempe, AZ, USA).
Retinas were superfused at ∼1 ml min−1 with a solution containing (in mm): 101 NaCl, 22 NaHCO3, 2.5 KCl, 2 CaCl2, 0.5 MgCl2, 9 glucose. The pH was 7.4 after bubbling with 95% O2–5% CO2. In some experiments, pH buffering capacity was increased by adding 10 mm Hepes. For these experiments, the pH of the Hepes-containing solution was first adjusted to 7.4 with 1 m NaOH after bubbling with 95% O2–5% CO2.
Mouse retinal slices
Mice (Mus musculus, C57BL6J, Jackson Laboratory, Bar Harbor, ME, USA) were killed by CO2 inhalation. The eye was then rapidly enucleated and retinal slices were prepared with techniques similar to those described for salamander above. One difference was that mouse retinas were isolated after adhering to the filter paper but before adding any additional saline solution to the chamber. Slices were superfused at room temperature at a rate of ∼1 ml min−1 with a solution containing (in mm): 120 NaCl, 22.6 NaHCO3, 3 KCl, 0.5 KH2PO4, 2 CaCl2, 0.5 MgSO4, 6 glucose. The saline solution was continuously bubbled with 95% O2 and 5% CO2. As with salamander, pH buffering capacity was increased in some experiments by adding 10 mm Hepes. The pH of the Hepes-containing solution was matched to that of the control solution (7.4) using 1 m NaOH after bubbling with 95% O2–5% CO2.
Electrophysiology
Whole cell recordings were obtained using 10–15 MΩ patch electrodes fabricated from borosilicate glass (1.2 mm o.d., 0.95 mm i.d., with internal filament, World Precision Instruments, Sarasota, FL, USA) on a PP-830 micropipette puller (Narishige USA, East Meadow, NY, USA).
The pipette solution for salamander HCs contained (in mm): 94 caesium gluconate, 9.4 TEACl, 1.9 MgCl2, 9.4 MgATP, 0.5 GTP, 5 EGTA, 32.9 Hepes (pH 7.2). The pipette solution for salamander rods contained (in mm): 15 caesium gluconate, 70 caesium glutamate, 10 TEACl, 3.5 NaCl, 10 Hepes, 5 EGTA, 1 CaCl2, 10 MgATP, 0.5 GTP, 2 glucose (pH 7.2). For current clamp recordings of salamander rod membrane potential, we used gramicidin (5 μg ml−1) as a perforating agent with a pipette solution containing (in mm): 54 KCl, 61.5 KCH3SO4, 3.5 NaCH3SO4, 10 Hepes (pH 7.2).
Whole cell voltage clamp recordings from mouse rods were obtained using perforated patch recording techniques. The perforating agent, amphotericin B, was prepared in a DMSO stock solution (23 mg ml−1) and dissolved at a final concentration of 25 μm into a pipette solution containing (in mm): 125 caesium gluconate, 10 TEACl, 10 Hepes, 3 EGTA, 1 ATP, 0.5 GTP, 3 MgCl2, 1 CaCl2 (pH 7.2). For current clamp recordings of mouse rod membrane potential, we used gramicidin (5 μg ml−1) as a perforating agent with a pipette solution containing (in mm): 98 KCH3SO4, 44 KCl, 3 NaCl, 5 Hepes, 3 EGTA, 3 MgCl2, 1 CaCl2, 2 glucose, 1 Mg-ATP, 1 GTP (pH 7.2).
In both species, rods were voltage clamped using a Multiclamp patch-clamp amplifier or Axopatch 200B amplifer (Axon Instruments, Molecular Devices, Sunnyvale, CA, USA). Currents were acquired using a Digidata 1200 or 1322A interface and pCLAMP 9.2 software (Axon Instruments). Rod ICa was measured using a ramp voltage protocol (−90 to +60 mV, 0.5 mV ms−1) applied from a steady holding potential of −70 mV. We compensated for junction potentials of 13.2 mV (salamander rods) or 14.3 mV (mouse rod voltage clamp recordings) calculated with pCLAMP software. Passive rod membrane resistance measured between −80 and −60 mV was subtracted post hoc.
Salamander rods were identified by shape and HCs by their characteristic responses and morphology (Thoreson et al. 1997). Analysis of charging curves in salamander rods lacking outer segments yielded Cm= 17.1 ± 1.3 pF, Rin= 370 ± 36 MΩ, and Ra= 35.7 ± 2.0 MΩ (n= 18).
In mouse retina, cone inner segments and somas are located in the most distal part of the outer nuclear layer as seen by using FITC-conjugated peanut agglutinin (Bionexus, Oakland, CA, USA) to stain cones in retinal slices (see online supplemental material, Supplemental Fig. 1). To avoid the possibility of recording from cones, we recorded from rod somas in the proximal half of the outer nuclear layer of mouse retina. In mouse rods, Cm= 7.17 ± 0.79 pF, Rin= 1.39 ± 0.56 GΩ, and Ra= 34.7 ± 5.6 MΩ (n= 6).
Imaging
Confocal images were acquired using a laser confocal scanhead (Perkin Elmer Ultraview LCI) equipped with a cooled CCD camera (Orca ER) and mounted to a fixed stage upright microscope (Nikon E600 FN). Excitation and emission were controlled by a Sutter Lambda 10-2 filter wheel and controller. Images were acquired and analysed using UltraView Imaging Suite software. The objective (60×; 1.0 NA) was controlled using a Z-axis controller (E662 Physik Instrumente, Karlsruhe, Germany) to acquire a series of z-slices (1 μm steps) at each time point. Frame duration ranged from 100 to 200 ms and each z-stack was acquired at 10 s intervals.
In salamander, rods were loaded with the calcium sensitive dye, Fluo-4 (KCa= 345 nm), by incubating retinal slices for 45 min in the dark with 10 μm Fluo-4-AM ± 5 μm pluronic F-127 (Invitrogen, Carlsbad, CA, USA), followed by an additional incubation in Fluo-4-AM alone for 1.5 h (5°C). For mouse retina, slices were incubated with 0.5 ml of 10 μm Fluo-4-AM dissolved in 0.5 ml Hibernate-A solution (Brain Bits LLC, Sringfield, IL, USA) for 1 h at room temperature in darkness.
