Abstract
It has been recently shown that endothelial platelet endothelial cell adhesion molecule-1 (PECAM-1) expression is pro-atherogenic. PECAM-1 is involved in sensing rapid changes in fluid shear stress but the mechanisms for activating signalling complexes at the endothelial cell junction have yet to be elucidated. Additional studies suggest the activation of membrane-bound G proteins Gαq/11 also mediate flow-induced responses. Here, we investigated whether PECAM-1 and Gαq/11 could act in unison to rapidly respond to fluid shear stress. With immunohistochemistry, we observed a co-localization of Gαq/11 and PECAM-1 at the cell–cell junction in the atheroprotected section of mouse aortae. In contrast, Gαq/11 was absent from junctions in atheroprone areas as well as in all arterial sections of PECAM-1 knockout mice. In primary human endothelial cells, temporal gradients in shear stress led to a rapid dissociation of the Gαq/11–PECAM-1 complex within 30 s and a partial relocalization of the Gαq/11 staining to perinuclear areas within 150 min, whereas transitioning fluid flow devoid of temporal gradients did not disrupt the complex. Inhibition of G protein activation eliminated temporal gradient flow-induced Gαq/11–PECAM-1 dissociation. These results allow us to conclude that Gαq/11–PECAM-1 forms a mechanosensitive complex and its localization suggests the Gαq/11–PECAM-1 complex is a critical mediator of vascular diseases.
Detailed analyses of blood flow in atherosclerosis- susceptible vascular regions suggest that the balance of temporal gradients and mean shear stress determines the atherogenicity of haemodynamic signal (Zarins et al. 1983). This pattern of plaque formation, independent of other associated risk factors, has led a number of investigators to suggest that high steady fluid flow within the vasculature is atheroprotective, whereas unsteady flow with a low mean is pro-atherogenic (White et al. 2007).
Given the pulsatile nature of the cardiac cycle, flow patterns are comprised of two distinct and superimposed components: steady and dynamic (temporal). Temporal gradients in shear stress are defined as a rapid increase or decrease of shear stress over a small period of time (<0.3 s) at the same location. For steady shear stress, although levels of shear vary throughout the cardiac cycle, time-averaged fluctuations lack rapid changes in absolute levels of shear stress.
The molecular basis of shear-induced mechanochemical signal transduction and the endothelium's ability to discriminate between flow profiles remain largely unclear. Given that fluid shear stress (FSS) does not involve a traditional receptor–ligand interaction, identification of the molecule(s) responsible for sensing fluid flow and mechanical force discrimination has been difficult (Dai et al. 2004). In addition to the glycocalyx (Tarbell & Ebong, 2008), it has been proposed that the lipid bilayer itself may act as a flow sensing receptor (Gudi et al. 1998). Fluid shear stress is a frictional force that acts on the apical surface of the endothelial monolayer. In an endothelial monolayer, the region of greatest tension has been shown to be at the cell–cell junction (Fung & Liu, 1993) where temporal gradients induce membrane deformation and subsequent signalling (White et al. 2001; Dusserre et al. 2004).
A number of membrane-associated proteins are specifically localized to the cell–cell junction. Platelet endothelial cell adhesion molecule-1 (PECAM-1) is the cell–cell adhesion molecule most abundantly expressed in endothelial cells. In endothelial cells forming a confluent monolayer, it is localized along the cell–cell border (Albelda et al. 1990). When endothelial cells are exposed to physiological levels of fluid shear stress, PECAM-1 has been shown to be tyrosine phosphorylated (Osawa et al. 2002). Given the subcellular localization of PECAM-1 to regions of high mechanical tension, shear-induced PECAM-1 signalling may result from force-induced deformational changes in the molecule (Osawa et al. 2002), suggesting a role in sensing atheroprone haemodynamic flow. This is supported by the recent demonstration that PECAM-1 is a critical mediator of atherosclerosis (Goel et al. 2008; Harry et al. 2008; Stevens et al. 2008). There is also increasing evidence that PECAM-1 serves as a scaffold for other mechanochemical signalling. Nitric oxidesynthase III (eNOS) is localized at the endothelial cell junction and complexes with PECAM-1 (Dusserre et al. 2004). The eNOS–PECAM-1 complex is rapidly disrupted by atherogenic temporal gradients in FSS but not by steady atheroprotective FSS. A mechanosensitive complex involving PECAM-1, the vascular endothelial growth factor-receptor 2 (VEGF-R2) and the vascular endothelial (VE) cadherin has also been shown (Tzima et al. 2005). Here we describe a mechanosensitive complex involving the G protein Gαq/11 with PECAM-1.