For Ca2+ imaging, rods were depolarized by bath application of solutions containing 20 mm KCl in salamander retina and 30 mm KCl in mouse retina. These solutions were formulated by replacing equimolar amounts of NaCl with KCl in the amphibian or mammalian superfusates described above. Previous measurements showed that high K+ solutions produced effects on rod membrane potential in salamander retina that are accurately described by a modified Goldman–Hodgkin–Katz equation which omits Cl− and assumes a Na+/K+ permeability ratio of 0.13 (Thoreson et al. 2003). After compensating for a 9.3 mV junction potential in the study by Thoreson et al. (2003), this relationship shows that 20 mm K+ depolarizes light-adapted salamander rods to ∼−35 mV. With gramicidin perforated patch whole cell recordings from mouse rods, we determined that application of 30 mm K+ depolarized light-adapted cells from −54.4 ± 2.84 mV (n= 10) to –35.7 ± 1.44 mV (n= 4) after compensating for a 10.4 mV junction potential. For experiments with 10 mm Hepes, the solution pH was adjusted to 7.4 after bubbling with 95% O2–5% CO2 to match the pH of the high K+ solution without Hepes.
Manipulating HC membrane potential
In eyecup experiments, HCs were hyperpolarized by light flashes. Light stimuli were generated using a 50 W halogen lamp focused onto a fibre optic and reflected through a beam splitter into the microscope condenser. Saturating responses were obtained with 580 nm lights ranging in intensity from 1.0 × 103 to 1.1 × 105 photons s−1μm−2 measured with a laser power meter (Metrologic, Blackwood, NJ, USA).
HCs possess non-NMDA ionotropic glutamate receptors (Yang, 2004). HCs were depolarized by applying a glutamate agonist, kainic acid (KA), or hyperpolarized with AMPA/KA antagonists, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione (NBQX) or 6,7-dinitroquinoxaline-2,3-dione (DNQX). For Ca2+ imaging studies, KA (15 μm) and NBQX (3 μm) were bath applied. For electrophysiology, KA (1 mm) and DNQX (100 μm) were puffed from patch pipettes (2 p.s.i., 500 ms) into the OPL using a custom-fabricated pressure ejection device.
Glutamate agonists and antagonists were obtained from Tocris Bioscience (Ellisville, MO, USA). Unless otherwise specified, other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA). The criterion for statistical significance was chosen to be P < 0.05 and evaluated using Student's t-test and GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). Variability is reported as ±s.e.m.
Results
Salamander retina
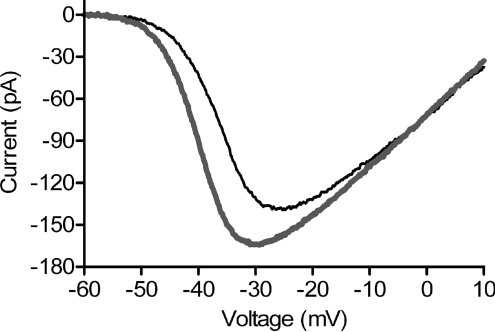
In salamander retinal slices, hyperpolarizing HCs with a bright flash of light caused a small negative shift in rod ICa voltage dependence and increase in ICa amplitude (Thoreson et al. 2008). However, many HCs are excised during the preparation of retinal slices. To assess the impact of HC to rod feedback when all of the HCs are present, we recorded from rods in flatmount retina. To avoid intrinsic light responses, we recorded from occasional rods that had accidentally lost their outer segments during isolation of the retina. Rod ICa was measured in both darkness and light using a ramp voltage protocol (Fig. 1). To quantify the shift in voltage dependence produced by light, we determined the voltage at which the current was half maximal (V50). The shift in V50 produced by hyperpolarizing HCs with a saturating light flash averaged −2.32 ± 0.41 mV (n= 14), roughly twice that observed in retinal slices. ICa amplitude increased by 13.3 ± 7.8%.
Figure 1. Hyperpolarizing HCs with a saturating light flash caused a leftward shift and increase in ICa amplitude of rods recorded from flatmount salamander retina.
Recording was obtained from a light-insensitive rod lacking its outer segment. In this and subsequent figures, ICa was recorded using a ramp voltage protocol (−90 to +60 mV, 0.5 mV ms−1). Thin black trace: dark. Thick grey trace: light.
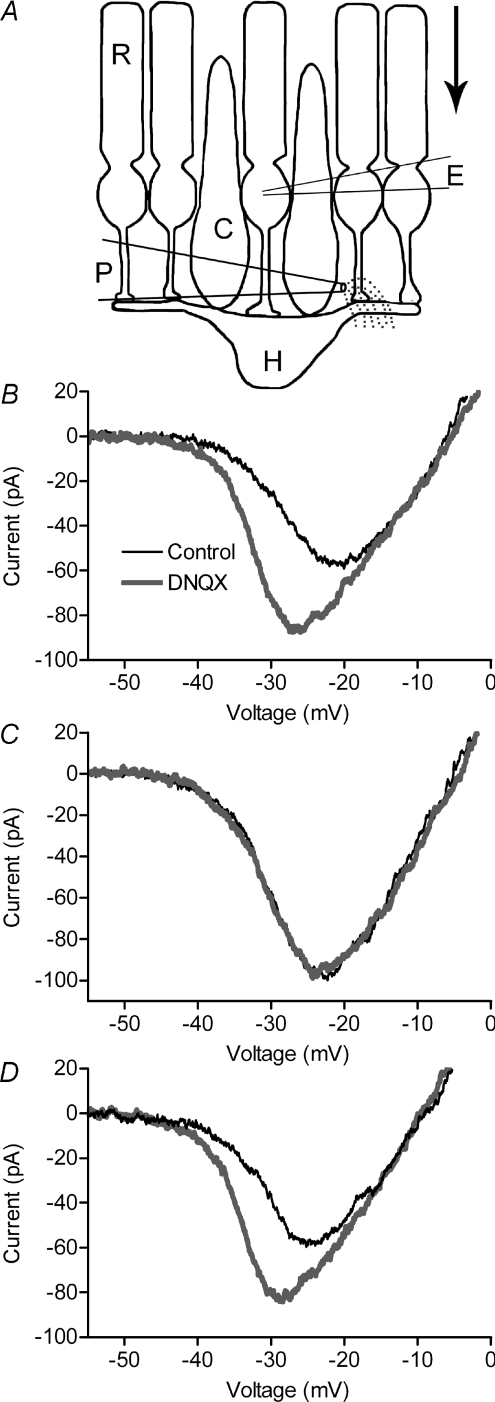
Adapting approaches used for the study of HC to cone feedback (Hirasawa & Kaneko, 2003), we tested HC to rod feedback by recording ICa from rods in retinal slices and manipulating HC membrane potential with glutamate agonists and antagonists. To hyperpolarize HCs, we used a glass pipette to pressure eject a glutamate antagonist, DNQX, onto horizontal cell processes. To depolarize HCs, we puffed on a glutamate agonist, KA. As illustrated schematically in Fig. 2A, the puffer pipette was placed above the OPL so that drugs would reach the dendrites of adjacent HCs. However, the puffer pipette was positioned beyond the synaptic terminal of the voltage-clamped rod so that drugs would be washed away by the flow of superfusate before they could diffuse to the voltage-clamped rod. As expected, puffing DNQX and KA into the OPL did not have any effect on the rod holding current (n= 5 and n= 16, respectively).