The rapid activation of heterotrimeric G proteins is known as one of the earliest flow-mediated responses in endothelial cells (Gudi et al. 1996, 2003), and may also play a role in the ability of the endothelium to discriminate between flow profiles. The sudden temporal onset of flow induces a burst of nitric oxide (NO) production (Frangos et al. 1996). This process is calcium-, G-protein- and PECAM-1-dependent (Dusserre et al. 2004; Bagi et al. 2005). In contrast, the prolonged steady shear stress that follows induces a sustained release of NO, and is calcium-, G-protein- and PECAM-1-independent (Kuchan et al. 1994; Dusserre et al. 2004; Bagi et al. 2005). This suggests that shear-induced stimulation of endothelial cells is derived from the superposition of two independent mechanical stimuli (steady shear and temporal changes in shear), which are in turn transduced by two different mechanochemical pathways. Furthermore, membrane- linked force transduction appears to be mediated, at least in part, by the Gαq/11 heterotrimeric G protein (Gudi et al. 2003).
In the present study, we hypothesized a combined role of Gαq/11 and PECAM-1 in mechanochemical transduction in endothelial cells. We observed that (1) both proteins are co-localized at the cell–cell junctions and (2) signalling events of Gαq/11 activation are associated with PECAM-1. This study is the first to suggest a role of the Gαq/11–PECAM-1 complex in the mechanotransduction response to fluid shear stress. We believe that this study provides additional understanding of how the endothelium senses haemodynamic forces in both normal physiology and vascular disease.
Methods
Cell culture and shear stress
Primary human umbilical vein endothelial cell (HUVEC) isolation was performed as previously described (Frangos et al. 1988). Human umbilical cords were obtained from Sharp Memorial Hospital (San Diego, CA, USA) under the auspices of Sharp Healthcare Institutional Review Board protocol no. 011081. Cells were seeded onto glass microscope slides and grown to confluence within 4 days in M199 media (Irvine Scientific). Prior to all experimental procedures, the HUVECs were serum-starved overnight in ATP-free M199 with 2% BSA to establish quiescence in the monolayer. Similar culture media was used in a conventional parallel-plate flow chamber where confluent HUVEC monolayers were subjected to varying periods of fluid shear stress via a computer-controlled syringe pump: 1 s square-wave (impulse) at 14 dynes cm−2, a continuous series of impulses (pulsatile) at a frequency of 1 Hz, or smoothly ramped transient FSS consisting of a 15 s increase in slowly transitioning FSS from 0–14 dynes cm−2, maintained for 1 s, and a 14–0 dynes cm−2 decrease over 14 s (White et al. 2001). For longer periods of FSS, HUVEC slides were mounted on similar flow chambers connected to a flow loop providing a sustained 19 dynes cm−2 shear stress, maintained at 37°C and ventilated with 95% humidified air with 5% CO2 (Frangos et al. 1988). An oscillatory flow was generated by the addition of an oscillator to create a mean FSS of ±19 dynes cm−2 at a frequency of 1 Hz. A minimal forward flow was provided every hour to deliver nutrients and remove waste products from cells. Slides were quickly transferred to ice-cold PBS (6.7 mm KH2PO4, 150 mm NaCl, pH 7.4) with 2 mm sodium orthovanadate for 1 min and harvested for lysate preparation (see below). Time-matched static or sham (slides mounted on chamber without flow) were performed for all experimental procedures. For G protein inhibition studies, HUVECs were incubated with 900 μm GDPβS (Calbiochem) 4 h prior to shear stress stimulation.