Figure 2. Feedback effects on rod ICa of hyperpolarizing HCs with DNQX.
Puffing a glutamate antagonist, DNQX (100 μm) into the OPL of salamander retina (A) caused a leftward shift and increase in peak amplitude of rod ICa (B). ICa was recorded in control conditions (thin black traces) and 525 ms after a puff (thick grey traces). As illustrated schematically in A, the puff pipette was positioned to eject DNQX into the OPL but avoid applying it to the terminal of the voltage-clamped rod. The direction of superfusate flow is indicated by the arrow. Bath application of 10 mm Hepes blocked effects of DNQX on ICa (C). Effects of DNQX recovered after washout of Hepes (D).
Puffing DNQX (100 μm) into the OPL hyperpolarized nearby HCs by an average of 12.3 ± 3.88 mV (n= 10) and they remained hyperpolarized for at least 5 s after the puff. DNQX presumably influenced nearby OFF bipolar cells, but membrane potential changes in bipolar cells do not affect rod ICa (Thoreson et al. 2008). Rod ICa was measured with a ramp voltage protocol (0.5 mV ms−1) applied 525 ms after the 1 s puff. Puffing DNQX caused a significant leftward shift (−2.94 ± 0.40 mV, n= 9, P < 0.0001) and increase (11.7 ± 2.5%, P= 0.0037) in rod ICa (Fig. 2B). The pH buffer Hepes blocks HC to cone feedback (Hirasawa & Kaneko, 2003) as well as feedback interactions between rods and HCs measured in paired simultaneous recordings (Thoreson et al. 2008).
Consistent with these results, addition of Hepes (10 mm) to the bathing solution blocked effects of the DNQX puff on rod ICa (Fig. 2C). In the presence of Hepes, puffing DNQX into the OPL caused a shift in rod ICa of only 0.26 ± 0.14 mV (n= 5, P= 0.14) and increased ICa amplitude by only 0.64 ± 0.81% (P= 0.48). Effects of DNQX on rod ICa recovered after Hepes was washed out of the recording chamber (Fig. 2D).
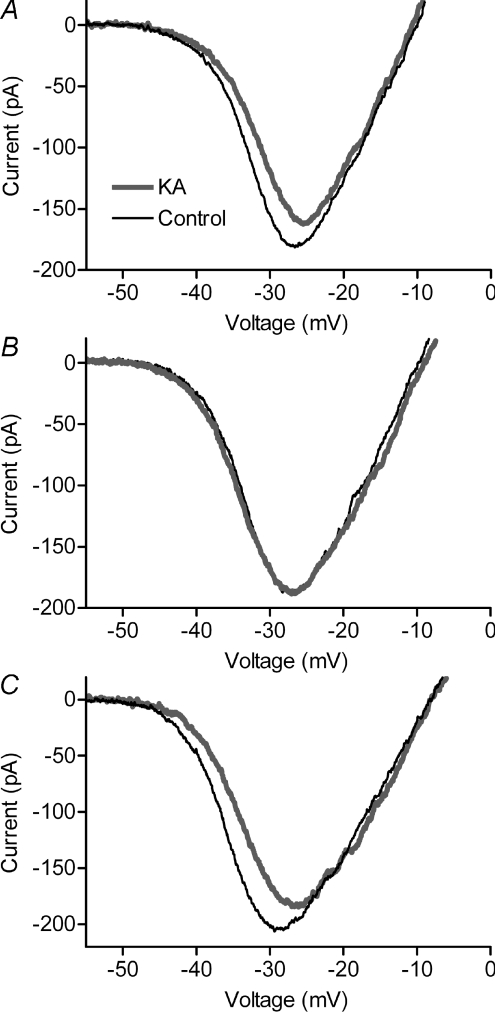
Puffing 1 mm KA into the OPL depolarized HCs by an average of 31.2 ± 4.9 mV (n= 16). Accompanying this HC depolarization was a significant rightward shift (2.94 ± 0.28 mV, n= 19, P < 0.0001) and decrease (–18.2 ± 2.8%, P < 0.0001) in rod ICa (Fig. 3A). Effects of KA on ICa were blocked by bath application of Hepes (Fig. 3B). In the presence of Hepes, puffing KA shifted the V50 by only 0.03 ± 0.13 mV (n= 9, P= 0.80) and reduced the amplitude of ICa by 0.82 ± 0.71% (P= 0.28). Effects of KA on ICa recovered after washout of Hepes (Fig. 3C).
Figure 3. Feedback effects on rod ICa of depolarizing HCs with KA.
Puffing a glutamate agonist, KA (1 mm), into the OPL of salamander retina caused a rightward shift and decrease in peak amplitude of rod ICa (A). Bath application of 10 mm Hepes blocked effects of KA on ICa (B). Effects of KA on ICa recovered after washout of Hepes (C). Thin black trace: control. Thick grey trace: KA.