En face and immunohistochemistry microcopy studies
All mouse procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of LJBI (Assurance number A3432) which is authorized by the Office of Laboratory Animal Welfare of the US National Institutes of Health, as required by the Health Resource Extension Act of 1985. PECAM-1 knockout mice (B6; 129S-PECAMtm1Lex) and wild-type mice (B6;129SF2/J) were purchased from Jackson Labs. Mice were anaesthetized with 90 mg kg−1 ketamine and 10 mg kg−1 xylazine, i.p., and then perfused with ice-cold Stefanini buffer (2% paraformaldehyde and 15% picric acid in 0.1 m phosphate buffer, pH 7.6) for 10 min. Aortas were carefully dissected, branches (renal artery bifurcation) and blood flow were recorded, and cross-sectional segments were cut and opened longitudinally, then permeabilized with 0.1% Triton X-100 for 10 min. For double labelling, aortae were successively incubated with: (1) 5% donkey serum to block for non-specific staining; (2) goat anti-PECAM-1 antibody (Santa Cruz Biotechnology, 1/50) and rabbit anti-Gαq/11 antibody (SC, 1/25); (3) donkey anti-goat IgG (Alexa Fluor 488, Molecular Probes) or donkey anti-rabbit (Alexa Fluor 568, Molecular Probes) and nuclei were stained with DAPI (Molecular Probes). Vessels were mounted in Gelvatol (Polyvinal Alcohol) mounting media, internal side up. All samples were observed under a DeltaVision deconvolution microscope system (Applied Precision Inc.) at the UCSD Cancer Center Digital Imaging Shared Resource.
For in vitro studies, HUVECs were fixed in ice-cold methanol/acetone for 10 min and processed as described above with a monoclonal anti-PECAM-1 antibody (R&D systems, 1/1000) and a rabbit anti-Gαq/11 antibody (Santa Cruz Biotechnology, 1/100). Slides were then examined under a confocal fluorescent microscope (Zeiss Pascal LSM5, Carl Zeiss, Germany) equipped with a Plan-Apochromatic 63/1.4 objective.
Preparation of cellular lysates
HUVECs were harvested in octyl glucoside (OG) lysis buffer (60 mmol l−1 OG, 50 mm Tris-HCl pH 7.5, 50 μmol l−1 EGTA, 125 mmol l−1 NaCl, 2 mmol l−1 dithiothreitol, 2 mmol l−1 sodium orthovanadate and protease inhibitors) and incubated on ice for 30 min. Lysates were centrifuged (14 000 g, 20 min, 4°C) and the detergent-soluble supernatant fractions retained.
Coimmunoprecipitation
Aliquots of cell lysates were pre-cleared for 45 min at 4°C with 20 μl of rabbit anti-goat beads (Sigma). Supernatants were incubated overnight with goat anti-PECAM-1 (Santa Cruz Biotechnology, clone M-20) at a final concentration of 8 μg ml−1. Rabbit anti-goat beads were then added and mixed for 1.5 h at 4°C. Bound immune complexes were washed 4 times with lysis buffer. To decrease non-specific binding, bound proteins were released from the beads with an acid elution buffer (0.1 m glycine, pH 2.5, 60 mmol l−1 OG) and immediately neutralized with 1 : 10 volume of 1 m Tris buffer, pH 8.0.
Immunoblot
Denatured immunoprecipitated proteins or crude lysates were separated on NuPAGE 4–12% Bis-Tris gels and transferred to PVDF membrane (Millipore). Membranes were blocked at 1 h with 5% non-fat milk in Tris-buffered saline with 0.1% (v/v) Tween 20 (TBST) and then incubated with primary antibodies (from Santa Cruz Biotechnology) against Gαq/11 (1 : 10 000), PECAM-1 (1 : 5000) for 1 h in 5% milk–TBST. Bound primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies: anti-rabbit IgG True Blot (eBioscience, 1 : 5000) and anti-goat IgG True Blot (eBioscience, 1 : 5000), respectively. Band intensity was quantified on unsaturated X-ray film by a digital image analyser (Bio-Rad Laboratories). All comparisons were made relative to static or sham controls.
Image analysis and statistics
Gαq/11 perinuclear staining was quantified in en face preparations using Image J software (NIH, USA) and each cell showing a full distinct ring of Gαq/11 staining around the nucleus was counted as a positive cell and a ratio was done over the total number of cells observed. A similar quantification was performed in HUVECs cultured in vitro and for each experiment 15 fields were counted with an average of 10 cells per field. The quantification of the Gαq/11 and PECAM-1 co-staining was performed by measuring the intensity of the co-staining (ranges of yellow).
All experimental values are given as mean and standard deviation of the mean. All reported values of n refer to the number of separate and independent experiments from multiple primary HUVEC cultures. Non-parametric statistical comparisons between groups were performed with significance level of P < 0.05.
Results
Gαq/11 localization in endothelial cells from mice aorta
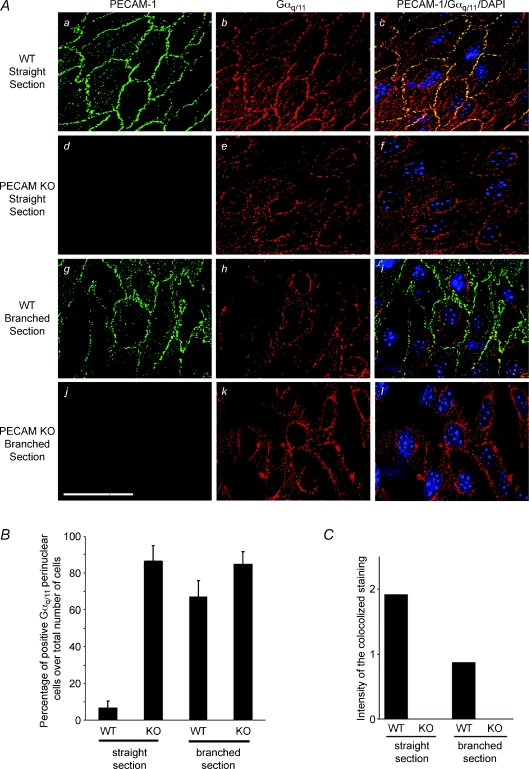
To determine whether Gαq/11 and PECAM-1 co-localize within endothelial cells in vivo, a deconvolution microscopy, double-label immunofluorescence study was performed in freshly isolated aortae from wild-type (WT) and PECAM-1 knockout (KO) mice. In wild-type mice, PECAM-1 was localized along the cell periphery in branch-free areas of the descending aorta (straight section, Fig. 1Aa), described as atheroprotective areas with unidirectional flow and small temporal gradients of fluid shear stress (Nakashima et al. 1998). Similarly, staining with anti-Gαq/11 also reveals strong Gαq/11 staining along the cell periphery and a predominant co-localization of the two proteins was observed at the cell–cell junction (Fig. 1Ab and c). No labelling of PECAM-1 was detected in KO mice and Gαq/11 was absent from the cell–cell junction and instead localized perinuclearly (Fig. 1Ae). Similarly, in WT mouse aortae, junctional Gαq/11 staining was absent at the renal artery bifurcation, which is an atheroprone area exposed to large temporal gradients (Fig. 1Ah). Quantitative studies showed that the percentage of cells with Gαq/11 perinuclear staining was significantly higher in KO mice (86.4 ± 8.5% (s.e.m.), n= 3; Fig. 1B) and renal artery bifurcation (WT: 67.2 ± 8.8%, n= 3; KO: 84.7 ± 6.9%) than WT descending aorta (6.5 ± 3.9%, n= 3). Even though some Gαq/11 staining was still localized to the cell–cell junction, co-localization of Gαq/11 and PECAM-1 in the WT was reduced by half at the renal artery bifurcation as compared to the descending aortae (Fig. 1C).
Figure 1. En face deconvolution microscopy of PECAM-1 and Gαq/11 staining in PECAM-1 knockout (KO) mouse aorta preparations.
A, staining was performed on the descending aorta (straight section) of wild-type (WT) mouse (a–c), PECAM-1 KO mouse (d–f) and branch points of the aorta (renal artery bifurcation) of WT (g–i) and KO mouse (j–l). Double staining was performed to localize PECAM-1 (green) and Gαq/11 (red). Superposition of PECAM-1 and Gαq/11 staining demonstrates co-localization in yellow–orange (right column). Cell nuclei were labelled using DAPI (blue). Scale bar is 20 μm. B, in WT descending aorta only a few cells expressed Gαq/11 staining in perinuclear areas. The number of cells with Gαq/11 perinuclear staining was significantly increased in PECAM-1-deficient mice and in branched regions of WT mice (n= 3 animals for each condition). C, the quantification of the Gαq/11 and PECAM-1 co-staining intensity (ranges of yellow) was reduced to half in branched section compared to straight section of the aorta. KO mice did not express PECAM-1.