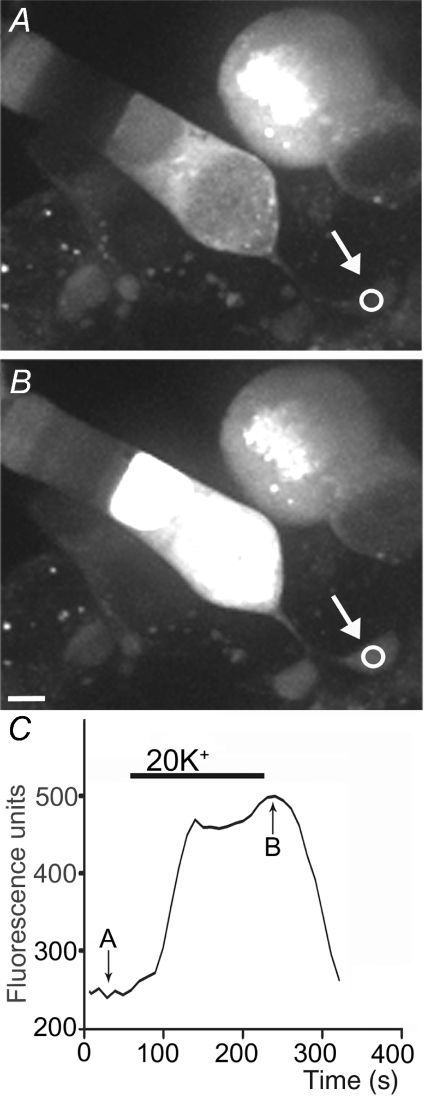
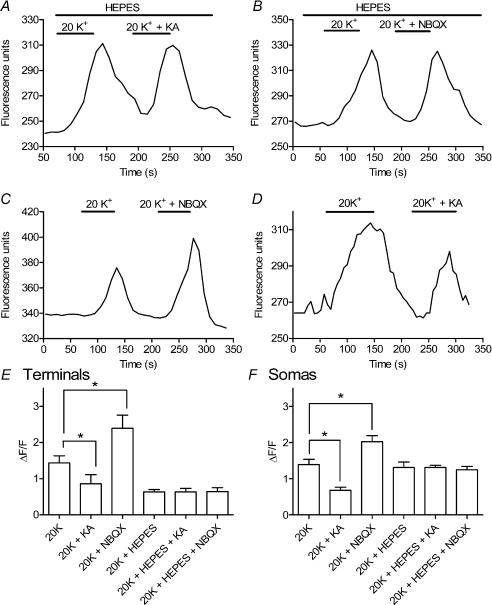
Using approaches developed by Vessey et al. (2005) to study HC to cone feedback, we examined the effects of changes in HC membrane potential on [Ca2+]i in rods by loading retinal slices with a Ca2+-sensitive dye, Fluo-4-AM. Somas and terminals of individual rods were visualized using confocal microscopy. Rods were depolarized to ∼−35 mV, near their dark resting potential, by bath applying a solution containing 20 mm K+ (Thoreson et al. 2003). Application of 20 mm K+ stimulated increases in [Ca2+]i in both the terminals and somas of rods (Fig. 4). ICa is not typically fully activated at a membrane potential of ca−35 mV and thus a leftward shift in voltage dependence should enhance increases in [Ca2+]i evoked by 20 mm KCl. By depolarizing HCs, high K+ solutions should also cause a feedback-induced rightward shift in ICa, opposing the direct actions of high K+ on rod [Ca2+]i. Consistent with these predictions, hyperpolarizing HCs by blocking AMPA/KA receptors with NBQX enhanced the [Ca2+]i increase in rod terminals evoked by 20 mm K+ (Fig. 5A). Conversely, depolarizing HCs by application of KA (15 μm) caused 20 mm K+ to stimulate a smaller intraterminal Ca2+ increase (Fig. 5B). Consistent with the absence of AMPA/KA glutamate receptors on rods (Yang, 2004), bath application of NBQX (3 μm) and KA (15 μm) had no significant effect on ICaV50 (NBQX: 0.2 ± 0.21 mV, n= 4, P= 0.42; KA: −0.2 ± 0.20 mV, n= 4, P= 0.34) or ICa amplitude (NBQX: −1.2 ± 1.0%, n= 4, P= 0.25; KA: −0.2 ± 1.0%, n= 4, P= 0.56) recorded from enzymatically isolated rods. NBQX and KA also had no significant effect on membrane potentials of rods recorded from retinal slices under current clamp conditions using perforated patch techniques (NBQX: −0.1 ± 0.4 mV, n= 8, P= 0.83; KA: 0.0 ± 0.6 mV, n= 8, P= 0.97).
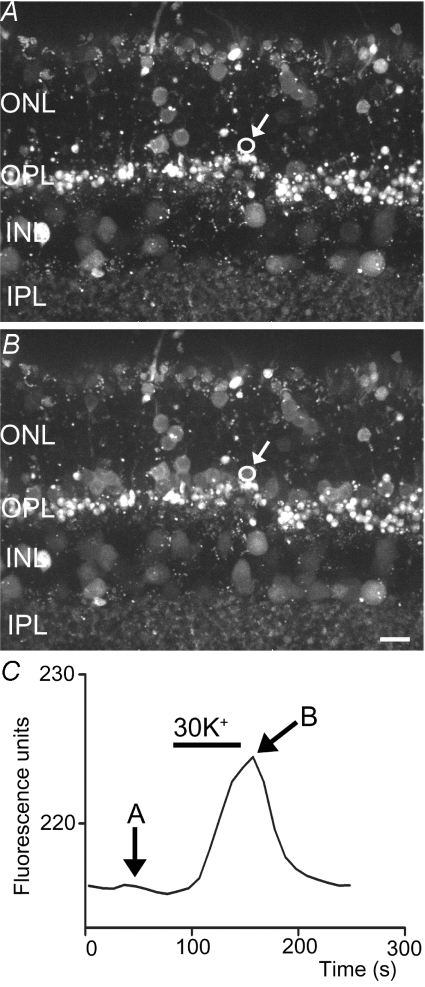
Figure 4. Bath application of 20 mm KCl stimulated an increase in Fluo-4 fluorescence indicating an increase in [Ca2+]i in the rod synaptic terminal (arrow) and soma.
Intraterminal Fluo-4 fluorescence measured in the region of interest (circle) is plotted in C. Images in A and B were obtained at the time points indicated in C. Scale bar = 5 μm.
Figure 5. Bath application of a glutamate antagonist NBQX or glutamate agonist KA caused [Ca2+]i changes in salamander rod terminals consistent with negative feedback effects of HC membrane potential on rod ICa.