Oscillatory flow induces Gαq/11 perinuclear localization in vitro
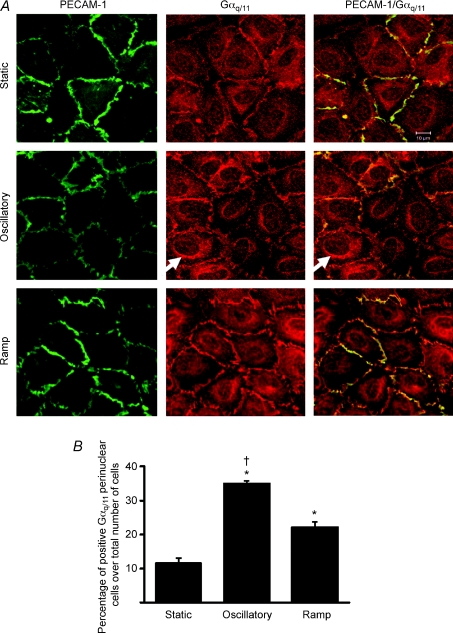
To determine whether shear stress has a role in mediating Gαq/11 translocation in vitro, confluent serum-starved HUVECs were subjected to temporal gradients in FSS for 150 min, a sufficient period of time to allow Gαq/11 translocation (Drmota et al. 1998). The immuno-co-localization of Gαq/11 and PECAM-1 at the cell–cell junction was seen in all conditions and no significant decrease in Gαq/11 junctional staining was detected after 2 h of stimulation. However, under oscillatory flow or slowly ramped FSS of similar value, distinct rings of Gαq/11 staining were detected perinuclearly (arrow, Fig. 2A). Quantification of the Gαq/11 staining shows a significant elevation of cells positive for Gαq/11 perinuclear staining under oscillatory condition versus ramp or static condition (Fig. 2B, P < 0.01, in all cases).
Figure 2. Perinuclear expression of Gαq/11 under shear stress in vitro.
A, coimmunostaining for Gαq/11 (red) and PECAM-1 (green) of serum-starved HUVECs after exposure for 150 min of no flow (Static, upper lane), oscillatory flow (±19 dynes cm−2, 1 Hz, middle lane) or ramped-transient flow (2 min slow ramping to 19 dynes cm−2, maintained for 146 min, then 2 min slow ramping down to 0 dynes cm−2, lower lane). Arrow indicates positive cells with a distinct ring of Gαq/11 perinuclear staining. B, the number of cells with Gαq/11 perinuclear staining increases under flow conditions. Values are mean ±s.d. (*P < 0.01 from static control, †P < 0.01 from ramp; n= 4, 3 and 3 experiments for static, oscillatory and ramp flow conditions, respectively). Scale bar in A is 10 μm.
A single impulse but not ramped-transient flow promotes time-dependent dissociation of Gαq/11–PECAM-1 complex
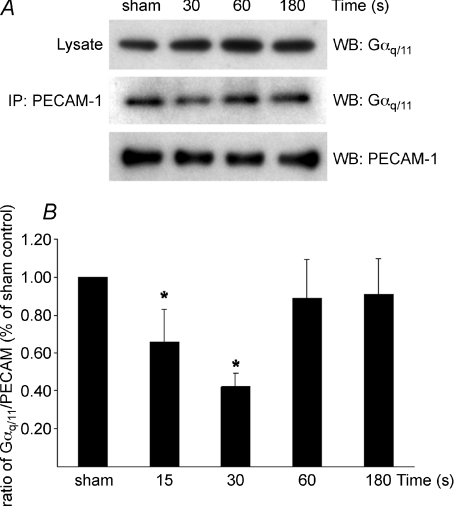
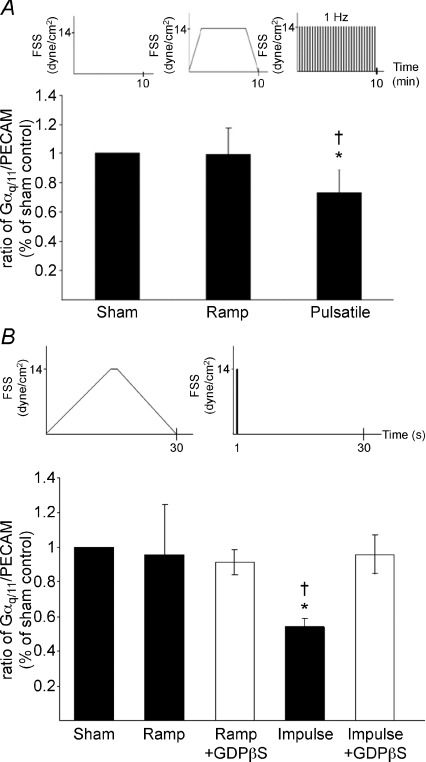
Co-immunoprecipitation of PECAM-1 and Gαq/11 in HUVEC in vitro demonstrates that they form a complex (sham, Fig. 3A). In order to dissect the kinetics of shear-induced dissociation of the Gαq/11–PECAM-1 complex, cells were subjected to a single impulse flow. A single impulse of flow represents a single cycle of pulsatile flow and has previously been shown to stimulate endothelial responses such as BrdU uptake and eNOS–PECAM-1 dissociation as longer term pulsatile flow (White et al. 2001; Dusserre et al. 2004). A rapid disruption of the Gαq/11–PECAM-1 complex was observed 15 s after exposure to a 1 s impulse flow and was maximal 30 s after impulse (42 ± 7% of the control, Fig. 3B). The complex rapidly re-associated after 30 s and the amount of Gαq/11 immunoprecipitated with PECAM-1 subsequently returned to the control (sham) level. Interestingly, Gαq/11–PECAM-1 dissociation was sustained when cells were exposed for 10 min pulsatile flow while a smoothly transitioning ramped flow devoid of temporal gradients did not induce dissociation (Fig. 4A). Similarly, ramped transient flow did not induce the dissociation of the Gαq/11–PECAM-1 complex at 30 s as compared to a single impulse (†P < 0.05, Fig. 4B, filled bars). As similar levels of FSS were achieved in ramped flow, it is clear that FSS itself did not induce complex dissociation. Rather, the dynamic component (temporal gradient in shear stress) appears responsible for the dissociation, making the in vitro findings consistent with the in vivo observations.
Figure 3. Time-dependent dissociation of Gαq/11–PECAM-1 complex in response to impulse flow.
Confluent serum-starved HUVECs were harvested 15, 30, 60 or 180 s after exposure to a single 1 s impulse (temporal gradient) using a syringe pump delivering a 14 dynes cm−2 FSS rate. A, representative Western blot (WB) of one experiment of sham and after 30, 60 and 180 s exposure to the impulse showing lysate Gαq/11, immunoprecipitated (IP) PECAM-1 blotted with Gαq/11 and PECAM-1. B, densitometric analysis of three independent experiments displays the kinetics of association–dissociation of the Gαq/11–PECAM-1 complex following impulse flow. Levels of Gαq/11 co-immunoprecipitated with PECAM-1 were normalized to lysate Gαq/11 levels and immunoprecipitated PECAM-1 levels. Results are percentage of sham controls. Values are mean ±s.d. (*P < 0.05 from sham control).
Figure 4. Impulse but not ramped-transient flow promotes dissociation of the Gαq/11–PECAM-1 complex after 30 s; this dissociation is dependent on G protein activation.
A, pulsatile FSS induces dissociation of the Gαq/11–PECAM-1 complex, whereas slowly transitioning ramped FSS does not. Confluent serum-starved HUVECs were subjected to ramped-transient flow (60 s slow ramping to 14 dynes cm−2, maintained for 8 min, 60 s slow ramping down to 0 dynes cm−2) or pulsatile flow (14 dynes cm−2, 400 pulses of 0.5 s with 1 s intervals for 10 min). Immunoprecipitated Gαq/11 levels were normalized to lysate Gαq/11 levels and immunoprecipitated PECAM-1 levels. Results are percentage of sham controls. Values are mean ±s.d. (*P < 0.01 from sham control, †P < 0.01 from ramp; n= 3 experiments). B, confluent serum-starved HUVECs were pretreated with or without GDPβS for 4 h, subjected to impulse flow (temporal gradient, 1 s at 14 dynes cm−2) or ramped-transient flow (devoid of temporal gradients). Immunoprecipitated Gαq levels were normalized to lysate Gαq/11 levels and immunoprecipitated PECAM-1 levels. Results are percentage of sham controls. Densitometric analysis of three independent experiments shows a significant dissociation of the Gαq/11–PECAM-1 complex following impulse flow in non-treated samples. GDPβS treatment had no effect on the association/dissociation of the complex in sham controls. Inhibition of G proteins with GDPβS completely eliminated impulse flow-induced Gαq/11–PECAM-1 complex dissociation. Values are mean ±s.d. (*P < 0.05 from sham control, †P < 0.05 from ramp).
Shear-induced Gαq/11–PECAM-1 complex dissociation is dependent on G protein activation
To determine whether G protein activation is required to mediate the dissociation of the Gαq/11–PECAM-1 complex, cells were treated with GDPβS, an inhibitor of G protein activation. GDPβS abolished impulse flow-induced Gαq/11–PECAM-1 dissociation (Fig. 4B, open bars). Complex dissociation, therefore, is dependent on G protein activation. GDPβS treatment had no significant effect on the association/dissociation of the complex in cells subjected to ramped flow or sham controls (87 ± 15% of the non-treated sham control; n.s., n= 3).