A, co-application of NBQX (3 μm) enhanced the [Ca2+]i increase in a salamander rod terminal evoked by bath application of 20 mm K+. B, co-application of KA (15 μm) inhibited the response to 20 mm K+ in a different salamander rod. Effects of NBQX and KA were both blocked by addition of 10 mm Hepes to the bathing medium (C and D). Recordings illustrated in A–D were obtained from different rod terminals in different retinal slices. E, summary of intraterminal Fluo-4 fluorescence changes evoked by high K+ relative to the basal fluorescence level measured prior to high K+ application (ΔF/F). NBQX (3 μm) significantly enhanced [Ca2+]i increases evoked by 20 mm K+ (n= 24 retinal slices, P= 0.012) whereas KA (15 μm) significantly reduced Ca2+ responses (n= 4, P= 0.041). Hepes (10 mm) blocked these effects of NBQX (n= 8, P= 0.016) and KA (n= 3, P= 0.0075). Responses to 20 K+ in the absence of these drugs were also significantly diminished by 10 mm Hepes (n= 18, P= 0.001). Data in the bar graph show changes in ΔF/F averaged from multiple retinal slices (1 data point per slice) and measurements from each slice were averaged from 1 to 8 (mean = 3.1 ± 0.23) individual rod terminals. F, similar effects were observed with high K+-evoked Fluo-4 fluorescence changes measured in rod cell bodies. NBQX (3 μm) significantly enhanced [Ca2+]i increases evoked by 20 mm K+ (n= 12 retinal slices, P= 0.011) whereas KA (15 μm) significantly reduced Ca2+ responses (n= 6, P= 0.006). Hepes (10 mm) blocked effects of NBQX (n= 7, P= 0.048) and KA (n= 9, P= 0.003). Unlike intraterminal fluorescence changes, responses to 20 mm K+ in control conditions were not diminished by 10 mm Hepes (n= 15, P= 0.80). Data in the bar graph show measurements of ΔF/F averaged from multiple retinal slices (1 data point per slice). Measurements from each slice were averaged from 1 to 5 (mean = 2.44 ± 0.16) individual rods.
Results from multiple Ca2+ imaging experiments are summarized in Fig. 5E. To compare results from different cells, we measured the change in Fluo-4 fluorescence evoked by high K+ relative to the basal fluorescence level measured prior to high K+ application (ΔF/F). ΔF/F provides a measure of [Ca2+]i independent of changes in Fluo-4 concentration (Helmchen, 2000). The bar graph shows measurements averaged from multiple retinal slices (1 data point per slice). Measurement data points from each slice were averaged from one to eight (mean = 3.1 ± 0.23) individual rod terminals. Application of NBQX significantly enhanced high K+-evoked Ca2+ increases (P= 0.012) whereas KA significantly inhibited these Ca2+ increases (P= 0.041; Fig. 5E). Application of NBQX or KA alone had no significant effect on Fluo-4 fluorescence (P= 0.14 and P= 0.24, respectively; data not shown). Consistent with effects of KA and NBQX being due to HC to rod feedback, bath application of 10 mm Hepes blocked the enhancement of high K+-evoked Ca2+ increases by NBQX and the reduction of Ca2+ increases by KA (Fig. 5C–E). Similar effects of NBQX, KA and Hepes were observed when Ca2+ increases were measured in rod cell somas rather than terminals (Fig. 5F). One difference between somatic and intraterminal measurements was that intraterminal responses to high K+ were reduced by the application of Hepes under all three conditions whereas somatic Ca2+ increases were not substantially reduced by Hepes alone. This effect of Hepes does not appear to be due to changes in ICa voltage dependence since application of Hepes caused an insignificant shift in ICaV50 of 0.25 ± 1.36 mV (n= 22; P= 0.86). An alternative possibility is that Hepes may alter intraterminal pH since intracellular acidification diminishes the amplitude of Ca2+-dependent changes in Fluo-4 fluorescence (Rohrbach et al. 2005).
Mouse retina
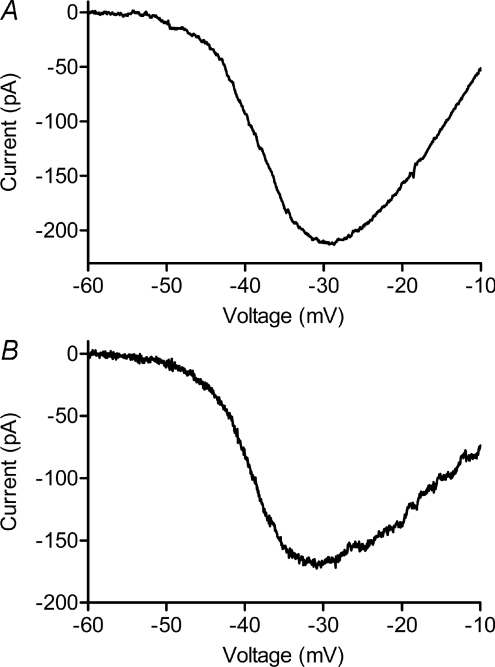
We recorded ICa from mouse rods in retinal slices using perforated patch whole cell recording techniques. The peak amplitude of ICa measured with a ramp voltage protocol (−90 to +60 mV, 0.5 mV ms−1) averaged 48.0 ± 17.8 pA (n= 11) with currents in some cells exceeding 100 pA (Fig. 6). Fitting ICa with a Boltzmann function adjusted for driving force yielded an average V50 of −38.8 ± 2.3 mV and slope factor of −8.34 ± 0.99, similar to properties of ICa reported previously for mouse and pig rods (Morgans et al. 2005; Cia et al. 2005). As illustrated in Fig. 6, the current–voltage relationship of mouse rod ICa was quite similar to ICa in salamander rods. The peak amplitude of salamander rod ICa averaged 88.9 ± 11.4 pA, V50 averaged −38.9 ± 1.6 mV, and slope factor averaged −7.17 ± 0.63 (n= 18).
Figure 6. ICa recorded from mouse (A) and salamander (B) rods were quite similar.
Fitting data in the figure with a Boltzmann function adjusted for reversal potential yielded V50=−37.5 mV with slope factor =−6.76 in mouse (A) and V50=−38.5 mV with slope factor =−5.88 in salamander (B). The salamander rod lacked its outer segment.