Discussion
Vascular mechanotransduction has long been recognized as mediating the localization of lesions in atherosclerosis.
There is a consensus that haemodynamic forces represent two distinct biomechanical stimuli: unidirectional fluid shear which is atheroprotective, while rapidly changing and/or reverse flow which is atherogenic (White et al. 2001). The ability of the endothelium to discriminate between these flow components strongly suggests the existence of distinct molecular mechanisms of mechanochemical signal transduction (Frangos et al. 1996). The molecular components distinguishing these transduction pathways, however, remain largely unclear. Both PECAM-1 and G proteins have independently been proposed as mechanosensors (Masuda et al. 1997).
This is the first study to demonstrate that PECAM-1 and Gαq/11 form a complex which is also mechanosensitive. In addition, the association between PECAM-1 and Gαq/11 was shown to discriminate between specific components of fluid flow both in vivo and in vitro. In the atheroprotected region of the descending aorta, Gαq/11 co-localized with PECAM-1 at the cell–cell junctions, yet was localized perinuclearly in atheroprone regions. In vitro, while flow devoid of temporal gradients of shear stress could not induce Gαq/11–PECAM-1 dissociation, dynamic flow was a potent and rapid mediator of PECAM-1 and Gαq/11 dissociation.
The Gαq/11–PECAM-1 complex may be part of a larger macromolecular structure assembled at the endothelial cell–cell junction. Regions of PECAM-1 form a scaffold for the G protein-coupled receptor bradykinin 2 receptor (Yeh et al. 2008), eNOS (Dusserre et al. 2004), VEGFR2 and VE-cadherin (Tzima et al. 2005). Inhibition of dissociation with GDPβS shows that flow-induced Gαq/11–PECAM-1 complex dissociation requires G protein activation and this mechanism may represent the initiation signal leading to activation of the macromolecular complex at the membrane. Indeed, heterotrimeric G protein activation is one of the earliest known responses to shear (Berthiaume & Frangos, 1992; Gudi et al. 1996), with activation occurring within 1 s of flow onset. The activation of G proteins may be direct (Gudi et al. 1998) or indirectly by flow-induced activation of the bradykinin B2 receptor (Chachisvilis et al. 2006).
Although no significant change in Gαq/11 junctional staining was observed in vitro we have shown here that dissociation of the Gαq/11–PECAM-1 complex is accompanied by increased localization of Gαq/11 in the perinuclear region upon 2 h of stimulation with dynamic shear stress. This may be an indication of an initial Gαq/11 translocation, consistent with the reversible trafficking of G protein subunits from the plasma membrane to intracellular sites upon activation (Marrari et al. 2007). In addition, G protein redistribution from the plasma membrane to the Golgi is correlated to the rate of GTP/GDP exchange (Azpiazu et al. 2006). Gαq/11 induces the transcription factor NFκB activation through a Ras–Raf–Erk pathway and subsequently stimulates production of pro-inflammatory cytokines (Gudi et al. 2003; Williams et al. 2007). Furthermore, Gαq/11 inhibition significantly reduces systemic blood pressure and neointima formation after carotid artery injury in mice (Kawasaki et al. 2005). Thus, G protein translocation may be an early step in endothelial pro-inflammatory activation. Conversely, the inability to form junctional Gαq/11 in animals deficient in the junctional scaffold PECAM-1 leads to disruption of Gαq/11 trafficking and pro-inflammatory signalling. This is consistent with the recent findings that PECAM-1 is a critical mediator of atherogenic signalling (Goel et al. 2008; Harry et al. 2008; Stevens et al. 2008).
In summary, this is the first study to demonstrate that PECAM-1 and Gαq/11 form a mechanosensitive complex. The localization of the Gαq/11–PECAM-1 complex to the endothelial cell–cell junction positions this mechanosensor complex to a region of greatest shear-induced mechanical tension (Fung & Liu, 1993; White & Frangos, 2007). Temporal gradients in shear stress lead to a rapid dissociation of the Gαq/11–PECAM-1 complex, whereas fluid flow devoid of temporal gradients does not disrupt the complex. Furthermore, G protein activation is required for Gαq/11–PECAM-1 dissociation. Together with the role of PECAM-1 as a critical mediator of atherogenic signalling, the finding of this flow-dependent complex dissociation may represent a missing link between the primary force-sensing elements of the cell and the downstream mechanochemical transduction pathway.
Acknowledgments
We thank Ronald Y. Kwon for critical reading of the manuscript. This study was supported by NIH-NHLBI Grant HL-40696.