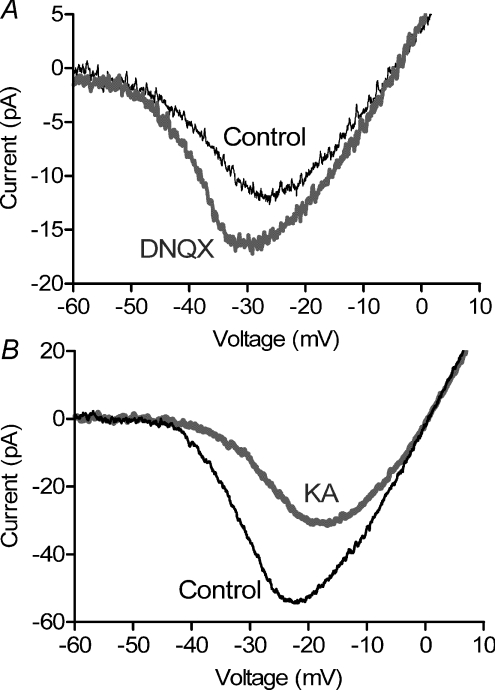
To test for feedback effects of HCs on mouse rod ICa, we performed puffing experiments similar to those described for salamander rods. Hyperpolarizing HCs by puff application of DNQX (100 μm) into the OPL shifted mouse rod ICa to more negative values (−2.67 ± 0.57 mV, P= 0.002, n= 9) and increased ICa peak amplitude by 44.9 ± 5.9% (P < 0.0001). Fig. 7A shows ICa averaged from nine rods in control conditions (thin trace) and 525 ms after the puff (thick trace). Conversely, puffing KA (1 mm) into the OPL to depolarize HCs caused a positive shift in voltage dependence (+4.20 ± 0.91 mV, P= 0.031, n= 5) and decrease in the peak amplitude (−42.3 ± 5.9%, P= 0.048) of rod ICa averaged from five cells (Fig. 7B). Recordings of mouse rod ICa were quite fragile and did not last long enough to test effects of Hepes or obtain recovery in most cases.
Figure 7. Effects of puffing DNQX and KA on mouse rod ICa.
Puffing a glutamate antagonist, DNQX (100 μm) into the OPL of mouse retina (A) caused a leftward shift and increase in peak amplitude of rod ICa. By contrast, puffing a glutamate agonist, KA (1 mm), into the OPL caused a rightward shift and decrease in peak amplitude of ICa (B). ICa was recorded in control conditions (thin black trace) and 525 ms after the puff (thick grey trace). As illustrated in Fig. 2A, the puff pipette was positioned beyond the cell so that DNQX and KA were not applied directly to the voltage-clamped rod. Data in A and B were averaged from 9 and 5 cells, respectively.
We measured Ca2+ levels in individual mouse rods by using confocal microscopy after loading retinal slices with Fluo-4-AM. We depolarized rods to ∼–35 mV by bath application of 30 mm K+. High K+-evoked Ca2+ increases could be observed in both terminals and somas but somatic Ca2+ changes were observed more often. Depolarization-evoked Ca2+ changes were also more frequently observed in somas close to the OPL (Fig. 8) perhaps because these somas are closer to their synaptic terminals. The small size of mouse rod terminals made accurate measurement difficult during small tissue movements and we were concerned about inadvertently measuring Ca2+ changes in cone terminals which also reside in the OPL (Supplemental Fig. 1). We therefore made Ca2+ measurements in the somas of mouse rods. In salamander retina, KA and NBQX produced the same effects on [Ca2+]i in somas and terminals suggesting that Ca2+ changes in mouse rod somas are likely to reflect intraterminal changes.
Figure 8. Bath application of 30 mm KCl stimulated an increase in Fluo-4 fluorescence, indicating an increase in [Ca2+]i in a mouse rod soma.
Fluo-4 fluorescence measured in the region of interest (circle) is plotted in C. The images in A and B were obtained at the time points indicated in C. Scale bar = 5 μm.
Consistent with the increase in Ca2+ influx predicted by a negative activation shift and increase in peak amplitude of ICa, application of 30 mm K+ in the presence of NBQX (3 μm) evoked a larger Ca2+ increase than application of 30 mm K+ alone (Fig. 9A). Conversely, consistent with a positive activation shift and decrease in peak amplitude of ICa, application of 30 mm K+ in the presence of KA (15 μm) evoked a smaller Ca2+ increase (Fig. 9B). As in salamander retina, effects of NBQX and KA were blocked by bath application of 10 mm Hepes (Fig. 9C and D).
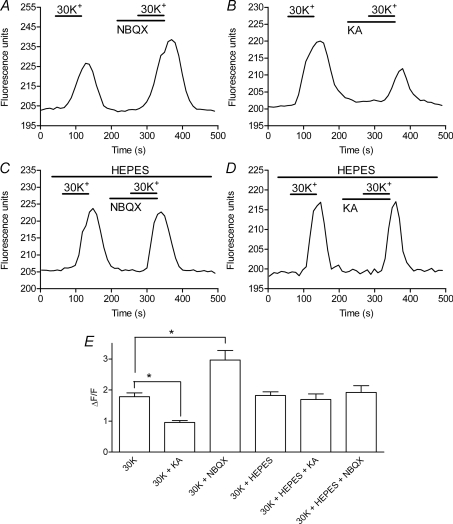
Figure 9. Bath application of a glutamate antagonist, NBQX, or glutamate agonist, KA, caused [Ca2+]i changes in mouse rods consistent with negative feedback effects of HC membrane potential on rod ICa.
A, co-application of NBQX (3 μm) enhanced the [Ca2+]i increase in a mouse rod soma evoked by bath application of 30 mm K+. B, co-application of KA (15 μm) inhibited the response to 30 mm K+. Effects of NBQX and KA were both blocked by addition of 10 mm Hepes to the bathing medium (C and D). Recordings illustrated in A–D were obtained from different rods in different retinal slices. E, intracellular Fluo-4 fluorescence changes evoked by high K+ relative to the basal fluorescence level measured prior to high K+ application (ΔF/F). NBQX (3 μm) significantly enhanced [Ca2+]i increases evoked in mouse rods by 30 mm K+ (n= 18 retinal slices, P= 0.029) whereas KA (15 μm) significantly reduced Ca2+ responses (n= 15, P= 0.0001). Hepes (10 mm) blocked these effects of NBQX (n= 18, P= 0.137) and KA (n= 17; P= 0.730). Changes in ΔF/F were averaged from multiple retinal slices (1 data point per slice) and measurements from each slice were averaged from 1 to 9 (mean = 4.7 ± 0.20) individual rods.
Results of Ca2+ imaging experiments in mouse are summarized in the bar graph in Fig. 9E. The format is the same as Fig. 5E. Measurements were averaged from multiple retinal slices (1 data point per slice) and measurements from each retinal slice represent the average of one to nine (mean = 4.7 ± 0.2) individual rods. As in salamander, application of KA significantly inhibited high K+-evoked Ca2+ increases in mouse rods (P= 0.001) whereas NBQX significantly enhanced Ca2+ increases (P= 0.003). NBQX and KA had no significant effect on [Ca2+]i when applied alone without high K+ (P= 0.14 and P= 0.73, respectively; data not shown). Consistent with effects in salamander and the presence of HC to rod feedback in mouse retina, addition of Hepes blocked the effects of KA or NBQX on the amplitude of Ca2+ responses.
Discussion
The present results provide further evidence for the presence of HC to rod feedback in amphibian retina (Thoreson et al. 2008) by showing that hyperpolarizing HCs with a glutamate antagonist caused a negative shift in rod ICa voltage dependence and an increase in ICa peak amplitude. Conversely, depolarizing HCs with a glutamate agonist caused a positive shift in rod ICa voltage dependence and a decrease in peak amplitude. In addition, at potentials near the dark resting membrane potential, bath application of a glutamate antagonist, NBQX, reduced [Ca2+]i in rod terminals whereas the glutamate agonist, KA, increased [Ca2+]i. We found similar effects on ICa and [Ca2+]i in mouse rods indicating that HCs also provide feedback in mammalian retina. The finding of HC feedback in mammalian rods is consistent with anatomical findings that axon terminals of B-type HCs make synaptic contacts exclusively with rods (Kolb, 1974; Boycott et al. 1978). The presence of HC to rod feedback in both amphibian and mammalian retina indicates that it is a highly conserved feature of vertebrate retinal function.
The properties of ICa were similar in both salamander and mouse rods and are also similar to those reported previously for mouse and pig rods (Morgans et al. 2005; Cia et al. 2005). Differences in pH (Barnes et al. 1993) and divalent cations (Piccolino et al. 1996) can have a significant impact on ICa voltage dependence due to their effects on surface charge. The similarity in the voltage dependence of mouse and salamander ICa is thus partly the result of using the same pH and Ca2+ concentration for both preparations but also indicates an underlying similarity in the Ca2+ channels of both species. The predominant ICa channel subtype in mouse rods is CaV1.4 although CaV1.3 channels are also present (Morgans et al. 2005). When expressed in HEK293 cells, single CaV1.4 channels exhibit a very low maximal open probability of <0.015 and single channel conductance fivefold smaller than other L-type channels (Doering et al. 2005), including single L-type Ca2+ channels recorded from salamander rod terminals (Thoreson et al. 2000). To generate ICa of 48 pA using channels with these properties would require an unrealistically large number of ∼15 000 Ca2+ channels per ribbon. This suggests that either these properties of CaV1.4 are not retained in vivo or there is a substantial contribution to the whole cell current from CaV1.3 channels (Morgans et al. 2005).
In cones, the exocytosis of protons from synaptic vesicles can acidify the synaptic cleft and thereby inhibit ICa (DeVries, 2001). Consistent with this, bath application of Hepes caused a negative shift in voltage dependence of ICa in salamander cones suggesting that Hepes alkalinized the cleft (Cadetti & Thoreson, 2006). By contrast with cones, exocytosed protons do not appear to influence rod ICa (Hosoi et al. 2005). Consistent with this, we found that bath application of Hepes did not significantly alter voltage dependence of rod ICa.
The role of feedback from HCs to rods in shaping visual perception has not been studied. Illumination hyperpolarizes HCs which in turn causes a negative voltage shift and reduction in rod ICa. As expected from these feedback-mediated changes in ICa, we found that [Ca2+]i was enhanced by HC hyperpolarization and reduced by HC depolarization. Given the properties of ICa and effects of HC feedback measured in rods from isolated retina (Fig. 1), HC to rod feedback should restore ∼30% of the decrease in ICa, and thus ∼30% of the reduction in synaptic release (Thoreson et al. 2004), produced by a 10 mV hyperpolarizing light response in rods. Feedback from HCs therefore counters the local reduction in synaptic output caused by the hyperpolarizing light response of an individual rod. Because HCs have large receptive fields, negative feedback from HCs provides a mechanism for subtracting spatially averaged luminance levels from local luminance changes detected by individual rods. In cones, HC feedback generates centre–surround receptive fields (Baylor et al. 1971) and contributes to light adaptation (Burkhardt, 1995). HC to rod feedback may also generate centre–surround receptive fields in salamander retina where bipolar cell receptive fields exhibit centre–surround antagonism under dim light conditions in which they are driven entirely by rods (Hare & Owen, 1990). Centre–surround receptive fields are also evident in the responses of AII amacrine cells from rabbit retina under scotopic conditions where they receive predominantly rod input. However, inner retinal interactions contribute to formation of centre–surround receptive fields in AII amacrine cells and centre–surround receptive fields were not observed in rod bipolar cells (Bloomfield & Xin, 2000). If HC to rod feedback contributes to the surround in AII amacrine cells and other rod-driven neurons then our results suggest that it would be inhibited by Hepes. HCs in some non-mammalian species can receive inputs from both rods and cones (Fain, 1975; Leeper & Copenhagen, 1979; Toyoda et al. 1978; Hanani & Vallerga, 1980) suggesting that HC feedback to rods could potentially generate colour opponent interactions in the rod pathway under mesopic conditions. However, in mammalian retina, rod and cone inputs into horizontal cells are strongly segregated, which would prevent colour-opponent interactions (Pan & Massey, 2007; Trümpler et al. 2008).
Rod and cone feedback appear to operate through similar mechanisms involving changes in ICa voltage dependence and amplitude that are blocked by increased pH buffering (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Cadetti & Thoreson, 2006; Davenport et al. 2008; Thoreson et al. 2008). The similarity between HC feedback in rods and cones suggests that studies on HC to rod feedback in mouse retina may provide insights into feedback mechanisms in cones. Proposed mechanisms of HC to cone feedback include GABAergic feedback from HCs, ephaptic feedback involving hemigap junctions or glutamate receptors, and changes in cleft pH (Byzov & Shura-Bura, 1986; Wu, 1991; Hirasawa & Kaneko, 2003; Fahrenfort et al. 2004; Kamermans & Fahrenfort, 2004; Tatsukawa et al. 2005; Vessey et al. 2005; Davenport et al. 2008). Although pH buffers like Hepes are effective blockers of HC feedback to both rods and cones, the cellular origins of this pH dependence are unclear. The discovery of HC to rod feedback in mouse retina opens up the possibility of using mouse genetics to analyse the mechanisms responsible for HC feedback in rods and cones and study the impact of HC to rod feedback on visual perception.
Acknowledgments
This research was supported by Research to Prevent Blindness and NEI grant EY10542. We thank Ted Bartoletti, Aaron Mercer and Paul Witkovsky for their advice and assistance.