Glossary
Abbreviation
- FSS
fluid shear stress
- HUVEC
human umbilical vein endothelial cell
- KO
knockout
- NO
nitric oxide
- PECAM-1
platelet endothelial cell adhesion molecule-1
- VEGF-R2
vascular endothelial growth factor-receptor 2
- TBST
Tris-buffered saline with Tween 20
- WT
wild-type
Author contributions
L.A.O., K.S.B., L.L., J.-C.Y., B.M., D.N.D, H.Y.S and C.R.W performed the experiments, L.A.O, B.M. and J.A.F prepared the manuscript, and J.A.F designed the experiments and secured the funding.
References
- Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiazu I, Akgoz M, Kalyanaraman V, Gautam N. G protein. βγ11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the a subunit type. Cell Signal. 2006;18:1190–1200. doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- Berthiaume F, Frangos JA. Flow-induced prostacyclin production is mediated by a pertussis toxin-sensitive G-protein. FEBS Lett. 1992;308:277–279. doi: 10.1016/0014-5793(92)81292-t. [DOI] [PubMed] [Google Scholar]
- Chachisvilis M, Zhang Y-L, Frangos JA. G protein-coupled receptors sens fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, García-Cardeña G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drmota T, Novotny J, Kim GD, Eidne KA, Milligan G, Svoboda P. Agonist-induced internalization of the G protein G11á and thyrotropin-releasing hormone receptors proceed on different time scales. J Biol Chem. 1998;273:21699–21707. doi: 10.1074/jbc.273.34.21699. [DOI] [PubMed] [Google Scholar]
- Dusserre N, L’Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA. PECAM-1 interacts with nitric oxide synthase in human endothelial cells, implication for flow-induced nitric oxide synthase activation. Arterioscler Thromb Vasc Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- Fung YC, Liu SQ. Elementary mechanics of the endothelium of blood vessels. J Biomech Eng. 1993;115:1–12. doi: 10.1115/1.2895465. [DOI] [PubMed] [Google Scholar]
- Frangos JA, Huang TY, Clark CB. Steady shear and step changes in shear stimulate endothelium via independent mechanisms–superposition of transient and sustained nitric oxide production. Biochem Biophys Res Commun. 1996;224:660–665. doi: 10.1006/bbrc.1996.1081. [DOI] [PubMed] [Google Scholar]
- Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;11:1996–2002. doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi S, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- Gudi S, Huvar I, White CR, McKnight NL, Dusserre N, Boss GR, Frangos JA. Rapid activation of ras by fluid flow is mediated by Gáq and Gbg subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:994–1000. doi: 10.1161/01.ATV.0000073314.51987.84. [DOI] [PubMed] [Google Scholar]
- Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008;11:2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Taniguchi M, Moritani Y, Uemura T, Shigenaga T, Takamatsu H, Hayashi K, Takasaki J, Saito T, Nagai K. Pharmacological properties of YM-254890, a specific Gáq/11 inhibitor, on thrombosis and neointima formation in mice. Thromb Haemost. 2005;94:184–192. doi: 10.1160/TH04-09-0635. [DOI] [PubMed] [Google Scholar]
- Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol Cell Physiol. 1994;267:C753–C758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7777. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HY, Melchior B, Bell KS, Yun S, Yeh J-C, Frangos JA. PECAM-1 is a critical mediator of atherosclerosis. Dis Model Mech. 2008;1:175–181. doi: 10.1242/dmm.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell JM, Ebong EE. The endothelial glycocalyz: a mechano-sensor and -transducer. Sci Signal. 2008;1:40. doi: 10.1126/scisignal.140pt8. pt8. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- White CR, Frangos JA. The shear stress of it all: all the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Haidekker M, Bao X, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation. 2001;103:2508–2513. doi: 10.1161/01.cir.103.20.2508. [DOI] [PubMed] [Google Scholar]
- Williams R, Zou X, Hoyle GW. Tachykinin-1 receptor stimulates proinflammatory gene expression in lung epithelial cells through activation of NF-κB via a Gq-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2007;292:L430–L437. doi: 10.1152/ajplung.00475.2005. [DOI] [PubMed] [Google Scholar]
- Yeh JC, Otte LA, Frangos JA. Regulation of G protein-coupled receptor activities by the platelet-endothelial cell adhesion molecule, PECAM-1. Biochemistry. 2008;47:9029–9039. doi: 10.1021/bi8003846. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Cir Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]