Glossary
Abbreviations
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- HC
horizontal cell
- ICa
calcium current
- KA
kainic acid
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione
- OPL
outer plexiform layer
- V50
voltage at which calcium current was half-maximal
Author contributions
W.B.T. conceived experiments and wrote the first draft of the article. N.B. conducted most of the experiments. Both authors contributed to design, analysis and interpretation of data as well as to revising the article. Both authors approve the final version.
Supplemental material
References
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci U S A. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, O’Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol. 2000;523:771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Peichl L, Wassle H. Morphological types of horizontal cell in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978;203:229–245. doi: 10.1098/rspb.1978.0103. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977;40:53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. The influence of center-surround antagonism on light adaptation in cones in the retina of the turtle. Vis Neurosci. 1995;12:877–885. doi: 10.1017/s0952523800009433. [DOI] [PubMed] [Google Scholar]
- Byzov AL, Shura-Bura TM. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 1986;26:33–44. doi: 10.1016/0042-6989(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cadetti L, Thoreson WB. Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J Neurophysiol. 2006;95:1992–1995. doi: 10.1152/jn.01042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cia D, Bordais A, Varela C, Forster V, Sahel JA, Rendon A, Picaud S. Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. J Neurophysiol. 2005;93:1468–1475. doi: 10.1152/jn.00874.2004. [DOI] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008;28:456–464. doi: 10.1523/JNEUROSCI.2735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Doering CJ, Hamid J, Simms B, McRory JE, Zamponi GW. Cav1.4 encodes a calcium channel with low open probability and unitary conductance. Biophys J. 2005;89:3042–3048. doi: 10.1529/biophysj.105.067124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort I, Sjoerdsma T, Ripps H, Kamermans M. Cobalt ions inhibit negative feedback in the outer retina by blocking hemichannels on horizontal cells. Vis Neurosci. 2004;21:501–511. doi: 10.1017/S095252380421402X. [DOI] [PubMed] [Google Scholar]
- Fain GL. Interactions of rod and cone signals in the mudpuppy retina. J Physiol. 1975;252:735–769. doi: 10.1113/jphysiol.1975.sp011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld HM, Piccolino M, Neyton J. Feed-back modulation of cone synapses by L-horizontal cells of turtle retina. J Exp Biol. 1980;89:177–192. doi: 10.1242/jeb.89.1.177. [DOI] [PubMed] [Google Scholar]
- Hanani M, Vallerga S. Rod and cone signals in the horizontal cells of the tiger salamander retina. J Physiol. 1980;298:397–405. doi: 10.1113/jphysiol.1980.sp013089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA, Owen WG. Spatial organization of the bipolar cell's receptive field in the retina of the tiger salamander. J Physiol. 1990;421:223–245. doi: 10.1113/jphysiol.1990.sp017942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F. Calibration of fluorescent calcium indicators. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2000. pp. 32.1–32.9. [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Arai I, Tachibana M. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J Neurosci. 2005;25:4062–4072. doi: 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol. 2004;14:531–541. doi: 10.1016/j.conb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J Comp Neurol. 1974;155:1–14. doi: 10.1002/cne.901550102. [DOI] [PubMed] [Google Scholar]
- Lasansky A, Vallerga S. Horizontal cell responses in the retina of the larval tiger salamander. J Physiol. 1975;251:145–165. doi: 10.1113/jphysiol.1975.sp011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper HF, Copenhagen DR. Mixed rod-cone responses in horizontal cells of snapping turtle retina. Vision Res. 1979;19:407–412. doi: 10.1016/0042-6989(79)90105-6. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: insight from night blindness. Vis Neurosci. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Normann RA, Pochobradsky J. Oscillations in rod and horizontal cell membrane potential: evidence for feed-back to rods in the vertebrate retina. J Physiol. 1976;261:15–29. doi: 10.1113/jphysiol.1976.sp011546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryan PM. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973;235:207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Massey SC. Rod and cone input to horizontal cells in the rabbit retina. J Comp Neurol. 2007;500:815–831. doi: 10.1002/cne.21127. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Byzov AL, Kurennyi DE, Pignatelli A, Sappia F, Wilkinson M, Barnes S. Low-calcium-induced enhancement of chemical synaptic transmission from photoreceptors to horizontal cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:2302–2306. doi: 10.1073/pnas.93.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach P, Friedrich O, Hentschel J, Plattner H, Fink RHA, Lanzer M. Quantitative calcium measurements in subcellular compartments of Plasmodium falciparum-infected erythrocytes. J Biol Chem. 2005;280:27960–27969. doi: 10.1074/jbc.M500777200. [DOI] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Vis Neurosci. 2005;22:317–324. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Reducing extracellular Cl− suppresses dihydropyridine-sensitive Ca2+ currents and synaptic transmission in amphibian photoreceptors. J Neurophysiol. 1997;77:2175–2190. doi: 10.1152/jn.1997.77.4.2175. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: chloride imaging and cell-attached patch clamp recordings. Vis Neurosci. 2000;17:197–206. doi: 10.1017/s0952523800172025. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ, Rabl K. Reciprocal interactions between calcium and chloride in rod photoreceptors. J Neurophysiol. 2003;90:1747–1753. doi: 10.1152/jn.00932.2002. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Rabl K, Townes-Anderson E, Heidelberger R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron. 2004;42:595–605. doi: 10.1016/s0896-6273(04)00254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Babai N, Bartoletti TM. Feedback from horizontal cells to rod photoreceptors in vertebrate retina. J Neurosci. 2008;28:5691–5695. doi: 10.1523/JNEUROSCI.0403-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda JI, Saito T, Kondo H. Three types of horizontal cells in the stingray retina: their morphology and physiology. J Comp Neurol. 1978;179:569–579. doi: 10.1002/cne.901790308. [DOI] [PubMed] [Google Scholar]
- Trümpler J, Dedek K, Schubert T, de Sevilla Müller LP, Seeliger M, Humphries P, Biel M, Weiler R. Rod and cone contributions to horizontal cell light responses in the mouse retina. J Neurosci. 2008;28:6818–6825. doi: 10.1523/JNEUROSCI.1564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM. Input-output relations of the feedback synapse between horizontal cells and cones in the tiger salamander retina. J Neurophysiol. 1991;65:1197–1206. doi: 10.1152/jn.1991.65.5.1197. [DOI] [PubMed] [Google Scholar]
- Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004;73:127–150. doi: 10.1016/j.pneurobio.2004.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.